Abstract
HIV-1 proteins, such as Tat and gp120 are believed to play a crucial role in the central nervous system (CNS) pathology of acquired immune deficiency syndrome (AIDS). The present study sought to determine the potential role of Tat and/or gp120 on behavioral development and the relationship with the assessed long-term effects of the HIV-1 proteins on the rat hippocampus. Male pups of 13 Sprague-Dawley litters were bilaterally injected on postnatal day (P)1. Every litter contributed an animal to each of four treatment condition: VEH (0.5µl sterile buffer), gp120 (100ng), Tat (25µg) or combined gp120+Tat (100ng+25µg). Body weight was not affected by either protein group. Tat revealed a transient effect on many of the behavioral assessments early in development as well as on preattentive processes and spatial memory in adulthood. Gp120 had more selective effects on negative geotaxis (P8–10) and on locomotor activity (P94–96). Combined gp120+Tat effects were noted for eye opening with potential interactive effects of gp120 and Tat on negative geotaxis. Anatomical assessment at ~7½ month of age was conducted by using design-based stereology to quantify the total cell number in five hippocampal subregions [granule layer (GL), hilus of the dentate gyrus (DGH), cornu ammonis fields (CA)2/3, CA1, and subiculum (SUB)] (Fitting et al., 2007a). A relationship between early reflex development and estimated cell number in the adult hippocampus was indicated by simple regression analyses. In addition, estimated number of neurons and astrocytes in the DGH explained 81% of the variance of the distribution of searching behavior in the probe test. Collectively, these data indicate that the DGH may participate in the spatial memory alterations observed in adulthood consequent to neonatal exposure to HIV-1 proteins. (Supported by DA013137, DA014401, HD043680).
Keywords: Tat, gp120, HIV-1, development, behavior, stereology, neuron, glia
1. Introduction
Since the first cases of human immunodeficiency virus (HIV) were identified 25 years ago (Gottlieb et al., 1981, Mildvan et al., 1982), the scale of the global HIV/AIDS epidemic has exceeded all expectations. Approximately 33 million people are currently living with HIV, and approximately 25 million men, women, and children have died of AIDS (UNAIDS/WHO, 2007).
The importance of studying perinatally acquired HIV/AIDS is derived from two key issues: First, the entry of the virus occurs in an immature immune and nervous system and interferes with a period that is most vulnerable to the developing system (Belman, 1997). The adverse effects of HIV-1 often result in more rapid onset of clinical symptoms and progression to death in infants relative to adults. The prevalence of children (< 15 years) dying of AIDS is 2.3-times greater compared to adults (UNAIDS/WHO, 2007). Second, in contrast to adult infection the peripheral nervous system is rarely involved in pediatric HIV-1. The CNS disease in children occurs often independent of other opportunistic infections (Mintz, 2005), indicating the importance of studying HIV-1 effects on the CNS independent of HIV-1 effects on the immune system. HIV-1 entry into the brain and HIV-1 infection of the CNS is a critical element in the development of neurological disorders. Domains affected by CNS HIV-1 infection include impaired brain growth (Wiley et al., 1990), delays in developmental milestones (Epstein et al., 1986), motor dysfunction (Mintz et al., 1996), attentional disorders and other potential cognitive dysfunction (Mintz et al., 1994). With the development of antiretroviral therapies, more severe cases, such as progressive encephalopathy, have been significantly reduced (Epstein, 1986, Tardieu et al., 2000). Nevertheless, HAART-treated children still develop CNS disease as studies show that the CNS can serve as a site for relentless viral replication (Martin et al., 2005).
Whether clinical and pathological substrates of HIV-1 infection can be attributed to the direct action of the virus on neurons itself, remains a matter of active debate (Bagasra et al., 1996, Torres-Munoz et al., 2001, Trillo-Pazos et al., 2003, Takahashi et al., 1996, Thompson et al., 2004). A more established finding is that the interaction of neurons with regulatory (e.g., Tat, rev) and structural (e.g., gp120, gp41) viral gene products plays an important role in the development of neurological abnormalities (Bansal et al., 2000, Behnisch et al., 2004, Brenneman et al., 1988, Catani et al., 2003, Nath et al., 2000a, 2000b, Cheng et al., 1998, Fauci, 2006). These gene products are believed to be released by activated HIV-1-infected cells, such as macrophages/microglia and astrocytes, and are then available extracellularly in the HIV-1-infected brain (Kramer-Hämmerle et al., 2005, Lannuzel et al., 1997, Mattson et al., 2005). Specifically, the envelope glycoprotein 120 (gp120) and the transactivator of transcription (Tat) have been measured in the brain tissue of patients with HIV-1-associated dementia (Jones et al., 2000, Del Valle et al., 2000) and are likely agents of the observed neuronal loss in the brains of AIDS patients.
Gp120-induced neurotoxicity has been reported, by both in vitro and in vivo studies, to be primarily mediated by N-methyl-D-aspartate (NMDA) receptor mechanisms (Barks et al., 1997, Lipton et al., 1995). By binding to and/or indirectly activating cell surface receptors, such as CXCR4 and NMDA, gp120 may trigger neuronal apoptosis and excitotoxicity as a result of oxidative stress, perturbed cellular calcium homeostasis and mitochondrial alterations (Corasaniti et al., 2001, Haughey & Mattson, 2002, Lipton, 1991). In contrast, Tat appears to promote HIV-1 neurotoxicity by interacting directly with neurons and causing oxidative stress as an early step in neuronal degeneration (Aksenov et al., 2001, 2003, 2006, Aksenova et al., 2005, 2006, Bansal et al., 2000). Neuronal cell culture studies demonstrated that exposure of neurons to Tat triggers an early increase in intracellular reactive oxygen species production followed by accumulation of oxidatively modified proteins and subsequent neuronal death (Aksenov et al., 2006, Aksenova et al., 2005, 2006). Studies examining effects of Tat on behavior and development indicate adverse Tat-induced effects on the developing CNS of perinatal animals (Barks et al., 1997, Fitting et al., 2006b). A developmental study examining inhibition of the auditory startle response (ASR) in developing rats demonstrated a shift in the peak inhibition and flattening of the latency to startle for males and females, respectively (Fitting et al., 2006b). The authors concluded that Tat is able to impair the cognitive processes involved in sensorimotor gating. Collectively, it is suggested that both protein neurotoxins, gp120 and Tat, play important roles in pediatric HIV-1 infection of the CNS and the development of HIV-1-associated neuropsychological complications in children.
The behavioral effects of Tat and gp120 may be attributed to alterations of neurobiological mechanisms of the hippocampus. A stereology design-based anatomical study assessed loss of different cell types in five subdivisions of the hippocampal formation [granule layer (GL), hilus of the dentate gyrus (DGH), cornu ammonis fields (CA)2/3, CA1, and subiculum (SUB)] (Fitting et al., 2007a). Differential effects of gp120 and Tat were noted; gp120 treatment reduced total neuron number in the CA2/3 field, whereas Tat decreased the number of neurons in both the CA2/3 field and the DGH. A corresponding Tat-induced significant increase in glial cell number was noted with no readily discernible effect for gp120. A key component of the present study was to test whether long-term alterations in neural systems were predictive of the behavioral outcomes observed in young and adult rats. Various studies have reported the involvement of the hippocampus in behavioral measures such as prepulse inhibition (PPI) (Koch & Schnitzler, 1997, Swerdlow et al., 2001) and spatial learning and memory (O’Keefe & Nadel, 1978). In the present study, conducting correlation and regression analyses directly tested the relationship between behavior and hippocampal anatomy. Additionally, the role of specific hippocampal subdivisions and their participation in behavioral measures, such as spatial memory, was assessed to potentially provide new insights for behavior-anatomy relationships.
The aims of the present study were two-fold: First, to determine the immediate and long-term impact of gp120- and Tat neurotoxicity, and their interaction, on behavior in young and adult rats following intrahippocampal injection on postnatal day (P)1. Behavioral assessments focused on the domains that have been reported to be affected by CNS HIV infection in children. Basic developmental milestones were examined, such as somatic growth and early reflex function, as well as performance measures of motor and cognitive functions, including locomotor activity, PPI, and spatial learning and memory. Ontogenic and longitudinal analyses were conducted to establish the nature and extent of behavioral alterations that occurred in response to virotoxin exposure (Tat/gp120) in neonates. It was hypothesized that both HIV-1 proteins should have an impact on the behavioral measures assessed, specifically those involving the hippocampus, such as PPI and spatial memory. Second, to determine the relationship between the behavioral measures and the recently reported long-term effects of the HIV-1 proteins on the estimated total number of cells in the five subregions of the rat hippocampus (Fitting et al., 2007a). It was hypothesized that the hippocampus plays an important role in behavioral measures such as PPI and spatial memory and that specific hippocampal subdivisions may differentially participate in the behavioral alterations observed in young and adult rats.
2. Results
Data analysis
As appropriate, data were expressed as mean (± S.E.M.) or median (± Interquartile Range). Data on a continuous scale were analyzed in a 2×2 factorial design, using analysis of variance (ANOVA) techniques (SYSTAT 11.0 for Windows, SYSTAT Inc.). ANOVAs included two between-subjects factors, gp120 treatment (yes, no) and Tat treatment (yes, no). The 2×2 factorial design and additional planned contrasts were employed to answer three basic questions: First, if there is a significant overall HIV-1 protein treatment effect? This question was answered by comparing the VEH control group with the three HIV-1 protein-treated groups (overall protein treatment effect). Second, if there is a significant gp120 or Tat treatment effect? This question was answered by ANOVAs that tested for significant main effects of gp120 or Tat treatment. Third, which of the three treatment groups differed significantly from the vehicle control group? To answer this question, the VEH control group was compared to each of the three HIV-1 protein groups.
Mixed model ANOVAs were conducted when appropriate, including a within-subjects factor (i.e. day and trial) in the 2×2 factorial design. The three main questions were maintained with the within-subjects factor as an interaction term (e.g., Tat × Day, Gp120 × Day, etc.). For the within-subjects terms, potential violations of sphericity were preferentially handled by the use of orthogonal decomposition (Winer, 1971).
The non-parametric Mann-Whitney U-Test was performed on the rank scores of the somatic growth index of eye opening. Using the same conceptual approach inherent in a 2×2 factorial design we used four Mann-Whitney U-tests to evaluate 1) an overall protein treatment effect, 2) a main effect for gp120 or Tat treatment, and 3) a comparison of the combined gp120+Tat group to the VEH control group. For categorical interstimulus interval (ISI) data from the prepulse inhibition (PPI) test, the Fisher’s exact test was applied. An alpha level of p ≤ 0.05 was considered significant for all statistical tests used.
The relationship between behavioral and anatomical measures was assessed conducting correlation and multiple regression analyses. The purpose of the last set of analyses was to determine if observed changes in the estimated total number of cells in specific hippocampal areas were significantly predictive of behavioral outcome.
Somatic Growth
Body Weight
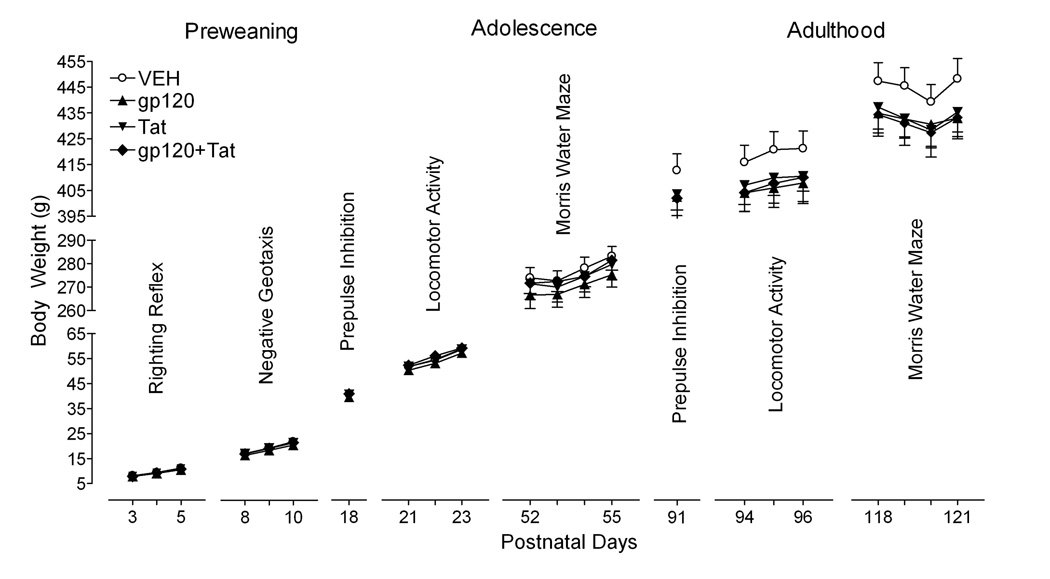
Figure 1 illustrates the mean (± S.E.M.) body weight data across the different test days for each treatment group. First, no overall protein treatment effect was noted for any of the test sets. Second, no gp120 or Tat treatment or treatment by day interaction was noted for any of the test sets. Even though HIV-1 protein treatments did not significantly affect body weight, the three HIV-1-protein groups started to show decreased weights (approximately 3%) in adulthood compared to the VEH control group.
Figure 1.
Mean (± S.E.M) body weight across the various test days by treatment group. No significant treatment effects and no significant treatment by test day interactions were noted.
Eye opening
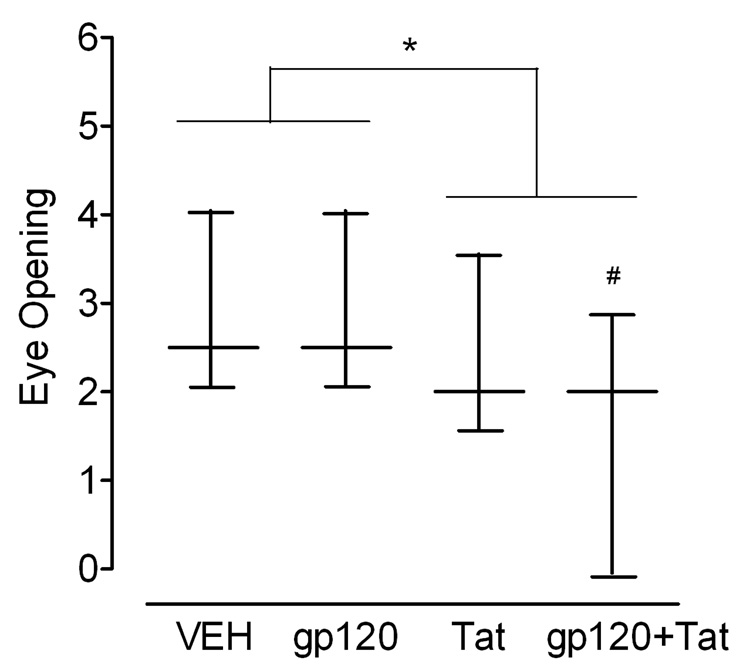
Figure 2 illustrates the median (± Interquartile Range) for the eye status collapsed across P14–16. First, no overall protein treatment effect was noted. A significant difference was revealed for Tat treatment [U = 396.5, p ≤ 0.05]. Second, the sum of the ranks equaled 696.5 for the non-Tat-treated animals and 528.5 for the Tat-treated animals. No effect was noted for gp120 treatment. Third, a significant difference was noted when comparing the ranks for the vehicle control group (190.5) to the combined gp120+Tat group (134.5) [U = 112.5, p ≤ 0.05]. In sum, the combined data (collapsed across days) indicated a significant deficit in eye status for those animals that received neonatal Tat. A specific effect was noted for the combined gp120+Tat group. The daily observation data failed to confirm any significant alterations as a function of treatment.
Figure 2.
Median (± Interquartile Range) for ranked eye opening scores collapsed across P14–16 by treatment group. Tat treatment induced significant deficits in eye opening (*p ≤ 0.05). VEH controls significantly differ from the combined gp120+Tat group (#p ≤ 0.05).
Early Reflex Development
Righting Reflex
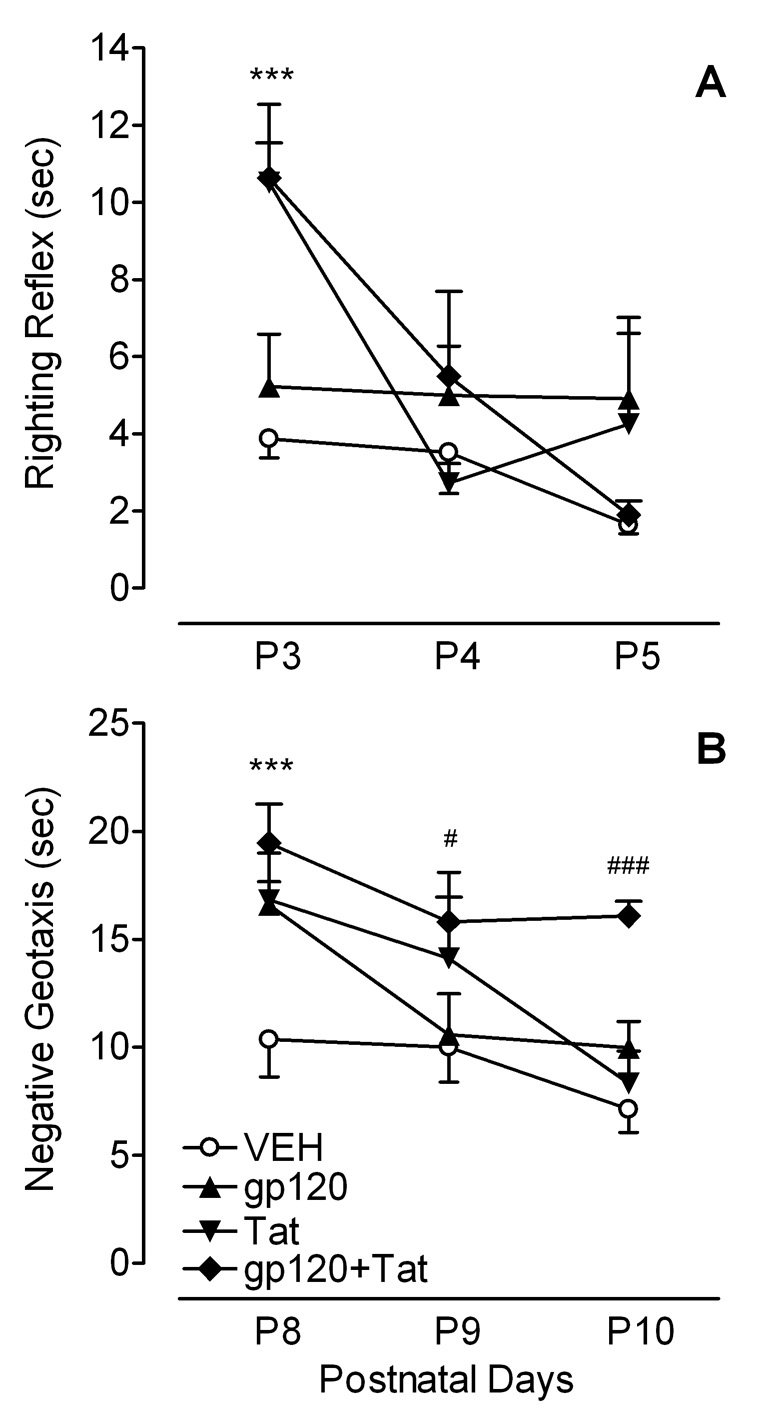
Figure 3A illustrates mean (± S.E.M.) response time for righting reflex across P3–4. First, a significant overall protein treatment effect conducted on latency (time in sec) was noted collapsed across the three test days [F(1, 45) = 6.0, p ≤ 0.05], primarily contributed by the first test day on P3 [F(1, 45) = 12.4, p ≤ 0.001]. Second, a 2 (Gp120) × 2 (Tat) × 3 (Test days) mixed-model ANOVA revealed a significant main effect of Tat [F(1, 45) = 6.9, p ≤ 0.05] and test day [F(2, 90) = 1835.0, p ≤ 0.001]. Further, a significant Tat × Day interaction was noted [F(2, 90) = 15.3, p ≤ 0.001], suggesting a test day-dependent alteration by Tat treatment. Third, planned contrast analyses revealed a significant difference in the progression of the righting reflex across the three test days between VEH controls and combined gp120+Tattreated animals [F(2, 46) = 8.5, p ≤ 0.01] and between VEH controls and Tat-treated animals [F(2, 45) = 9.3, p ≤ 0.001]. Whereas, VEH control group displayed a fast righting reflex for all three test days, Tat treatment significantly delayed the onset (P3) of the developmental sensory-motor reflex [F(1, 45) = 26.3, p ≤ 0.001]. However, on the last day of testing (P5) all HIV-1 protein-treated groups approximated the performance level of the VEH controls (see Figure 3A).
Figure 3.
Mean (± S.E.M.) response time across three days by treatment group. (A) Righting reflex indicates a significant Tat effect for the first test day (***p ≤ 0.001). No treatment effect was noted for the other two test days. (B) Negative geotaxis indicates a significant overall protein treatment effect for the first test day (***p ≤ 0.001). For P9 and P10 only combined gp120+Tat group continued to differ from the VEH controls (#p ≤ 0.05 and ###p ≤ 0.001, respectively).
Negative Geotaxis
Figure 3B illustrates the mean (± S.E.M.) response time for negative geotaxis across P8–10. First, a significant overall protein treatment effect conducted on latency (time in sec) was noted collapsed across the three test days [F(1, 45) = 13.8, p ≤ 0.001], primarily contributed by the first (P8) and last (P10) test days [F(1, 45) = 21.8, p ≤ 0.001 and F(1, 45) = 9.9 p ≤ 0.01, respectively]. Second, a 2 (Gp120) × 2 (Tat) × 3 (Test days) mixed-model ANOVA revealed significant main effects for Tat [F(1, 45) = 10.5, p ≤ 0.01] and for gp120 [F(1, 45) = 15.8, p ≤ 0.001], with overall slower response latencies for both treatment conditions. Additionally, a significant main effect of test day [F(2, 90) = 16.0, p ≤ 0.001] and a three-way interaction of Gp120 × Tat × Day was revealed [F(2, 90) = 4.0, p ≤ 0.05]. As illustrated in Figure 3B, the three-way interaction suggested persisting slow response latencies across test days for the combined gp120+Tat group; in contrast to a transient single test day alteration with either protein alone. This finding is confirmed by a third set of analyses. Planned contrast analyses revealed differences in the progression of negative geotaxis across the three test days. Whereas the gp120- and the Tat-treated groups revealed linear response learning curves [F(1, 11) = 20.8, p ≤ 0.001 and F(1, 11) = 12.5, p ≤ 0.01, respectively], the combined gp120+Tat group showed no significant learning. Compared to VEH controls each of the three HIV-1 protein groups differed on test day one (P8) [gp120 group: F(1, 45) = 12.8, p ≤ 0.001, Tat group: F(1, 45) = 11.4, p ≤ 0.01, combined gp120+Tat: F(1, 45) = 20.6, p ≤ 0.001], but only the combined gp120+Tat group continued to differ from the VEH controls on P9 and P10 [F(1, 45) = 5.3, p ≤ 0.05 and F(1, 45) = 26.5, p ≤ 0.001, respectively].
Sensorimotor function (PPI of the ASR)
Control Trials (0, 4000msec ISI combined)
For preweanlings (P18) and adults (P91), no overall protein treatment effect was noted on peak ASR amplitude. A two-way ANOVA, with gp120 and Tat as between-subjects factors, revealed no significant effects, suggesting that the treatment groups did not differ in their baseline startle response.
PPI trials (8–120msec ISI)
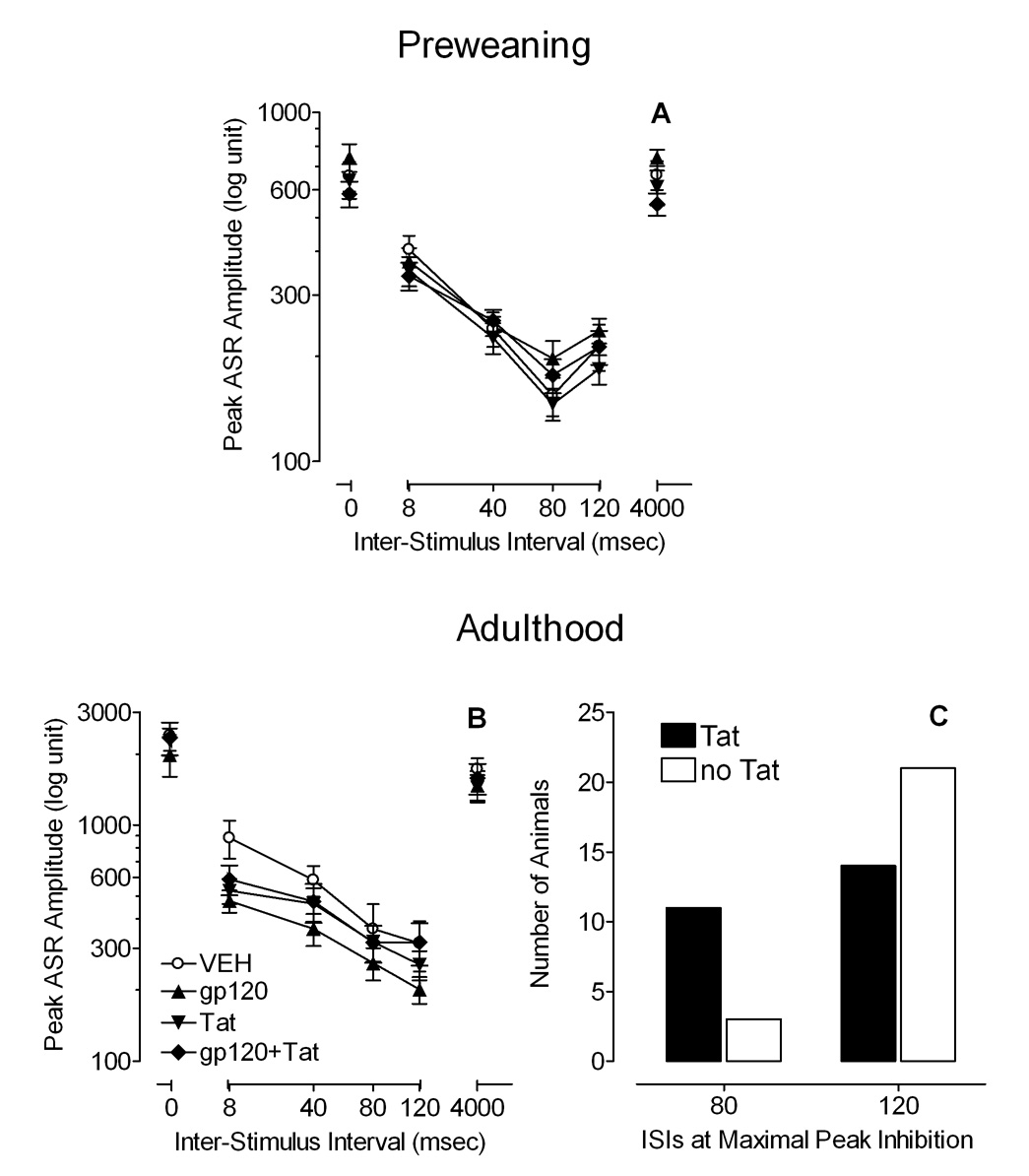
For preweanlings, Figure 4A illustrates mean (± S.E.M.) peak ASR amplitude across ISI (0–4000msec ISIs). First, no overall protein treatment effect was noted on peak ASR amplitude of the PPI trials. Second, a 2 (Gp120) × 2 (Tat) × 4 (08, 40, 80, 120msec) mixed-model ANOVA revealed no effects or interactions of Tat and/or gp120. A significant main effect of ISI was noted [F(3, 135) = 55.9, p ≤ 0.001] with a prominent linear component [F(1, 45) = 65.0, p ≤ 0.001]. For the ISI data, maximal peak inhibition occurred at 80msec with no significant differences across treatment.
Figure 4.
Mean (± S.E.M.) peak ASR amplitude across ISIs (0–4000msec ISI), (A) in preweaning development at P18, and (B) in adulthood at P91. (C) In adulthood, ISI data of the maximal peak response inhibition demonstrated a significant flattening of the peak inhibition function for Tat treatment (p ≤ 0.05).
For adults, Figure 4B illustrates mean (± S.E.M.) peak ASR amplitude across ISI (0–4000msec ISIs). First, no overall protein treatment effect was noted on peak ASR amplitude of the PPI trials. Second, a 2 (Gp120) × 2 (Tat) × 4 (08, 40, 80, 120msec) mixed-model ANOVA revealed no main effect of gp120 or Tat, but a significant main effect of ISI [F(3, 135) = 54.6, p ≤ 0.001] with a prominent linear component [F(1, 45) = 76.7, p ≤ 0.001]. Further, a significant ISI × Gp120 × Tat interaction was noted [F(3, 135) = 3.5, p ≤ 0.05], indicating that the Tat-treated animals were less responsive to manipulation of ISI in the PPI trials then the other groups; i.e., the inhibition curve is flatter. Third, for the ISI data (see Figure 4C), maximal peak inhibition occurred only on PPI trials 80 and 120msec ISIs. A Fisher’s Exact test revealed a significant flattening of the peak inhibition function for Tat relative to the VEH- and the gp120-treated animals (p ≤ 0.05). Thus, whereas most of the non-Tat treatment animals indicated a maximum peak inhibition response at 120msec ISI, Tat treatment flattened the peak inhibition function with a significant shift of maximum peak inhibition response to 80msec.
Locomotor Activity
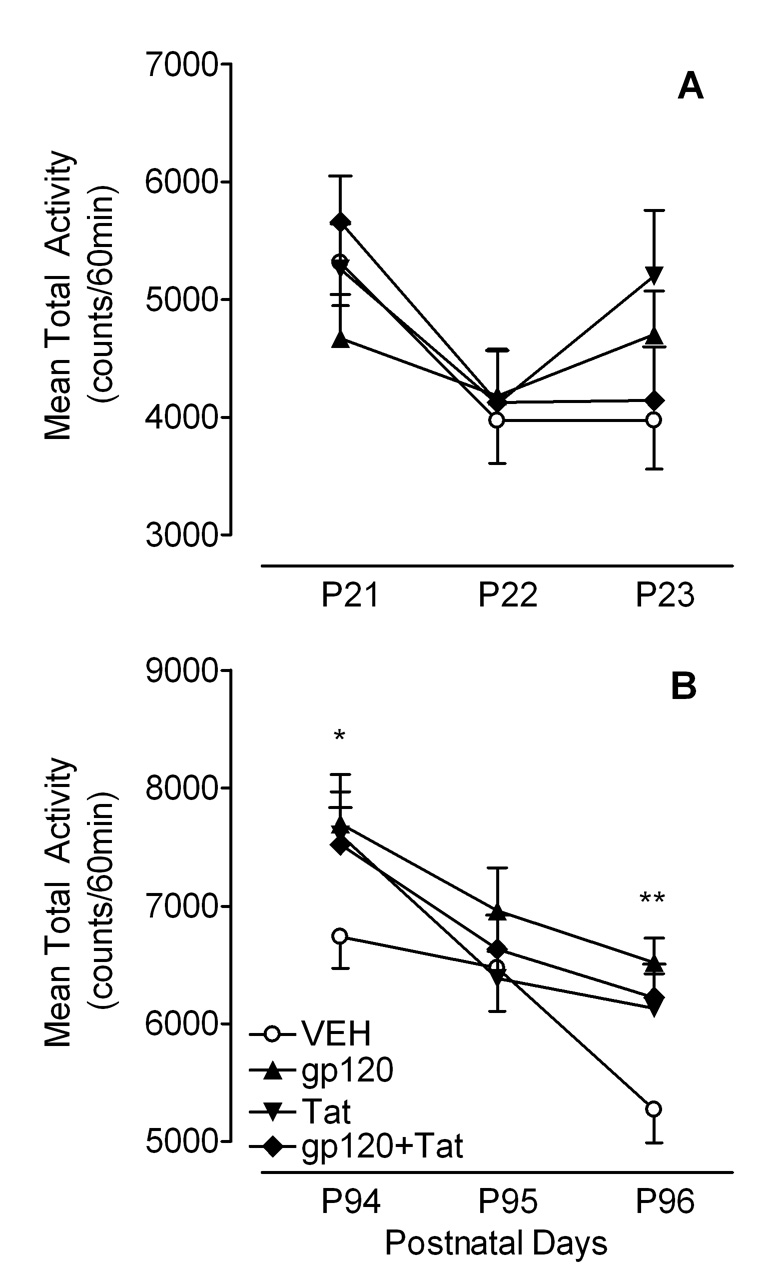
For weanlings, the mean total activity data (counts/60 min) across P21–23 are illustrated in Figure 5A. First, no overall protein treatment effect was noted. Second, a 2 (Gp120) × 2 (Tat) × 3 (Test days) mixed-model ANOVA revealed a significant main effect of test day [F(2, 90) = 8.8, p ≤ 0.001] and a significant Gp120 × Tat × Day interaction [F(2, 90) = 3.4, p ≤ 0.05]. Third, planned contrast analyses indicated significant test day effects with linear components for the VEH controls and the combined gp120+Tat-treated animals [F(1, 11) = 10.3, p ≤ 0.01 and F(1, 12) = 8.6, p ≤ 0.01, respectively], demonstrating habituation. In contrast, no test day effects were noted for gp120- and Tat-treated animals, suggesting no habituation across the three test days (see Figure 5A).
Figure 5.
Mean (± S.E.M.) total activity across the three test days, (A) in preweaning development across P21–23, and (B) in adulthood across P94–96. In adulthood a significant overall protein treatment effect was noted on the first test day (*p ≤ 0.05) and the last test day (**p ≤ 0.01).
For adults, the mean total activity data (counts/60 min) collapsed across P94–96 are illustrated in Figure 5B. First, a significant overall protein treatment effect conducted on total activity was noted collapsed across the three test days [F(1, 45) = 6.7, p ≤ 0.05], primarily contributed by the first (P94) and last (P96) test day [F(1, 45) = 4.7, p ≤ 0.05 and F(1, 45) = 0.5, p ≤ 0.01, respectively]. Second, a 2 (Gp120) × 2 (Tat) × 3 (Test days) mixed-model ANOVA revealed a significant main effect of gp120 [F(1, 45) = 4.6, p ≤ 0.05], demonstrating higher total activity for animals treated with gp120 compared to animals not treated with gp120. Further, all animals exhibited habituation across the three test days indicated by a significant test day effect [F(2, 90) = 27.3, p ≤ 0.001]. Third, planned contrast analyses revealed significant differences on the last test day between the VEH controls and the gp120 group [F(1, 45) = 10.4, p ≤ 0.01], between the VEH controls and the Tat group [F(1, 45) = 4.9, p ≤ 0.05], and between the VEH controls and the combined gp10+Tat group [F(1, 45) = 6.3, p ≤ 0.05]. As illustrated in Figure 5B all HIV-1 protein-treated animals exhibited higher activity levels compared to the VEH control group.
Spatial Learning and Memory
Adolescent Rats (P49–55)
Acquisition Training
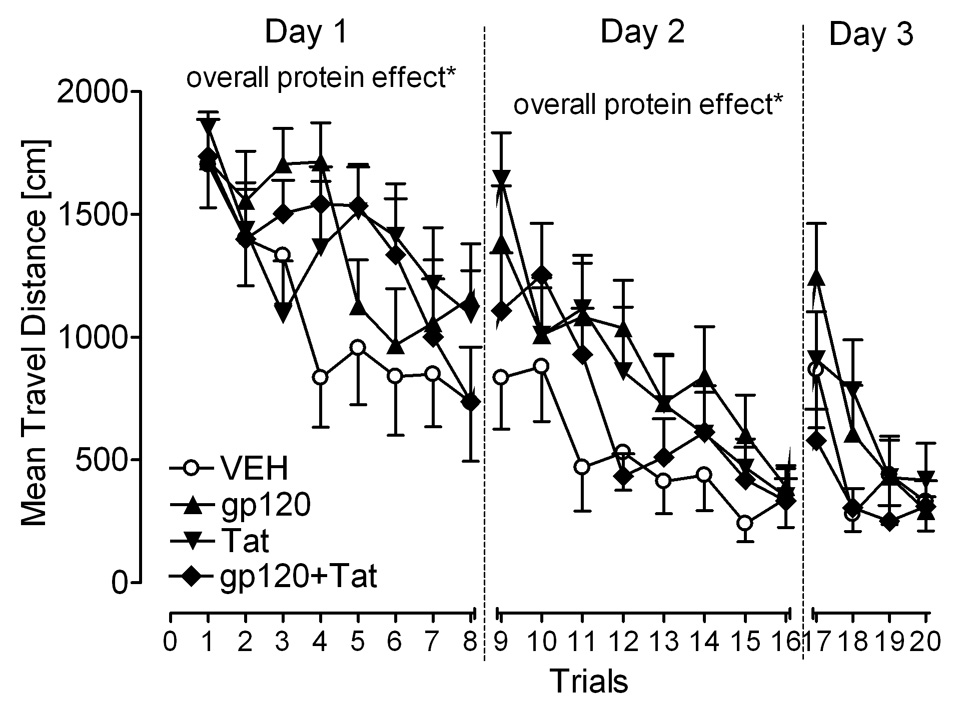
Travel distance during acquisition training across the 20 training trials is shown in Figure 6. First, an overall protein treatment effect on travel distance was noted collapsed across all 20 training trials, and for individual training days 1 (training trials 1–8) and 2 (training trials 9–16). Table 1 illustrates the results. Second, mixed-model ANOVAs were conducted for each training day. On day 1, a main effect for trial was revealed [F(7, 315) = 8.3, p ≤ 0.001] with a prominent linear component [F(1, 45) = 33.4, p ≤ 0.001]. Further, significant Gp120 × Trial [F(1, 45) = 5.3, p ≤ 0.05, quadratic component] and Tat × Trial interactions [F(1, 45) = 7.9, p ≤ 0.01, cubic component] were noted. HIV-1-protein-treated animals displayed a different learning pattern to find the target platform location, traveling longer distances across trials 1–8. On days 2 and 3, significant main effects of trial [F(7, 315) = 15.2, p ≤ 0.001 and F(3, 135) = 14.5, p ≤ 0.001, respectively] and significant Gp120 × Tat interactions were noted [F(1, 45) = 6.0, p ≤ 0.05 and F(1, 45) = 4.5, p ≤ 0.05, respectively]. The Gp120 × Tat interactions on days 2 and 3, suggest that both HIV-1 proteins act differently on spatial learning in the presence of the second HIV-1 protein. Third, results of planned contrast analyses are shown in Table 1. The three HIV-1 protein-treated animals indicated significant differences from the VEH controls on training days 1 and 2. No significant differences were noted on the last training day 3. As supported in Figure 6, continued training revealed that performance of the HIV-1-treated animals approximated that of VEH controls. All groups achieved the performance level of the VEH controls at the end of day 3 in acquisition training.
Figure 6.
Adolescence. Mean (± S.E.M.) on travel distance for each of the four HIV-1-protein treatments in acquisition training. Significant overall protein treatment effects (VEH controls vs. the three HIV-1 protein groups) were noted for training days 1 and 2, but not for day 3., *p ≤ 0.05.
Table 1.
Planned Contrast Analyses on Acquisition Training for Adolescent and Adult Rats
| Adolescence | |||||
|---|---|---|---|---|---|
| Planned Contrasts | Df | Days 1–3 | Day 1 | Day 2 | Day 3 |
| Overall Protein Treatment | (1, 45) | 6.2* | 5.6* | 5.8* | 0.3 |
| Effect (VEH controls vs. the three HIV-1 protein groups) | |||||
| VEH controls vs. gp120 group | (1, 45) | 6.0* | 3.9* | 6.0* | 1.3 |
| VEH controls vs. Tat group | (1, 45) | 5.3* | 3.9 | 4.9* | 1.1 |
| VEH controls vs. combined gp120+Tat group |
(1, 45) | 1.8 | 3.4 | 1.5 | 0.7 |
| Adulthood | |||||
| Overall Protein Treatment | (1, 45) | 0.1 | 0.0 | 0.1 | 0.5 |
| Effect (VEH controls vs. the three HIV-1 protein groups) | |||||
| VEH controls vs. gp120 group | (1, 45) | 0.0 | 0.0 | 0.1 | 0.0 |
| VEH controls vs. Tat group | (1, 45) | 0.3 | 0.4 | 0.0 | 0.3 |
| VEH controls vs. combined gp120+Tat group |
(1, 45) | 0.0 | 0.2 | 0.5 | 1.1 |
p ≤ 0.05
Probe Test
Quadrant preference in the probe test represents the amount of time spent in each of the four quadrants when no platform was present (data are not shown). First, no significant overall protein treatment effect was noted for any of the four quadrants. Second, a 2 (Gp120) × 2 (Tat) × 4 (Quadrant) mixed-model ANOVA conducted on the time spent in each of the four quadrants revealed a significant quadrant effect [F(3, 135) = 78.5, p ≤ 0.001], with no other effects or interactions being significant. All animals spent most of the time in the target quadrant relative to the opposite quadrant [discrimination effect: F(1, 45) = 111.6, p ≤ 0.001] and thus, were able to discriminate and able to remember the prior platform location. In addition, a significant effect of evasion was revealed [F(1, 45) = 29.6, p ≤ 0.001], with all rats spending less time in the opposite quadrant relative to the adjacent quadrants. All animals therefore avoided the opposite quadrant relative to the adjacent quadrants and remembered were not to go in the pool. In sum, the results indicated all groups of rats developed an understanding about where to go and where not to go in the testing environment, suggesting the preservation of spatial memory in the adolescent HIV-1-protein treated animals.
Adult Rats (P113–121)
Acquisition training
First, no overall protein treatment effect on travel distance was noted with Table 1 illustrating the results. Second, mixed-model ANOVAs on travel distance for each of the three training days revealed significant main effects of trial [day 1: F(7, 315) = 9.5, p ≤ 0.001, day 2: F(7, 315) = 17.7, p ≤ 0.001, day 3: F(3, 135) = 21.8, p ≤ 0.001], indicating that the animals learned the spatial location of the platform. No treatment effects or interactions were noted (data are not shown.)
Probe Test
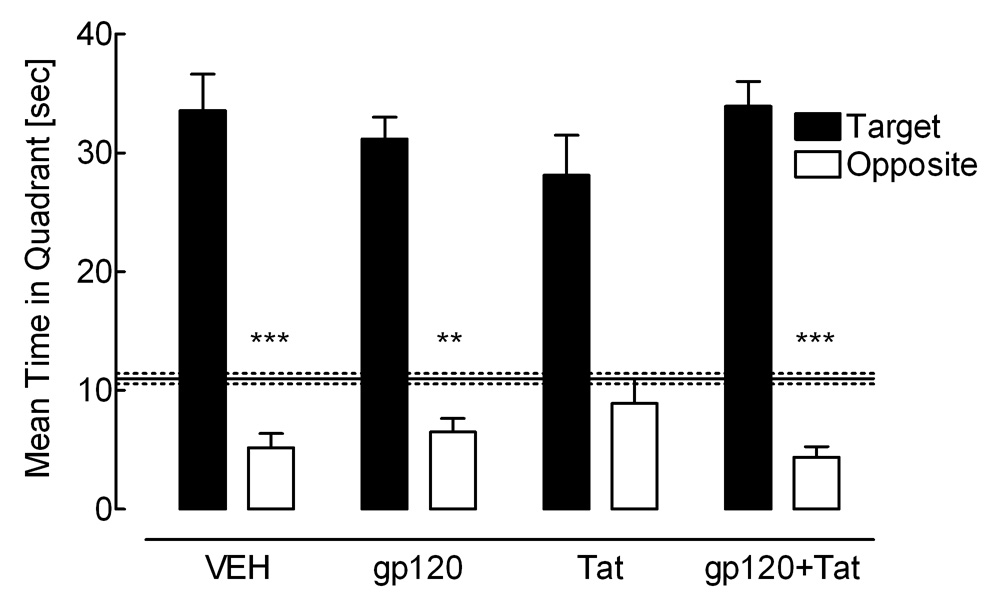
Figure 7 illustrates quadrant preference in the probe test, with the amount of time spent in each of the four quadrants when no platform was present. First, no significant overall protein treatment effect was noted for any of the four quadrants. Second, a 2 (Gp120) × 2 (Tat) × 4 (Quadrant) mixed-model ANOVA conducted on the time spent in each of the four quadrants revealed a significant quadrant effect [F(3, 135) = 105.6, p ≤ 0.001]. As illustrated in Figure 7 all animals were able to discriminate and spent most of the time in the target quadrant relative to the opposite quadrant [F(1, 45) = 178.5, p ≤ 0.001], suggesting that all animals displayed spatial memory for the prior platform location. However, a significant Tat × Gp120 interaction [F(1, 45) = 5.1, p ≤ 0.05] and a significant cubic Gp120 × Tat × Quadrant interaction was revealed [F(1, 45) = 4.0, p ≤ 0.05], suggesting that HIV-1 protein effects had at least some impact on the distribution of searching behavior in adulthood. Third, planned contrast analyses demonstrated a significant effect on the evasion measure for all but the Tat-treated group, [VEH controls: F(1, 11) = 22.6, p ≤ 0.001, gp120-treated animals: F(1, 11) = 14.1, p ≤ 0.01, Tat-treated animals: F(1, 11) = 1.7, p > 0.05, combined gp120+Tat-treated animals: F(1, 12) = 55.2, p ≤ 0.001]. As illustrated in Figure 7, the Tat-treated animals did not avoid the opposite quadrant relative to the adjacent quadrants, thus, failed to learn where not to go in the pool.
Figure 7.
Adulthood. Mean (± S.E.M.) time spent in the platform and opposite quadrants by treatment group during the probe test. Time spent in the quadrants adjacent to the platform is represented by the solid (mean) and dashed horizontal lines (± S.E.M.). Significant effects on the evasion measure are demonstrated for all, but the Tat-treated group., ***p ≤ 0.001, **p ≤ 0.01.
Relationship between behavior and anatomy
To test whether there is a relationship between the anatomical measures of the hippocampus and the behavioral measures two approaches were employed. First, a correlation matrix (Pearson) is displayed in Table 2 between the estimated total number of cells in the five subdivisions of the hippocampus combined, expressed as a percentage from the mean of the VEH control group, and the behavioral measures in preweanling, adolescent and adult rats. The significant correlations were furtherassessed by separate stepwise multiple regression analyses using percent number of neurons and glial cells as predictor variables. Results indicate predictability of behavioral scores by anatomical measures accounting for 22% – 42% of total variance in the data (summarized in Table 3 and illustrated in Figure 8).
Table 2.
Pearson Intercorrelation Matrix between the Different Behavioral Measures and Percent Cell Number from the Mean of the VEH Control Group for the Five Hippocampal Subfields Combined (N = 20).
| Neurons (in %) | Glia (in %) | ||
|---|---|---|---|
| Righting Reflex (in sec) | −0.648** | −0.191 | |
| Negative Geotaxis (in sec) | −0.049 | 0.479* | |
| Eye Opening (Ranking )+ | 0.340 | 0.005 | |
| Prepulse Inhibition (amplitude) | young | 0.347 | 0.138 |
| adult | 0.368 | 0.345 | |
| Locomotor Activity (counts/60min) | young | −0.471* | 0.243 |
| adult | −0.300 | 0.196 | |
| Distribution in searching behavior during the | young | −0.086 | 0.032 |
| probe test in the MWM (in sec) | adult | 0.479* | 0.191 |
Note: MWM = Morris water maze
p ≤ 0.01
p ≤ 0.01
Spearman Correlation
Table 3.
Stepwise Multiple Regression Analyses Predicting Different Behavioral Measures with Percent Neurons and Glial Cells as the Two Anatomical Predictors.
| Behavior | Predictor(s) | Coefficient | S.E.M. | t-statistics | Model Fit | R2 |
|---|---|---|---|---|---|---|
| Righting Reflex | % neurons | −0.136 | 0.038 | −3.6, p ≤ 0.002 | F(1, 18) = 13.0, | 42% |
| p ≤ 0.002 | ||||||
| Negative Geotaxis | % glia | 0.186 | 0.059 | 3.2, p ≤ 0.006 | F(2, 17) = 5.0, | 37% |
| % neurons | −0.219 | 0.111 | −2.0, p ≤ 0.066 | p ≤ 0.019 | ||
| Locomotor Activity | % neurons | −37.8 | 16.694 | −2.3, p ≤ 0.036 | F(1, 18) = 5.1, | 22% |
| p ≤ 0.036 | ||||||
| Distribution in searching | % neurons | 0.550 | 0.238 | 2.3, p ≤ 0.033 | F(1, 18) = 5.3, | 23% |
| behavior during the | p ≤ 0.033 | |||||
| probe test in the MWM |
Note: MWM = Morris water maze
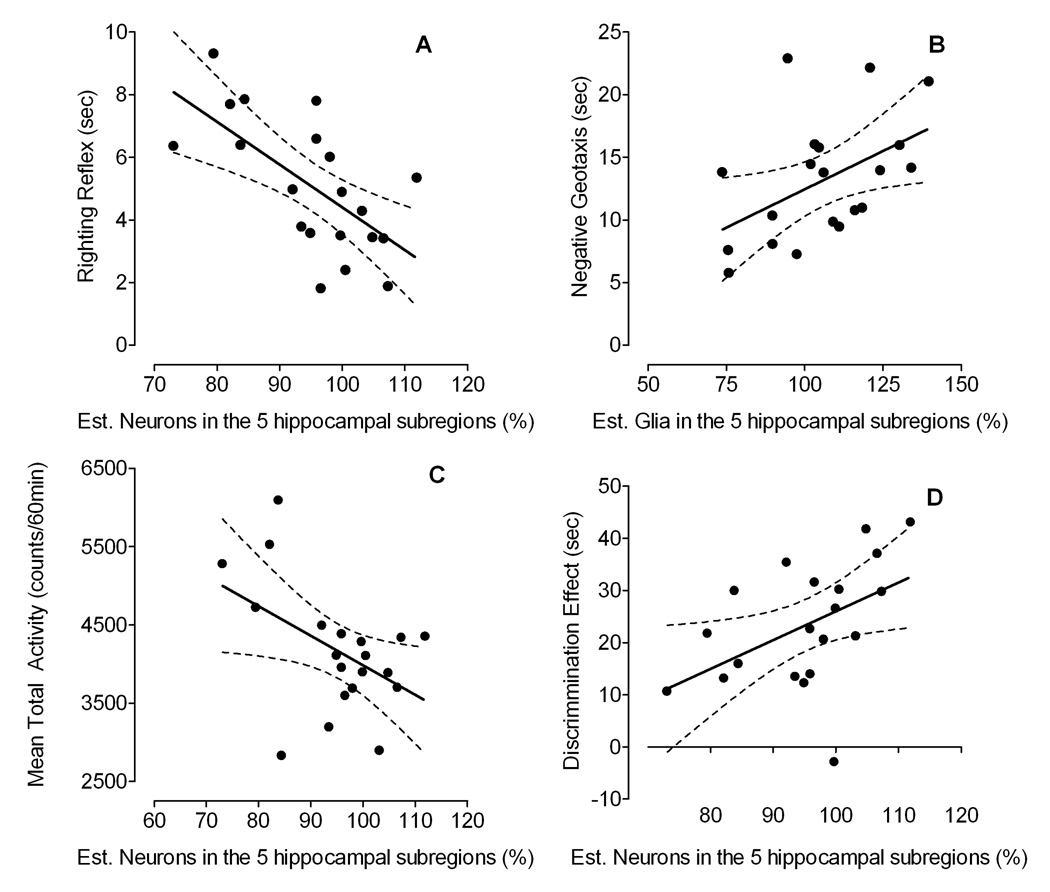
Figure 8.
Simple linear regression models indicating the relationship between, (A) righting reflex and % neurons in the hippocampus, (B) negative geotaxis and % glia in the hippocampus, (C) locomotor activity in young rats and % neurons in the hippocampus, and (D) distributional searching behavior during the probe test for adult rats and % neurons in the hippocampus.
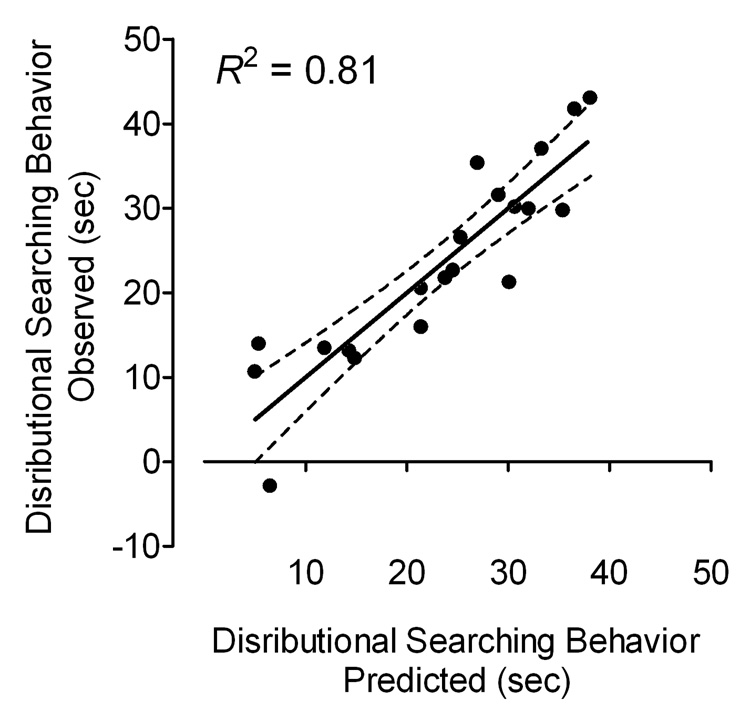
Second, because the hippocampus is well known to be involved in spatial memory, the relationship between the five hippocampal subdivisions and the distribution of searching behavior assessed during the probe test in adulthood was further explored. Pearson correlation analyses indicated a significant relationship between the behavioral measure in the spatial memory task and three anatomical measures, including neurons and astrocytes in the DGH, and oligodendrocytes in the SUB. To test for predictability of the distribution of searching behavior by these anatomical measures we used a linear multiple regression analysis. The best model version was an 8-parameter model, including the two predictor variables, neurons and astrocytes in the DGH. The model included four intercepts b0 that varied with condition and four weights that depended on the two anatomical measures in the DGH and the corresponding conditions (see Table 4). As illustrated in Figure 9, the model provided a very good fit of the distributional searching behavior explaining 81% variance of the data (R2 = 0.808).
Table 4.
Parameter Values and Fit Indices of the 8-Parameter Model for the Distribution in Searching Behavior during the Probe Test in Adulthood.
| b0 | bDGHN | bDGHA | |
|---|---|---|---|
| VEH | 25.8 | 0.001 | −0.001 |
| Gp120 | 66.8 | −0.000 | bDGHA VEH |
| Tat | −110.4 | 0.002 | bDGHN VEH |
| Tat + Gp120 | 6.7 | bDGHN TAT | bDGHA VEH |
Note: b0 = intercept or constant, bDGHN = weight of the total number of neurons in the DGH, bDGHA = weight of the total number of astrocytes in the DGH.
Figure 9.
Actual scores of the distributional searching behavior during the probe test are illustrated on the y-axis against the predicted searching behavior scores (x-axis) derived from the 8-parameter model explaining 81% of the data variance.
3. Discussion
The present experiment investigated the effects of the HIV-1 proteins Tat and gp120 on a set of behavioral tests in young and adult male rats. The HIV-1 proteins were bilaterally injected into the hippocampus of P1 rats. No effects on body weight were noted for any of the HIV-1 protein treated groups, indicating that the HIV-1 protein doses used in the present study produced no general growth deficits. Alterations were observed in eye opening, early reflex development (i.e. righting reflex and negative geotaxis), locomotor activity and cognitive functions, including preattentive processes and spatial learning and memory. The effects of Tat were more pervasive relative to those of gp120. A more general involvement of the hippocampus in early reflex development was suggested by a relationship between performance in the righting reflex/negative geotaxis and estimated cell number in the hippocampus. Additionally, the estimated number of neurons and astrocytes in the DGH explained 81% variance of the distributional searching behavior in the MWM probe test, suggesting that cell types in the DGH may participate in the spatial memory alterations observed in adulthood.
Somatic Growth
Poor growth patterns have been reported in the pediatric HIV population with infected children growing considerably slower compared to uninfected children; weight differences increased after the second year of life (Newell et al., 2003). In the present study, no significant effects on body weight were noted, even though the three HIV-1-protein groups started to show decreased weights (approximately 3%) in adulthood compared to the VEH control group. The lack of HIV-1 protein effects on body weight might suggest that the HIV-1 protein doses were too low to produce general growth deficits. Alternatively, the reported growth defects in HIV-1 infected children might be due to the effects of the virus on the immune system, rather than the effects of the viral proteins on the CNS system. This suggestion is supported by the finding that virologic control appears to be related to sustained growth and that antiretroviral therapies, such as highly active antiretroviral therapy (HAART), have greatly improved the average weight gain of HIV-infected children (Guillén et al., 2007, Nachman et al., 2005).
HIV-1 proteins, however, have some developmental effects on somatic growth, as indicated by the combined data for eye opening (collapsed across days). Animals that received neonatal Tat treatment indicated a significant deficit in eye status (closed or open); with the effect being most prominent for the combined gp120+Tat group. The observed alterations on the overall eye status measure, however, were never large enough within a reasonable sample size to be confirmed as a delay in development.
Early Reflex Development
The behavioral tests for early reflex development assessed simple neurological abilities that have been reported to be altered in the pediatric HIV population.
In the present study, significantly slower latency responses were noted for Tat treatment at the first test day for both the righting reflex as well as negative geotaxis, whereas gp120 treatment only affected negative geotaxis. Reflex performance for gp120- and/or Tat-treated animals, however, approximated that of VEH controls across test days, with the most parsimonious inference of gp120 and/or Tat causing a delay, rather than a disruption, of early reflex development. In contrast, potential interactive effects of gp120 and Tat were noted on negative geotaxis. As illustrated in Figure 3B, the combined gp120+Tat group indicated persisting slow response latencies across test days; in contrast to a transient single test day alteration with either protein alone. The contrast to the progressive improvement seen across test days when neonatally treated with either protein alone, suggested a potentiated effect of the combined HIV-1 proteins on simple neurological processes.
Preattentive Processes
Deficits on attentional processes have been reported as one of the most often affected higher cognitive functions in HIV-1 infected children. In the present study, preattentive processes were assessed with the behavioral measure prepulse inhibition (PPI) of the acoustic startle response (ASR). hereas no effects were noted for gp120, Tat treatment significantly altered the maximum peak inhibition response in adulthood, suggesting long-lasting effects of Tat on the process of sensorimotor gating. Nearly all of the VEH control animals displayed a maximum peak inhibition response at 120msec ISI; in contrast, Tat treatment flattened the peak inhibition function with approximately half of the animals shifting their maximum peak inhibition response to 80msec ISI. Tat-induced alterations on preattentive processes in adult and young rats have been recently reported (Fitting et al., 2006a, 2006b). The observed Tat-induced sensorimotor gating alterations may relate to the neurotoxic effects of Tat observed in in vitro studies (Aksenova et al., 2006, Kruman et al., 1998). In vitro exposure of neurons to Tat triggers an early increase in intracellular reactive oxidant species production, which is followed by an accumulation of oxidatively modified proteins, and subsequent neuronal death (Aksenov et al., 2006).
Motor Function
For motor function in weanlings, a lack of a linear decrease of activity across days was noted for gp120- and Tat-treated animals. The increased activity levels at the last test day indicate that the animals did not get familiar with the new environment in contrast to the VEH control group, suggesting some disruption of habituation. The disruption of habituation was also noted in adulthood, with the viral protein gp120 producing a long-term increase in locomotor activity. Gp120-treated animals were much more active at the first test day and exhibited significantly less habituation across the three test days, compared to the VEH controls. The hippocampus has been shown to be involved in background contextual learning, and further, hippocampal damage being disruptive to association learning (Phillips & LeDoux, 1994). This evidence might indicate a protein-induced neurotoxicity in the CNS and an alteration of the hippocampus. It has been suggested that Tat affects the hippocampal formation structurally and functionally (Maragos et al., 2003). Alteration observed in locomotor activity during weaning development may therefore reflect a deficit in learning contextual cues and remembering the new environment.
Spatial Learning and Memory
The hippocampus has been demonstrated to be the major brain structure that is involved in learning and memory. Due to the reported elevated viral load in the hippocampus (Wiley et al., 1998), HIV-1 infected children often display impairments on these potential higher cognitive functions, disrupting day-to-day life. The present study also gave evidence for a gp120- and Tat-induced delay in spatial learning in adolescent rats. However, with continued training the performance of the HIV-1 protein groups approximated that of the VEH controls, a recovery similar to that was seen in the assessment of early reflex development. Dissociation of repeated testing effects from ontogenic changes would require a separate same-age control group that was not assessed in adolescence. The use of such a design with PPI has previously demonstrated that persistent alterations were dissociable from repeated testing effects (Fitting et al., 2006b).
For spatial memory, Tat treatment produced long-lasting deficits on the distribution in searching behavior, suggesting CNS toxicity to the hippocampus. Even though Tat-treated animals displayed a preference for the target platform quadrant, they failed to learn where not to go in the pool, as indexed by the evasion measure. These data are consistent with the observations that Tat causes functional and anatomical impairments of the hippocampal formation (Fitting et al., 2006b, 2007).
For spatial learning and memory, it is not clear how and why the combined HIV-1 protein treatment failed to show further evidence of adverse interactive effects relative to either protein alone. Separate injections were not pursued because of the constraints of injection volume for neonatal brains. Further investigations are necessary to understand the interactions of gp120 and Tat.
Relationship between Behavior and Anatomy
Regression analyses of the behavioral and stereological measures suggested that the hippocampus plays an important role in behavioral measures such as early reflex development and spatial memory. In contrast to our hypothesis, no relationship was noted between PPI and the stereological hippocampal measures. Additional results indicated that the DGH subdivision of the hippocampus may participate in the behavioral alterations observed in the adult spatial memory assessment. The estimated number of neurons and astrocytes in the DGH explained 81% variance of the distribution in searching behavior in the adult MWM probe test suggesting that the relative lack of integrity of the DGH determined the spatial memory alterations observed in adulthood. The interpretation of the relationship between behavior in preweanling animals and anatomy might be a little less compelling as the anatomical measures were assessed in adulthood. Proliferation of cells in adulthood might mask potential effects on the anatomy of the developing hippocampus in young rats. Nevertheless, the strength of the relationship presented in the current study argues that the observed long-term alterations on the hippocampal formation are predictable of specific alterations seen in behavior early in development as well as in adulthood.
Conclusion
Collectively, the present results suggest that the viral HIV-1 protein Tat had an overall effect on many of the behavioral assessments, whereas gp120 had more selective effects on negative geotaxis and on locomotor activity in adulthood. The differential nature of Tat and gp120 is supported by a recent in vitro study, which indicated that the nature of the apoptotic events preceding death differed for both HIV-1 proteins (Singh et al., 2004). Although Tat 1–72 and gp120 induced significant neurotoxicity in mouse striatal neurons, gp120 acted in large part through the activation of caspase(s), whereas Tat 1–72-induced neurotoxicity was accompanied by activating an alternative pathway involving endo G (Singh et al., 2004). For the combined effects of Tat and gp120, the in vivo findings revealed some evidence of interactive effects, specifically on the early reflex development negative geotaxis. Further, the contribution of the viral proteins, gp120 and Tat, to spatial memory deficits observed in the probe test might be due to a loss of neurons and increase gliosis as noted in the DGH. The assessment of effects of Tat and gp120 on cytokines is currently under investigation to ascertain the potential involvement of inflammatory responses. This aspect can give important insights into HIV-1 protein-induced neurotoxicity and is critical in determining the contribution of the viral proteins, Tat and gp120, to the neurological and neuropsychiatric impairment consequent to HIV-1 infection.
4. Experimental Procedure
Animals
Pregnant Sprague-Dawley female rats (n = 13) were obtained from Harlan Laboratories, Inc. (Indianapolis, IN) and delivered to the vivarium before embryonic day seven. Dams were housed singly with food (Pro-Lab Rat, Mouse Hamster Chow #3000, NIH diet #31) and water available ad libitum. The day pups were found in the cage was designated as P0. On P1, litters were culled to 10 offspring of equal sexes, if possible. Only one male per litter was assigned to a single condition. At 21 days of age, animals were weaned and separated by sex; at 28 days of age males were pair-housed with their littermates for the remainder of the experiment. The animal facility was maintained at 20±2°C, 50±10% relative humidity and had a 12h light: 12h dark cycle with lights on at 0700h (EST). The animals were maintained according to the National Institute of Health (NIH) guidelines in AAALAC-accredited facilities. The experimental protocol was approved by the Institutional Animal Care and Use Committee (IACUC) at the University of South Carolina, Columbia.
HIV-1 proteins
For the gp120 treatment condition the purified gp120 LAV (T-tropic) envelope protein was purchased from Protein Sciences Corp. (Meriden, CT). Gp120 solutions of 100ng in 0.5µl were prepared immediately prior to injection. For the Tat treatment condition a recombinant biologically active Tat1–72 was produced as previously described (Ma & Nath, 1997). Tat solutions of 25µg in 0.5µl were prepared immediately prior to injection. Combined gp120+Tat was prepared by mixing both proteins together immediately prior to injection (0.5µl of gp120 100ng and Tat 25µg). Separate injection might have been preferable but was not pursued due to the constraint of injection volume.
In the present experiment, we specifically choose intermediate doses of Tat (25µg) and gp120 (100ng) in order to facilitate the detection of potential interactive effects when combining both HIV-1 proteins. The selection of HIV-1 protein doses followed prior establishments of dose-response functions for gp120/Tat neurotoxicity. For Tat, we have previously established the dose-response function for the neurotoxic effects of 5, 15, and 50µg of Tat on the response of glial fibrillary acidic protein (GFAP), an indirect measure of neuronal damage that is associated with the pathogenesis of HIV encephalitis (Aksenov et al., 2001). The 5µg dose injected into the rat striatum did not result in a detectable GFAP response. The 15µg doses of Tat produced statistically significant GFAP levels 7 days. The highest dose (50µg) produced observable striatal lesions, which lasted between 7 and 30 days. For gp120, we have established the dose-response function for the neurotoxic effects of 1.29, 12.9, and 129ng of gp120 on the sensory-motor measure and preattentive processes early in development (Fitting et al., 2007b). A linear gp120-dose dependent slowing of the latency response with increased gp120 treatment was noted for righting reflex on P3. Similarly, for preattentive processes, a linear gp120-dose dependent slowing of the latency response with increased gp120 treatment was noted for prepulse inhibition. We have previously established the dose-response function for the neurotoxic effects of 1.29, 12.9, and 129ng of gp120 on the preattentive processes in adulthood (Fitting et al., 2006c). Response inhibition was significantly reduced as a function of gp120 dose, and the inflection of the inhibition curve was significantly altered for the high-gp120 dose-treated animals.
Surgical techniques and protein treatment
Standard stereotaxic surgery techniques, modified for neonates, were used for treatment injection. Individual pups were removed from the dam and cryogenically anesthetized (AVMA, 2001) before being placed in a modified stereotaxic holder for surgery of neonates (Kopf, Inc.), which included a chilled base to maintain cryogenic anesthesia. Rubber head bars held the skull in place while bilateral microinjections (Hamilton Co., Nevada, USA) were made directly into the hippocampus using stereotaxic coordinates. The set of coordinates used for the left and right hippocampus were 0.65mm posterior to the bregma, ±1.0mm lateral to bregma, −2.2mm dorsal from dura. Preliminary data obtained from analyses of Nissl-stained sections through the hippocampus, confirmed the sites of injection for the experimental protocol. The injection volume was 0.5µl released over two minutes followed by a one-minute resting period that allowed the tissue to return to its original conformation. Animals were bilaterally injected with sterile buffer (vehicle (VEH); 10mM Tris HCl, 300nM NaCl, ph = 7.58, sterile), 100ng gp120, 25µg Tat or combined 100ng gp120 and 25µg Tat. The injection needle was withdrawn over two minutes to prevent reflux. The piercings in the skin of the head were closed with surgical glue and the pups warmed under a heat lamp (35°C) before being returned to the dam, where they were closely observed for any indications of rejection. No pups were rejected or abused by the dam.
Experimental Design
Four male rats per litter were randomly chosen; one was assigned to each of the four treatment groups that received bilateral hippocampal injections on P1 in a 2×2 design: VEH control, gp120, Tat, or combined gp120+Tat. Twelve animals were injected per condition, except for the combined gp120+Tat group (n = 13). Weighing started on P1 and behavioral assessment started on P3. All rats were tested for (1) somatic growth, indexed by body weight at the specific test days and eye opening on P13–17, (2) early reflex development, indexed by righting reflex on P3–5 and negative geotaxis on P8–10; (3) sensorimotor function, indexed by PPI of the ASR on P18 and on P91; (4) motor function, indexed by locomotor activity on P21–23 and on P94–96; and (5) spatial learning and memory by assessing performance in the Morris water maze on P50–55 and on P113–121.
Somatic Growth
Somatic growth, including body weight and eye opening were assessed to test whether HIV-1 proteins themselves induced significant impairments on these measures. Poor growth pattern has been reported in HIV-infected children, with weight differences increasing after the second year of life (Newell et al., 2003).
Body Weight
Somatic growth was measured by body weight as an index of general development. Pups were weighed on a daily basis from P1 to P13. Thereafter, starting on P14, weighing was continued on a weekly basis. Body weight was also taken on the animal’s test days.
Eye opening
Eye status (open or closed) was examined as a second index of somatic growth on P13–17. No eyes were open on P13 for any of the rats; eyes were fully open for all rats by P17. Eye status was assessed separately for the right and left eye between 0800–1000 h (EST). Eye not open was coded as zero and eye open was coded as one. The overall measure was then calculated collapsed across P14–16 by computing the sum of left and right eye, with a maximum rank score of six and a minimum rank score of zero.
Early Reflex Development
Early reflex development, including righting reflex and negative geotaxis, was assessed to test for simple neurological abilities that have been reported to be altered in the pediatric HIV population.
Righting Reflex
The righting reflex was examined on P3–5 to assess preweaning alterations in sensory-motor systems very early in development. The goal was to assess the development of the sensory-motor system at a time point when other developmental milestones were still immature and thus, not confounded by secondary variables, such as eye opening and ear opening. The righting reflex is defined as “the ability to assume an upright position when there has been a departure from it” (Walton et al., 2005). Pups were tested between 1600–1800h (EST) on a wire grid. A pup was placed on its back and time in seconds was recorded to return to the upright position by turning over onto its belly.
Negative Geotaxis
Another test to determine the development of sensory-motor systems was negative geotaxis on P8 – P10. Negative geotaxis is defined as an “orienting response and movement expressed in opposition to cues of a gravitational vector” (Motz & Alberts, 2005). Pups were tested between 1600–1800 h (EST). The test was conducted by placing the pup oriented facing head downward on a 25° tilted plane on a metallic wire grid. The dependent variable was time in seconds to turn 180° with orienting toward the high end of the plane.
Sensorimotor function (PPI of the ASR)
To test whether HIV-1 proteins themselves induce effects on higher order functions, preattentive processes were assessed. The animal’s ability to gate sensorimotor function was tested. Attentional processes have often been reported to be impaired in pediatric HIV-1 infection, interrupting day-to-day functioning. All rats were tested as preweanlings on P18 and as adults on P91.
Apparatus
The startle chamber (SR-Lab Startle Reflex System, San Diego Instruments, Inc.) was enclosed in a 10cm thick double-walled, 81×81×116cm isolation cabinet (external dimensions) (Industrial Acoustic Company, INC., Bronx, NY). The startle chamber consisted of a Plexiglas cylinder 3.9cm in internal diameter for preweanling rats and 8.75cm in internal diameter for adult rats. For each of the ages the Plexiglas cylinder was resting on a 12.5×20cm Plexiglas stand. Each animal was tested individually in the dark when a high-frequency loudspeaker was mounted inside the chamber 31cm above the Plexiglas cylinder. The startle stimulus was 100dB(A) and the prepulse stimulus was 85dB(A) in intensity. Both stimuli had a duration of 20msec. The animal’s response to the startle stimulus produced deflection of the Plexiglas cylinder, which was converted into an analog signal by a piezoelectric accelerometer. Response sensitivities were calibrated using a SR-LAB Startle Calibration System. Acoustic stimulus intensities were measured and calibrated with a sound level meter (Extech Instruments: Waltham, MA) with the microphone placed inside the Plexiglas cylinder. Response signals were digitized (12 bit A to D) and saved to a hard disk on a Pentium class computer.
Testing Procedures
All rats were tested for approximately 20min. Animals were first exposed to a five-min acclimation period of 70dB(A) background of white noise, followed by six single white noise stimuli of 100dB(A) as adaptation trials, and 36 PPI trials with 0, 8, 40, 80, 120, and 4000msec interstimulus intervals (ISIs), assigned by a Latin-square design. The ISI represents the time from the offset of the prepulse stimulus to the onset of the startle stimulus. The stimulus duration was 20msec. The dependent measures analyzed were (1) the baseline ASR indexed by control trials (0 and 4000msec ISIs), (2) PPI trials (8–120msec ISIs), and (3) ISI in which the maximal peak response inhibition occurred across the PPI trials (8–120msec ISIs).
Locomotor Activity
Movement as a measure of activity was obtained in locomotor activity chambers on P21–23 and on P94–96. The activity test was conducted to test for potential motoric dysfunctions that are often seen among the neuropsychological deficits in HIV-infected children.
The activity monitors were square (40×40cm) chambers (Hamilton-Kinder Inc., Poway, CA) that detected free movement of animals by infrared photocell interruptions. This equipment used an infrared photocell grid (32 emitter/detector pairs) with total locomotor activity being measured by assessing the number and type of photocell interruptions within a 60min period. The chambers were converted into round (~ 40cm diameter) compartments by adding clear Plexiglas inserts; photocell emitter/detector pairs were tuned by the manufacturer to handle the extra perspex width. All activity monitors were located in an isolated room. Testing occurred between 1500–1700h (EST) under dim light conditions, in the absence of direct overhead lighting (< 10lx). The dependent variable was the animal’s total activity reflected as the sum of the x and y photocell interruptions.
Spatial Learning and Memory
All rats were tested in the Morris water maze on spatial learning and memory over four consecutive days. Animals were tested in adolescence between P49–55 and in adulthood between P113–121. The Morris water maze task was conducted to assess deficits on potential higher cognitive functions often reported in the pediatric HIV population.
Apparatus
The Morris water maze used during the experiment was an aluminum tank measuring 1.8m in diameter by 0.60m high and filled with water to a depth of 0.40m. The water temperature was maintained at 28±1°C. The escape platform measured 10×10cm and was located 2.5cm below the surface of the water on a radius of 45cm in one of four arbitrarily designated quadrants, north (N), South (S), East (E), or west (W). The tank, in a 3x3m room, was surrounded by two sets of cues. One set consisted of four distinct background curtains, composing the walls of the environment: solid navy in the northern direction, solid white in the southern direction, vertical navy and white stripes (7.6cm wide) in the eastern direction, and horizontal navy and white stripes (7.6cm wide) in the western direction. The second set of cues consisted of 12 objects located outside the tank perimeter. There were four hanging objects suspended from the ceiling ranging in size from a 20cm coffee can to a 60 cm mailbox and eight objects on the curtains, including circles and quadrangles. The starting points used were NW, NE, SE, SW and varied by using two different entry point diagonal to each other in a counterbalanced order, ABBAABBA. A closed circuit video system, recessed mounted in the ceiling above the center of the pool, recorded the swimming behavior of the animals.
Procedure
The experiment was divided into three training phases consisting of acquisition training on days 1, 2, and 3, followed by a probe test on day 3 (Morris, 1981).
In acquisition training, all animals received 20 training trials across 3 days: eight trials on days 1 and 2 followed by four training trials on day 3. The platform was located in a fixed location for each subject during training; however, platform location (N, S, E, or W) was balanced across subjects. Animals were trained to different platform locations as juveniles and adults. A training trial began with the rat being placed into the pool along the edge at one of the entry points and the animal’s swimming behavior was recorded for 60 sec. If the animal failed to find the platform within 60 sec, the trial ended and the rat was directed to the platform. No animal was removed directly from the pool, only from the platform. The animal remained on the platform for 15 sec and was then returned to a holding cage. Because results for escape latency and travel distance were similar and highly correlated (r = 0.96, p ≤ 0.001) we only report travel distance as a measure.
The probe test immediately followed the last acquisition trial on day 3. In the probe test, the platform was removed from the pool and the visual-spatial environment was left unaltered. The probe test was conducted like the acquisition training trials with the exception that a novel starting position was used, i.e. one of the two starting points not used in training. The animal’s swimming behavior was recorded for 60 sec. At the end of the 60 sec period, the platform was placed in the pool and the animal permitted to find and remain on it for 15 sec. The dependent variable used was quadrant preference, i.e., the relative distribution of swimming time in each of the quadrants. Quadrant preference was analyzed by comparing the target and the opposite quadrant areas to provide an index of the animal’s spatial discrimination ability. An additional comparison was conducted taking the opposite and adjacent quadrant areas into account, which yields an evasion measure (Carman et al., 2003, Carman & Mactutus, 2002). The two latter measures indicate the degree of spatial discriminability animals have developed with the testing environment. Typically, animals learn to discriminate, which is indicated by spending more time in the target quadrant relative to the opposite quadrant. In other words, the animals learn where to go in the pool, demonstrating a discrimination effect. Contrary, the evasion measure indicates animals that learn to avoid the opposite quadrant relative to the adjacent quadrants (average of left and right quadrants), demonstrating an evasion effect. In this example, rats have learned where not to go in the pool. Taking into account the rat’s familiarity with the platform quadrant and identifying the rat’s position with respect to the platform location throughout the probe test it is possible to assess the rat’s more ‘implicit’ knowledge of the hidden platform location.
Anatomy
At 221 to 226 days of age (~7 ½ month of age) animals were sacrificed and five animals of each treatment condition were randomly chosen by an investigator, naïve to treatment condition, to be used for anatomical studies. The hippocampus was taken and sectioned in the transverse/horizontal plane using a microtome cryostat (Microm HM500M, Walldorf, Germany). The detailed procedure for tissue processing, sectioning, cresyl-violet (Nissl) staining and mounting, as well as information about the systematic random sampling scheme used for design-based stereology can be found in Fitting et al. (2007). Briefly, the design-based stereology procedure used the optical fractionator method (West et al., 1991). After sampling systematically at all levels, the number of a specific cell type in a known fraction of the different subdivisions was counted (Fitting et al., 2007a), which led to an unbiased stereological estimation of total number of cells in each of the five subdivision. We quantified total neurons in the five hippocampal subregions, granule layer (GL), hilus of the dentate gyrus (DGH), cornu ammonis fields (CA)2/3, CA1, and subiculum (SUB), and total glia (astrocytes and oligodendrocytes) in two subregions (DGH and SUB). To obtain a measure of the total number of cells for the five subfields combined, the percentage from the mean of the VEH control group for each of the subdivision was calculated and the average was taken. These anatomical data were thus available to correlate with the present behavioral findings.
Acknowledgments
This work was supported by the National Institute on Drug Abuse Grants DA013137 and DA014401 and by the National Institute of Child Health and Human Development Grant HD043680.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aksenov MY, Aksenova MV, Nath A, Ray PD, Mactutus CF, Booze RM. Cocaine-mediated enhancement of Tat toxicity in rat hippocampal cell cultures: the role of oxidative stress and D1 dopamine receptor. Neurotoxicology. 2006;27(2):217–228. doi: 10.1016/j.neuro.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Aksenov MY, Hasselrot U, Bansal AK, Wu G, Nath A, Anderson C, Mactutus CF, Booze RM. Oxidative damage induced by the injection of HIV-1 Tat protein in the rat striatum. Neurosci Lett. 2001;305(1):5–8. doi: 10.1016/s0304-3940(01)01786-4. [DOI] [PubMed] [Google Scholar]
- Aksenov MY, Hasselrot U, Wu G, Nath A, Anderson C, Mactutus CF, Booze RM. Temporal relationships between HIV-1 Tat-induced neuronal degeneration, OX-42 immunoreactivity, reactive astrocytosis, and protein oxidation in the rat striatum. Brain Res. 2003;987(1):1–9. doi: 10.1016/s0006-8993(03)03194-9. [DOI] [PubMed] [Google Scholar]
- Aksenova MV, Aksenov MY, Mactutus CF, Booze RM. Cell culture models of oxidative stress and injury in the central nervous system. Curr Neurovascr Res. 2005;2(1):73–89. doi: 10.2174/1567202052773463. [DOI] [PubMed] [Google Scholar]
- Aksenova MV, Silvers JM, Aksenov MY, Nath A, Ray PD, Mactutus CF, Booze RM. HIV-1 Tat neurotoxicity in primary cultures of rat midbrain fetal neurons: changes in dopamine transporter binding and immunoreactivity. Neurosci Lett. 2006;395(3):235–239. doi: 10.1016/j.neulet.2005.10.095. [DOI] [PubMed] [Google Scholar]
- AVMA. Report of the AVMA panel of euthanasia. J Am Vet Med Assoc. 2001;218:671–696. doi: 10.2460/javma.2001.218.669. [DOI] [PubMed] [Google Scholar]
- Bagasra O, Lavi E, Bobroski L, Khalili K, Pestaner JP, Tawadros R, Pomernatz RJ. Cellular reservoirs of HIV-1 in the central nervous system of infected individuals: identification by the combination of in situ polymerase chain reaction and immunohistochemistry. AIDS. 1996;10(6):573–585. doi: 10.1097/00002030-199606000-00002. [DOI] [PubMed] [Google Scholar]
- Bansal AK, Mactutus CF, Nath A, Maragos W, Hauser KF, Booze RM. Neurotoxicity of HIV-1 proteins gp120 and Tat in the rat striatum. Brain Res. 2000;879(1–2):42–49. doi: 10.1016/s0006-8993(00)02725-6. [DOI] [PubMed] [Google Scholar]
- Barks JD, Liu XH, Sun R, Silverstein FS. gp120, a human immunodeficiency virus-1 coat protein, augments excitotoxic hippocampal injury in perinatal rats. Neuroscience. 1997;76(2):397–409. doi: 10.1016/s0306-4522(96)00373-9. [DOI] [PubMed] [Google Scholar]
- Behnisch T, Francesconi W, Sanna PP. HIV secreted protein Tat prevents long-term potentiation in the hippocampal CA1 region. Brain Res. 2004;1012(1–2):187–189. doi: 10.1016/j.brainres.2004.03.037. [DOI] [PubMed] [Google Scholar]
- Belman AL. Infants, children, and adolescents. In: Berger JR, Levy RM, editors. AIDS and the nervous system. 2nd ed. Philadelphia: Lippincott-Raven; 1997. pp. 223–253. [Google Scholar]
- Belman AL, Diamond G, Dickson D, Horoupian D, Llena J, Lantos G, Rubinstein A. Pediatric acquired immunodeficiency syndrome. Neurologic syndromes. Am J Dis Child. 1988;142(1):29–35. doi: 10.1001/archpedi.1988.02150010039017. [DOI] [PubMed] [Google Scholar]
- Brenneman DE, Westbrook GL, Fitzgerald SP, Ennist DL, Elkins KL, Ruff MR, Pert CB. Neuronal cell killing by the envelope protein of HIV and its prevention by vasoactive intestinal peptide. Nature. 1988;335(6191):639–642. doi: 10.1038/335639a0. [DOI] [PubMed] [Google Scholar]
- Carman HM, Booze RM, Snow DM, Mactutus CF. Proximal versus distal cue utilization in preweanling spatial localization: the influence of cue number and location. Physiol Behav. 2003;79(2):157–165. doi: 10.1016/s0031-9384(03)00089-1. [DOI] [PubMed] [Google Scholar]
- Carman HM, Mactutus CF. Proximal versus distal cue utilization in spatial navigation: the role of visual acuity? Neurobiol Learn Mem. 2002;78(2):332–346. doi: 10.1006/nlme.2002.4062. [DOI] [PubMed] [Google Scholar]
- Catani MV, Corasaniti MT, Ranalli M, Amantea D, Litovchick A, Lapidot A, Melino G. The Tat antagonist neomycin B hexa-arginine conjugate inhibits gp-120-induced death of human neuroblastoma cells. J Neurochem. 2003;84(6):1237–1245. doi: 10.1046/j.1471-4159.2003.01620.x. [DOI] [PubMed] [Google Scholar]
- Cheng J, Nath A, Knudsen B, Hochman S, Geiger JD, Ma M, Magnuson DS. Neuronal excitatory properties of human immunodeficiency virus type 1 Tat protein. Neuroscience. 1998;82(1):97–106. doi: 10.1016/s0306-4522(97)00174-7. [DOI] [PubMed] [Google Scholar]
- Corasaniti MT, Piccirilli S, Paoletti A, Nisticò R, Stringaro A, Malorni W, Finazzi-Agrò A, Bagetta G. Evidence that the HIV-1 coat protein gp120 causes neuronal apoptosis in the neocortex of rat via a mechanism involving CXCR4 chemokine receptor. Neurosci Lett. 2001;312(2):67–70. doi: 10.1016/s0304-3940(01)02191-7. [DOI] [PubMed] [Google Scholar]
- Del Valle L, Croul S, Morgello S, Amini S, Rappaport J, Khalili K. Detection of HIV-1 Tat and JCV capsid protein, VP1, in AIDS brain with progressive multifocal leukoencephalopathy. J Neurovirol. 2000;6(3):221–228. doi: 10.3109/13550280009015824. [DOI] [PubMed] [Google Scholar]
- Epstein LG, Sharer LR, Oleske JM, Connor EM, Goudsmit J, Bagdon L, Robert-Guroff M, Koenigsberger MR. Neurologic manifestations of human immunodeficiency virus infection in children. Pediatrics. 1986;78(4):678–687. [PubMed] [Google Scholar]
- Fauci AS. The human immunodeficiency virus: infectivity and mechanisms of pathogenesis. Science. 1988;239(4840):617–622. doi: 10.1126/science.3277274. [DOI] [PubMed] [Google Scholar]
- Fitting S, Booze RM, Hasselrot U, Mactutus CF. Intrahippocampal injections of Tat: effects on prepulse inhibition of the auditory startle response in adult male rats. Pharmacol, Biochem Behav. 2006a;84(2):189–196. doi: 10.1016/j.pbb.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Fitting S, Booze RM, Hasselrot U, Mactutus CF. Differential long-term neurotoxicity of HIV-1 proteins in the rat hippocampal formation: a design-based stereological study. Hippocampus. 2007a;18(2):135–147. doi: 10.1002/hipo.20376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Booze RM, Mactutus CF. Neonatal hippocampal Tat injections: developmental effects on prepulse inhibition (PPI) of the auditory startle response. Int J Dev Neurosci. 2006b;24(4):275–283. doi: 10.1016/j.ijdevneu.2006.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Booze RM, Mactutus CF. Neonatal intrahippocampal glycoprotein 120 injection: the role of dopaminergic alterations in prepulse inhibition in adult rats. Pharmacol Exp Ther. 2006c;318(3):1352–1358. doi: 10.1124/jpet.106.105742. [DOI] [PubMed] [Google Scholar]
- Fitting S, Booze RM, Mactutus CF. Neonatal intrahippocampal gp120 injection: an examination early in development. Neurotoxicology. 2007b;28(1):101–107. doi: 10.1016/j.neuro.2006.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb MS, Schroff R, Schanker HM, Weisman JD, Fan PT, Wolf RA, Saxon A. Pneumocystis carinii pneumonia and mucosal candidiasis in previously healthy homosexual men: evidence of a new acquired cellular immunodeficiency. N Engl J Med. 1981;305(24):1425–1431. doi: 10.1056/NEJM198112103052401. [DOI] [PubMed] [Google Scholar]
- Guillén S, Ramos JT, Resino R, Bellón JM, Muñoz MA. Impact on weight and height with the use of HAART in HIV-infected children. Pediatr Infect Dis J. 2007;26(4):334–338. doi: 10.1097/01.inf.0000257427.19764.ff. [DOI] [PubMed] [Google Scholar]
- Haughey NJ, Mattson MP. Calcium dysregulation and neuronal apoptosis by the HIV-1 proteins Tat and gp120. J Acquir Immune Defic Syndr. 2002;31 Suppl 2:S55–S61. doi: 10.1097/00126334-200210012-00005. [DOI] [PubMed] [Google Scholar]
- Jones MV, Bell JE, Nath A. Immunolocalization of HIV envelope gp120 in HIV encephalitis with dementia. AIDS. 2000;14(17):2709–2713. doi: 10.1097/00002030-200012010-00010. [DOI] [PubMed] [Google Scholar]
- Koch M, Schnitzler HU. The acoustic startle response in rats--circuits mediating evocation, inhibition and potentiation. Behav Brain Res. 1997;89(1–2):35–49. doi: 10.1016/s0166-4328(97)02296-1. [DOI] [PubMed] [Google Scholar]
- Kramer-Hämmerle S, Rothenaigner I, Wolff H, Bell JE, Brack-Werner R. Cells of the central nervous system as targets and reservoirs of the human immunodeficiency virus. Virus Res. 2005;111(2):194–213. doi: 10.1016/j.virusres.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Kruman II, Nath A, Mattson MP. HIV-1 protein Tat induces apoptosis of hippocampal neurons by a mechanism involving caspase activation, calcium overload, and oxidative stress. Exp Neurol. 1998;154(2):276–288. doi: 10.1006/exnr.1998.6958. [DOI] [PubMed] [Google Scholar]
- Lannuzel A, Barnier JV, Hery C, Huynh VT, Guibert B, Gray F, Vincent JD, Tardieu M. Human immunodeficiency virus type 1 and its coat protein gp120 induce apoptosis and activate JNK and ERK mitogen-activated protein kinases in human neurons. Ann Neurol. 1997;42(6):847–856. doi: 10.1002/ana.410420605. [DOI] [PubMed] [Google Scholar]
- Lipton SA. HIV-related neurotoxicity. Brain Pathol. 1991;1(3):193–199. doi: 10.1111/j.1750-3639.1991.tb00659.x. [DOI] [PubMed] [Google Scholar]
- Lipton SA, Brenneman DE, Silverstein FS, Masliah E, Mucke L. gp120 and neurotoxicity in vivo. Trends Pharmacol Sci. 1995;16(4):122. doi: 10.1016/s0165-6147(00)88998-1. [DOI] [PubMed] [Google Scholar]
- Ma M, Nath A. Molecular determinants for cellular uptake of Tat protein of human immunodeficiency virus type 1 in brain cells. J Virol. 1997;71(3):2495–2499. doi: 10.1128/jvi.71.3.2495-2499.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maragos WF, Tillman P, Jones M, Bruce-Keller AJ, Roth S, Bell JE, Nath A. Neuronal injury in hippocampus with human immunodeficiency virus transactivating protein, Tat. Neuroscience. 2003;117(1):43–53. doi: 10.1016/s0306-4522(02)00713-3. [DOI] [PubMed] [Google Scholar]
- Martin SC, Wolters PL, Toledo-Tamula MA, Zeichner SL, Hazra R, Civitello L. Cognitive functioning in school-aged children with vertically acquired HIV infection being treated with highly active antiretroviral therapy (HAART) Dev Neuropsychol. 2006;30(2):633–657. doi: 10.1207/s15326942dn3002_1. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Haughey NJ, Nath A. Cell death in HIV dementia. Cell Death Differ. 2005;12 Suppl 1:893–904. doi: 10.1038/sj.cdd.4401577. [DOI] [PubMed] [Google Scholar]
- Mildvan D, Mathur U, Enlow RW, Romain PL, Winchester RJ, Colp C, Singman H, Adelsberg BR, Spigland I. Opportunistic infections and immune deficiency in homosexual men. Ann Intern Med. 1982;96(6 Pt 1):700–704. doi: 10.7326/0003-4819-96-6-700. [DOI] [PubMed] [Google Scholar]
- Mintz M. Clinical comparison of adult and pediatric NeuroAIDS. Adv Neuroimmunol. 1994;4(3):207–221. doi: 10.1016/s0960-5428(06)80259-7. [DOI] [PubMed] [Google Scholar]
- Mintz M. Neurological findings in pediatric HIV/AIDS. Clinical features. In: Gendelman HE, Grant I, Everall I-P, Lipton SA, Swindells Susan S, editors. The Neurology of AIDS. 2nd ed. Oxford: University Press; 2005. pp. 639–658. [Google Scholar]
- Mintz M, Tardieu M, Hoyt L, McSherry G, Mendelson J, Oleske J. Levodopa therapy improves motor function in HIV-infected children with extrapyramidal syndromes. Neurology. 1996;47(6):1583–1585. doi: 10.1212/wnl.47.6.1583. [DOI] [PubMed] [Google Scholar]
- Morris RGM. Spatial localization does not require the presence of local cues. Learn Motiv. 1981;12:239–260. [Google Scholar]
- Motz BA, Alberts JR. The validity and utility of geotaxis in young rodents. Neurotoxicol Teratol. 2005;27(4):529–533. doi: 10.1016/j.ntt.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Nachman SA, Lindsey JC, Moye J, Stanley KE, Johnson GM, Krogstad PA, Wiznia AA Pediatric AIDS Clinical Trials Group 377 Study Team. Growth of human immunodeficiency virus-infected children receiving highly active antiretroviral therapy. Pediatr Infect Dis J. 2005;24(4):352–357. doi: 10.1097/01.inf.0000157095.75081.43. [DOI] [PubMed] [Google Scholar]
- Nath A, Anderson C, Jones M, Maragos W, Booze R, Mactutus C, Bell J, Hauser KF, Mattson M. Neurotoxicity and dysfunction of dopaminergic systems associated with AIDS dementia. J Psychopharmacol. 2000a;14(3):222–227. doi: 10.1177/026988110001400305. [DOI] [PubMed] [Google Scholar]
- Nath A, Haughey NJ, Jones M, Anderson C, Bell JE, Geiger JD. Synergistic neurotoxicity by human immunodeficiency virus proteins Tat and gp120: protection by memantine. Ann Neurol. 2000b;47(2):186–194. [PubMed] [Google Scholar]
- Newell ML, Borja MC, Peckham C European Collaborative Study. Height, weight, and growth in children born to mothers with HIV-1 infection in Europe. Pediatrics. 2003;111(1):e52–e60. doi: 10.1542/peds.111.1.e52. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford University Press; 1978. [Google Scholar]
- Phillips RG, LeDoux JE. Lesions of the dorsal hippocampal formation interfere with background but not foreground contextual fear conditioning. Learn Mem. 1994;1(1):33–44. [PubMed] [Google Scholar]
- Sharer LR. Pathology of HIV-1 infection of the central nervous system. J Neuropathol Exp Neurol. 1992;51(1):3–11. doi: 10.1097/00005072-199201000-00002. [DOI] [PubMed] [Google Scholar]
- Singh IN, Goody RJ, Dean C, Ahmad NM, Lutz SE, Knapp PE, Nath A, Hauser KF. Apoptotic death of striatal neurons induced by human immunodeficiency virus-1 Tat and gp120: differential involvement of caspase-3 and endonuclease G. J Neurovirol. 2004;10(3):141–151. doi: 10.1080/13550280490441103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swedlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology. 2001;156(2–3):194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Wesselingh SL, Griffin DE, McArthur JC, Johnson RT, Glass JD. Localization of HIV-1 in human brain using polymerase chain reaction/in situ hybridization and immunocytochemistry. Ann Neurol. 1996;39(6):705–711. doi: 10.1002/ana.410390606. [DOI] [PubMed] [Google Scholar]
- Tardieu M, Le Chenadec J, Persoz A, Meyer L, Blanche S, Mayaux MJ. HIV-1-related encephalopathy in infants compared with children and adults. Neurology. 2000;54(5):1089–1095. doi: 10.1212/wnl.54.5.1089. [DOI] [PubMed] [Google Scholar]
- Thompson KA, Churchill MJ, Gorry PR, Sterjovski J, Oelrichs RB, Wesselingh SL, McLean CA. Astrocyte specific viral strains in HIV dementia. Ann Neurol. 2004;56(6):873–877. doi: 10.1002/ana.20304. [DOI] [PubMed] [Google Scholar]
- Torres-Muñoz J, Stockton P, Tacoronte N, Roberts B, Maronpot RR, Petito CK. Detection of HIV-1 gene sequences in hippocampal neurons isolated from postmortem AIDS brains by laser capture microdissection. J Neuropathol Exp Neurol. 2001;60(9):885–892. doi: 10.1093/jnen/60.9.885. [DOI] [PubMed] [Google Scholar]
- Trillo-Pazos G, Diamanturos A, Rislove L, Menza T, Chao W, Belem P, Sadiq S, Morgello S, Sharer L, Volsky DJ. Detection of HIV-1 DNA in microglia/macrophages, astrocytes and neurons isolated from brain tissue with HIV-1 encephalitis by laser capture microdissection. Brain Pathol. 2003;13(2):144–154. doi: 10.1111/j.1750-3639.2003.tb00014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNAIDS/WHO. AIDS Epidemic Update. UNAIDS. [Online] 2007 Available from http://www.unaids.org/en/HIV_data/epi2007/default.asp.
- Walton KD, Harding S, Anschel D, Harris YT, Llinás R. The effects of microgravity on the development of surface righting in rats. J Physiol. 2005;565(Pt 2):593–608. doi: 10.1113/jphysiol.2004.074385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231(4):482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Wiley CA, Belman AL, Dickson DW, Rubinstein A, Nelson JA. Human immunodeficiency virus within the brains of children with AIDS. Clin Neuropathol. 1990;9(1):1–6. [PubMed] [Google Scholar]
- Wiley CA, Soontornniyomkij V, Radhakrishnan L, Masliah E, Mellors J, Hermann SA, Dailey P, Achim CL. Distribution of brain HIV load in AIDS. Brain Pathol. 1998;8(2):277–284. doi: 10.1111/j.1750-3639.1998.tb00153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer BJ. Statistical Principles in Experimental Design. New York: McGraw-Hill; 1971. [Google Scholar]