Abstract
Structured RNAs embedded in the untranslated regions (UTRs) of messenger RNAs can regulate gene expression. In bacteria, control of a metabolite gene is mediated by the self-cleaving activity of a ribozyme embedded in its 5′ UTR1. This discovery has raised the question of whether gene-regulating ribozymes also exist in eukaryotic mRNAs. Here we show that highly active hammerhead ribozymes2,3 are present in the 3′ UTRs of rodent C-type lectin type II (Clec2) genes4–7. Using a hammerhead RNA motif search with relaxed delimitation of the non-conserved regions, we detected ribozyme sequences in which the invariant regions, in contrast to the previously identified continuous hammerheads8–10, occur as two fragments separated by hundreds of nucleotides. Notably, a fragment pair can assemble to form an active hammerhead ribozyme structure between the translation termination and the poly-adenylation signals within the 3′ UTR. We demonstrate that this hammerhead structure can self-cleave both in vitro and in vivo, and is able to reduce protein expression in mouse cells. These results indicate that an unrecognized mechanism of post-transcriptional gene regulation involving association of discontinuous ribozyme sequences within an mRNA may be modulating the expression of several CLEC2 proteins that function in bone remodelling and the immune response of several mammals.
The hammerhead ribozyme is a small, self-cleaving motif composed of a three-helical junction with a core of invariant nucleotides required for activity. To identify hammerhead ribozymes in mammalian mRNAs, we searched mRNA sequence databases using a pattern descriptor that allowed for insertions of up to 5,000 nucleotides at the ends of stem 1 or stem 3 (Supplementary Fig. 1)11,12. Three hammerhead ribozymes were identified in the 3′ UTRs of known rodent mRNAs. Two are found embedded in the transcripts of mouse Clec2d (osteoclast inhibitory lectin, also known as Ocil, Clr-b and Clec2d8)13 and its paralogue Clec2e (also known as Clra and Clec2d7)14, genes that belong to a group of phylogenetically related sequences within the natural killer receptor gene complex of chromosome 6. The third ribozyme is found in rat CLEC2D11 (ref. 7)—a homologue of mouse Clec2d—which resides in the syntenic natural killer receptor gene complex region on chromosome 4. We extended our search to the genomic sequences of other organisms using the UCSC genome browser’s comparative genomics tool15. Alignments using the natural killer receptor gene complex regions of mouse and rat led to the identification of nine candidate hammerhead ribozymes: four in the 3′ regions of predicted rat, horse and platypus Clec2-like genes, and five in the unannotated regions of five other mammalian genomes (Supplementary Fig. 2).
Unlike most known self-cleaving RNA motifs that are contiguous8–10,16–19, the hammerhead ribozymes identified here (referred to as CLEC2 ribozymes) are split into two fragments separated by a long ribozyme-unrelated insertion in the stem-1-capping loop. The insertion (which is 250 and 696 nucleotides in mouse Clec2d and Clec2e, respectively, and 145–1,739 nucleotides in other candidate ribozyme sequences) segregates the upstream substrate region, residing within 7–44 nucleotides of the stop codon, from the downstream enzyme fragment (Fig. 1 and Supplementary Fig. 2). When assembled from substrate and enzyme fragments, the secondary structure reflects a characteristic hammerhead ribozyme core of fifteen conserved nucleotides flanked by three helices. Comparison of all twelve CLEC2 ribozymes showed conservation of stem 2 as well as the presence of compensatory mutations maintaining stem 1 and the atypically long stem 3. Stem 1 and stem 2 contain secondary structure elements required to form a tertiary contact known to greatly enhance catalysis (Fig. 1 and Supplementary Fig. 2)3,20,21. Six loop and bulge residues (Fig. 1)—necessary for the active structure stabilization and optimal catalysis in the Schistosoma mansoni hammerhead ribozyme22—are also found in most CLEC2 ribozyme sequences. Taken together, the secondary structure features suggest that the 3′ UTR-embedded hammerhead ribozyme motifs form catalytically active structures despite their discontinuity.
Figure 1. Sequence arrangement and secondary structure model of the rodent Clec2d hammerhead ribozymes.
The mouse ribozyme sequence is shown in black and the rat ribozyme sequence length, single nucleotide, and base pair differences are denoted in red. The stop codon is shown in white. The substrate and enzyme sequences are shown on orange and blue backgrounds, respectively. The insertion sequence separating two ribozyme parts is abridged with a thick arrow. The predicted cleavage site (white arrowhead) is 3′ of the active site cytosine (circled). The three-helical junction, composed of conserved (with the exception of 2.1 and 1.1) nucleotides (nt) that make catalytically important interactions, is shown in greater detail (together with canonical numbering scheme) in the inset. The small black arrowheads indicate conserved nucleotides of the catalytically important loop/bulge interactions.
To test whether the hammerhead ribozymes embedded within the 3′ UTRs possess catalytic activity, we examined the in vitro transcription products of mouse 3′ UTRs from Clec2d (D-hcu, hammerhead containing UTR) and Clec2e (E-hcu). Cleavage of both 3′ UTR RNAs occurs during the course of the transcription reaction (data not shown) and at the predicted hammerhead ribozyme cleavage sites (Fig. 2). The DM1-hcu construct—which is expected to create an inactive hammerhead ribozyme due to a triple mutation that prevents the ‘substrate’ from being cleaved—yields an intact 3′ UTR transcript, thus confirming that the cleavage is due to the embedded hammerhead ribozyme activity. The mouse Clec2e-derived 3′ UTR cleaves to a lesser extent than D-hcu, perhaps because of misfolding effects due to a ~450 nucleotide longer intervening sequence and/or weakened secondary structure in the stem 1 and stem 3 regions (Supplementary Fig. 3). These results demonstrate that the disconnected ‘substrate’ and ‘enzyme’ fragments form a fully active hammerhead ribozyme.
Figure 2. The discontinuous hammerhead ribozyme-containing 3′ UTRs (hcu) self-cleave in vitro.
Reverse transcriptase primer extensions of in vitro transcribed mouse Clec2d (D-hcu, lane 5) and Clec2e (E-hcu, lane 7) 3′ UTRs show that both RNAs are cleaved at a single location (open arrowheads).D-hcu cleavage site 5′ of U1.1 was identified by dideoxy sequencing of transcription products. Uncleaved E-hcu and triple mutant DM1-hcu are indicated with a black arrowhead. Asterisks indicate non-extended labelled primers.
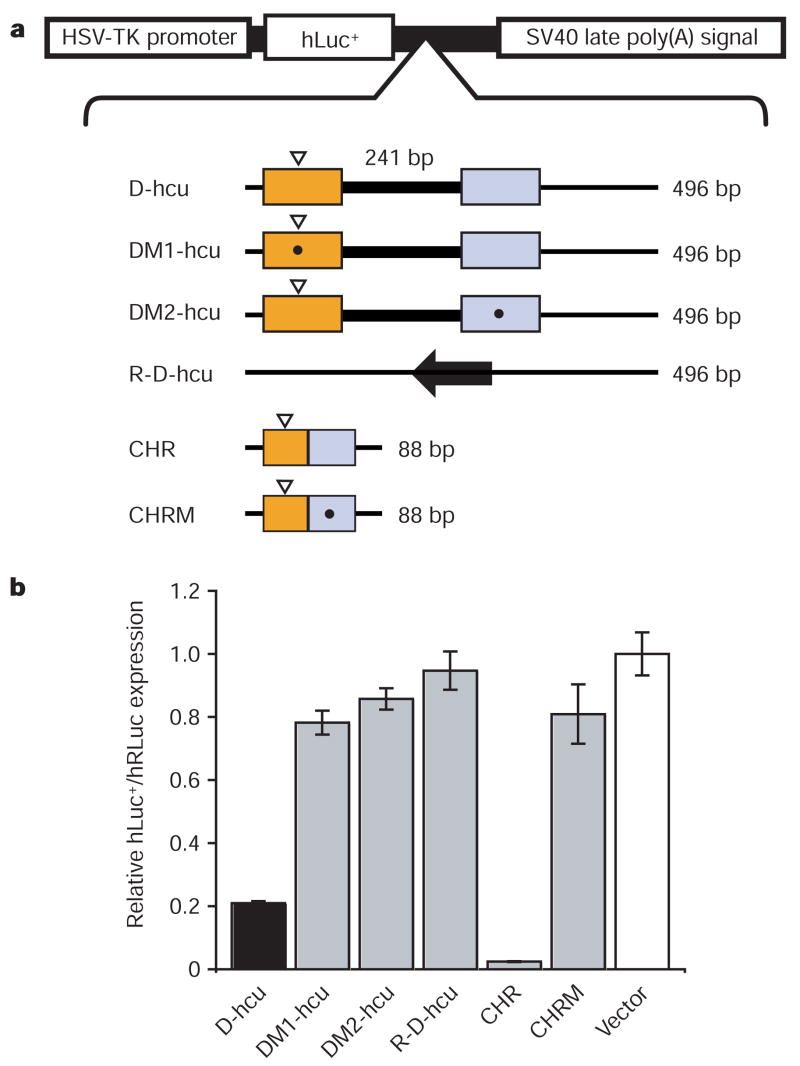
To determine whether the D-hcu sequence can reduce gene expression by forming an active ribozyme in vivo, it was incorporated into a dual-luciferase gene expression vector and assayed for activity in NIH 3T3 cells (Fig. 3). D-hcu reduces luciferase expression by 80% when placed downstream from the luciferase reporter (Fig. 3b). Nonspecific downregulation effects were ruled out because inversion of the same sequence (R-D-hcu) restores luciferase expression levels to that of the positive control. To verify that the reduction of gene expression was caused by the embedded ribozyme, mutations specifically targeting hammerhead ribozyme activity were introduced into D-hcu. Both a single transversion mutation at position G8 in the enzyme fragment (DM2-hcu), previously shown to compromise hammerhead ribozyme activity3, and a triple mutation in the substrate fragment (DM1-hcu) restore gene expression to control levels, indicating that the hammerhead ribozyme embedded in the mouse Clec2d 3′ UTR is responsible for reducing reporter protein expression in mouse cells.
Figure 3. The discontinuous mouse Clec2 hammerhead ribozymes embedded in the 3′ UTRs downregulate protein expression in vivo.
a, Layout of the constructs (top) and the schematic of hammerhead ribozyme-containing 3′ UTR (hcu) sequences used for expression. The colours of substrate (orange) and enzyme (blue) regions correspond to those of the structure in Fig. 1 and the insertion is highlighted by a thick line. The cleavage sites are indicated by white arrowheads and asterisks denote the positions of mutations. CHR (control hammerhead ribozyme) and CHRM (G8 to C8 mutant of CHR) are the controls; bp, base pairs. b, In vivo analysis of firefly luciferase protein expression from different ‘hcu’ constructs. Black denotes expression from the vector containing wild type 3′ UTR; grey represents mutants and controls; and white indicates a vector without an insert as a negative control. Relative firefly luciferase (hLuc+) expression was determined using a dual luciferase assay using the Renilla luciferase (hRLuc) expression for normalization. The results shown are means ± s.e.m. of triplicate experiments; the error bars for the vector value are averages of s.e.m. from three assays.
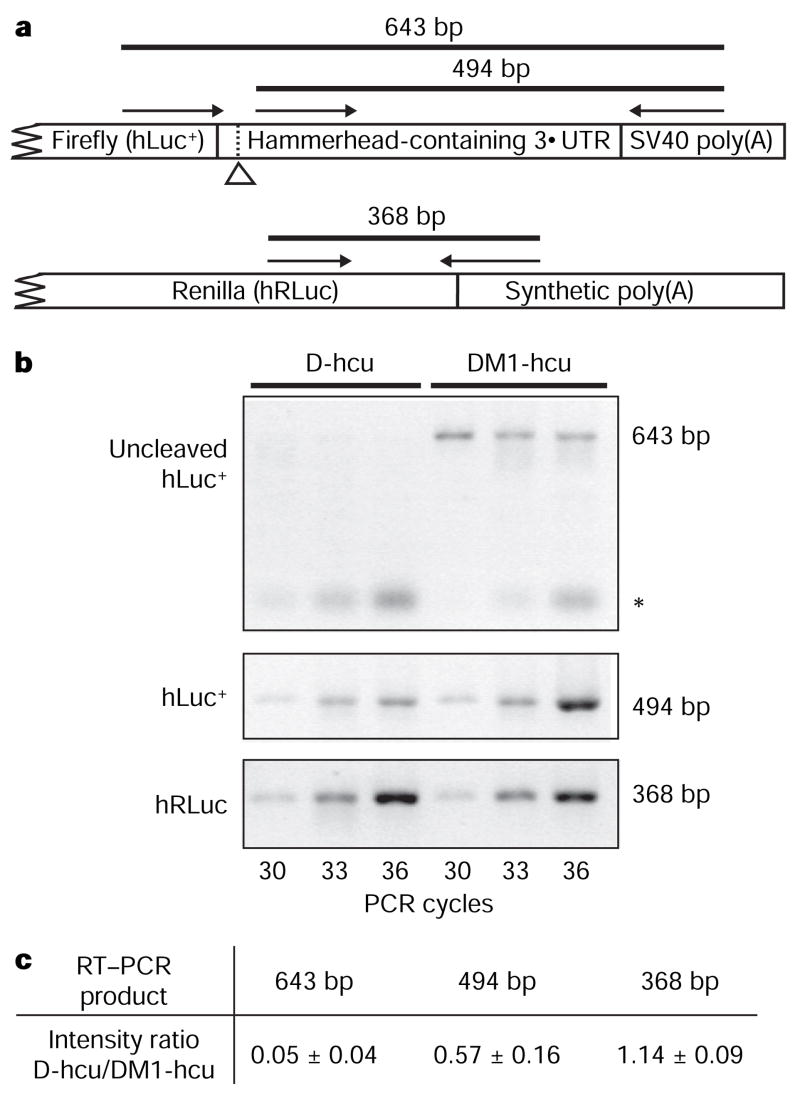
RNA from cells transfected with either wild-type (D-hcu) or mutant (DM1-hcu) dual luciferase reporter vectors was compared using reverse transcription–polymerase chain reaction (RT–PCR) to assess the effect of the discontinuous ribozyme-containing 3′ UTR on mRNA integrity. A mixture of two primers, each recognizing one of two reporter genes transcribed from the same vector, was used for first-strand complementary DNA synthesis (Fig. 4a). Amplification of the region spanning the ribozyme cleavage site indicates that mouse Clec2d 3′ UTR self-cleaves via the hammerhead ribozyme activity (Fig. 4b). The uncleaved product of D-hcu is at least tenfold reduced from that of DM1-hcu, in accordance with results of the protein expression assay (Fig. 4c). Previous studies have shown that ribozymes artificially engineered into 3′ UTRs can downregulate gene expression23 by promoting rapid destruction of the transcript by cytoplasmic RNA degradation machinery24. Consistent with these observations, the catalytic activity of mouse Clec2d 3′ UTR correlates with the overall reduction of firefly luciferase transcript levels, as compared to those of the mutant UTR-containing mRNA, indicating that the cleaved Clec2 transcript is degraded in vivo.
Figure 4. RT–PCR analysis of the in vivo expression products.
a, Schematic of the relative positions of the RT–PCR primers. The hammerhead-specific cleavage site is indicated by an open arrowhead. The 368 base pair (bp) product acts as the internal control for transfection efficiency and the extent of transcription. b, RT–PCR of RNA isolated from NIH 3T3 cells that have been transfected with pD-hcu or pDM1-hcu. The asterisk indicates nonspecific PCR products. c, Quantification of the difference in PCR product intensity between D-hcu and DM1-hcu. The product intensity was used to determine the ratio of D-hcu/DM1-hcu at each listed cycle. Each value represents the mean ± s.d. of intensity ratios from three listed cycles.
Structural conservation observed throughout the identified sequences, in conjunction with the tight association of the ribozyme with Clec2-like genes, suggests that the discontinuous ribozymes are orthologous to the mouse hammerhead motif characterized here. Within the rodent lineage, the rat CLEC2D11 ribozyme sequence shares 86% homology with the mouse Clec2d ribozyme. Similarly, two predicted rat Clec2d paralogues are closely (61% and 67%) related to the mouse Clec2e ribozyme (Supplementary Fig. 2). These sequence relationships positively correlate with phylogenetic evidence for multiple duplication events of rodent Clec2 genes7. Further genome searches using separate parts of the hammerhead ribozyme detected four additional mouse Clec2 paralogues that contain substrate-like, but not enzyme, sequences in their 3′ UTRs (data not shown). These partial motifs are also positioned proximally to the stop codon and share extensive primary sequence identity with the substrate regions of functional ribozymes of either mouse Clec2d or Clec2e. However, it is not clear if the incomplete ribozyme sequences are functional substrates for the Clec2d- and Clec2e-derived trans-acting ribozyme elements, or are non-functional evolutionary relics of the rodent Clec2 gene expansion.
Identification of CLEC2 ribozymes in the genomes of mammalian species other than rodents (notably platypus) indicates that these ribozymes share an early mammalian ancestor. Active hammerhead motifs have been found in several divergent species, such as newts10, plants8 and viroids10. However, only the Schistosoma satellite DNA is known to encode hammerhead ribozyme sequences that have significant homology to the CLEC2 ribozymes3,18. Although schistosomes parasitize rodents, their ribozymes are found in lineages more modern than the time of divergence of mouse and rat species18,25, suggesting a horizontal gene transfer of the hammerhead sequence from host to the parasite genome as the probable scenario.
In eukaryotes, post-transcriptional regulation of gene expression through the 3′ UTR involves an interaction between mRNA-stabilizing and -destabilizing protein-based processes26. Together with evidence of cross-species secondary structure conservation, our results indicate that the CLEC2 hammerhead ribozymes have roles analogous to those of destabilizing protein factors. Paired with a yet undiscovered antagonistic process, the hammerhead motif may constitute a system of rapid regulation for CLEC2d, a transmembrane C-type lectin that has a dual role: the inhibition of natural killer (NK)-cell-mediated lysis through the interaction with the NK cell receptor NKR-P1, and the inhibition of osteoclast formation4,6.
The core sequences of CLEC2 ribozymes resemble those of the smaller members of its ribozyme class, but the discontinuous arrangement of the substrate and enzyme regions exhibit properties previously associated only with catalytic introns. Although functioning through a fundamentally different mechanism and residing in transcripts distinct from processed mRNA, catalytic sequences of some self-splicing group I and II introns span long stretches of RNA19 on a scale analogous to that of the ribozymes described here. A question remains as to whether CLEC2 ribozymes can also process substrates encoded on detached transcripts similarly to RNase P, which possesses trans-cleaving properties unique among naturally occurring ribozymes19. Most self-cleaving RNAs, however, are considered to be compact sequences suitable for single-step functions such as multimer-to-monomer conversion of genomes in the course of viroid and satellite RNA replication, or for response to small molecule binding such as in metabolite-dependent gene regulation in bacteria. However, in the context of complex gene regulatory systems such as those of mammalian cells, the discontinuous configuration of the CLEC2 ribozyme sequences may provide an opportunity for allosteric regulation.
METHODS SUMMARY
mRNA sequence data from the Ensembl database (release 48, http://www.ensembl.org) were searched using the RNABOB program11,12 with a descriptor (Supplementary Fig. 1) for the hammerhead ribozyme motif. Orthologues of the rodent CLEC2 ribozymes were identified using BLAT and the UCSC Genome Browser (http://genome.ucsc.edu/)15,27. For in vitro RNA synthesis and analysis, the Clec2 3′ UTR-containing plasmids were linearized and transcribed using T7 RNA polymerase at a pH of 8.0 and in the presence of 28 mM MgCl2. Reverse transcriptase primer extensions and RNA sequencing were adapted for a [32P]-end-labelled primer from previously published methods28. For the expression assays, the NIH 3T3 mouse fibroblast cells were transiently transfected with Clec2 3′ UTR-containing psiCHECK-2 reporter plasmids (Promega) and analysed using the Dual-Glo Luciferase Assay System (Promega). Firefly reporter expression was normalized using expression of the Renilla luciferase encoded on the same plasmid. RNA from the transfected cells was purified using Trizol (Sigma). For RT–PCR, cDNA was synthesized using primers specific to plasmid-derived transcripts using previous methods28. PCR was performed with Accuprime Taq (Invitrogen). PCR products were resolved on an agarose gel and the product quantification was performed using ImageQuant 5.2 (Molecular Dynamics).
Supplementary Material
Full Methods and any associated references are available in the online version of the paper at www.nature.com/nature.
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Acknowledgments
We thank M. Hall and K. Chakrabarti for assistance with cell culture, members of Haussler and Ares laboratories for sharing tissue culture space, M. Robertson for the transcriptase and A. Zahler for discussion. This work was supported by the National Institutes of Health grant R01043393.
Footnotes
Author Contributions L.H.H. and M.M. did the sequence searches, designed the study, performed the experiments, analysed the data and wrote the manuscript.
References
- 1.Winkler WC, et al. Control of gene expression by a natural metabolite-responsive ribozyme. Nature. 2004;428:281–286. doi: 10.1038/nature02362. [DOI] [PubMed] [Google Scholar]
- 2.Khvorova A, Lescoute A, Westhof E, Jayasena SD. Sequence elements outside the hammerhead ribozyme catalytic core enable intracellular activity. Nature Struct Biol. 2003;10:708–712. doi: 10.1038/nsb959. [DOI] [PubMed] [Google Scholar]
- 3.Martick M, Scott WG. Tertiary contacts distant from the active site prime a ribozyme for catalysis. Cell. 2006;126:309–320. doi: 10.1016/j.cell.2006.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou H, et al. Osteoclast inhibitory lectin, a family of new osteoclast inhibitors. J Biol Chem. 2002;277:48808–48815. doi: 10.1074/jbc.M209059200. [DOI] [PubMed] [Google Scholar]
- 5.Plougastel B, Dubbelde C, Yokoyama WM. Cloning of Clr, a new family of lectin-like genes localized between mouse Nkrp1a and Cd69. Immunogenetics. 2001;53:209–214. doi: 10.1007/s002510100319. [DOI] [PubMed] [Google Scholar]
- 6.Carlyle JR, et al. Missing self-recognition of Ocil/Clr-b by inhibitory NKR-P1 natural killer cell receptors. Proc Natl Acad Sci USA. 2004;101:3527–3532. doi: 10.1073/pnas.0308304101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hao L, Klein J, Nei M. Heterogeneous but conserved natural killer receptor gene complexes in four major orders of mammals. Proc Natl Acad Sci USA. 2006;103:3192–3197. doi: 10.1073/pnas.0511280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Przybilski R, et al. Functional hammerhead ribozymes naturally encoded in the genome of Arabidopsis thaliana. Plant Cell. 2005;17:1877–1885. doi: 10.1105/tpc.105.032730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rojas AA, et al. Hammerhead-mediated processing of satellite pDo500 family transcripts from Dolichopoda cave crickets. Nucleic Acids Res. 2000;28:4037–4043. doi: 10.1093/nar/28.20.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flores R, et al. Hammerhead ribozyme structure and function in plant RNA replication. Methods Enzymol. 2001;341:540–552. doi: 10.1016/s0076-6879(01)41175-x. [DOI] [PubMed] [Google Scholar]
- 11.Gautheret D, Major F, Cedergren R. Pattern searching/alignment with RNA primary and secondary structures: an effective descriptor for tRNA. Comput Appl Biosci. 1990;6:325–331. doi: 10.1093/bioinformatics/6.4.325. [DOI] [PubMed] [Google Scholar]
- 12.Eddy SR. RNABOB: a program to search for RNA secondary structure motifs in sequence databases. 2008 http://selab.janelia.org/software.html.
- 13.Zhou H, et al. A novel osteoblast-derived C-type lectin that inhibits osteoclast formation. J Biol Chem. 2001;276:14916–14923. doi: 10.1074/jbc.M011554200. [DOI] [PubMed] [Google Scholar]
- 14.Carlyle JR, et al. Molecular and genetic basis for strain-dependent NK1.1 alloreactivity of mouse NK cells. J Immunol. 2006;176:7511–7524. doi: 10.4049/jimmunol.176.12.7511. [DOI] [PubMed] [Google Scholar]
- 15.Kent WJ, et al. The human genome browser at UCSC. Genome Res. 2002;12:996–1006. doi: 10.1101/gr.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salehi-Ashtiani K, Luptak A, Litovchick A, Szostak JW. A genomewide search for ribozymes reveals an HDV-like sequence in the human CPEB3 gene. Science. 2006;313:1788–1792. doi: 10.1126/science.1129308. [DOI] [PubMed] [Google Scholar]
- 17.Teixeira A, et al. Autocatalytic RNA cleavage in the human β-globin pre-mRNA promotes transcription termination. Nature. 2004;432:526–530. doi: 10.1038/nature03032. [DOI] [PubMed] [Google Scholar]
- 18.Ferbeyre G, Smith JM, Cedergren R. Schistosome satellite DNA encodes active hammerhead ribozymes. Mol Cell Biol. 1998;18:3880–3888. doi: 10.1128/mcb.18.7.3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Serganov A, Patel DJ. Ribozymes, riboswitches and beyond: regulation of gene expression without proteins. Nature Rev Genet. 2007;8:776–790. doi: 10.1038/nrg2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Canny MD, et al. Fast cleavage kinetics of a natural hammerhead ribozyme. J Am Chem Soc. 2004;126:10848–10849. doi: 10.1021/ja046848v. [DOI] [PubMed] [Google Scholar]
- 21.Osborne EM, Schaak JE, Derose VJ. Characterization of a native hammerhead ribozyme derived from schistosomes. RNA. 2005;11:187–196. doi: 10.1261/rna.7950605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martick M. PhD thesis. Univ. California; 2007. Structural Study of how the Hammerhead Ribozyme Solves the Problem of Catalysis. http://www.proquest.com/ [Google Scholar]
- 23.Yen L, et al. Exogenous control of mammalian gene expression through modulation of RNA self-cleavage. Nature. 2004;431:471–476. doi: 10.1038/nature02844. [DOI] [PubMed] [Google Scholar]
- 24.Meaux S, Van Hoof A. Yeast transcripts cleaved by an internal ribozyme provide new insight into the role of the cap and poly(A) tail in translation and mRNA decay. RNA. 2006;12:1323–1337. doi: 10.1261/rna.46306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lockyer AE, et al. The phylogeny of the Schistosomatidae based on three genes with emphasis on the interrelationships of Schistosoma Weinland, 1858. Parasitology. 2003;126:203–224. doi: 10.1017/s0031182002002792. [DOI] [PubMed] [Google Scholar]
- 26.Garneau NL, Wilusz J, Wilusz CJ. The highways and byways of mRNA decay. Nature Rev Mol Cell Biol. 2007;8:113–126. doi: 10.1038/nrm2104. [DOI] [PubMed] [Google Scholar]
- 27.Kent WJ. BLAT–the BLAST-like alignment tool. Genome Res. 2002;12:656–664. doi: 10.1101/gr.229202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stern S, Moazed D, Noller HF. Structural analysis of RNA using chemical and enzymatic probing monitored by primer extension. Methods Enzymol. 1988;164:481–489. doi: 10.1016/s0076-6879(88)64064-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Full Methods and any associated references are available in the online version of the paper at www.nature.com/nature.
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.