Abstract
Alcoholism pharmacotherapies are underutilized in community addiction treatment settings, in part because individuals who practice in these settings—non-medical addiction counselors and administrators—lack knowledge about and confidence in the value of adjunctive alcohol pharmacotherapies. We developed and tested an intervention to improve knowledge and attitudes about naltrexone. A team of researchers, physicians, addiction treatment counselors and administrators collaborated to develop a naltrexone educational intervention designed for non-medical addiction professionals. The intervention was compared to a control condition in a pilot study with six addiction treatment agencies (3 agencies per group). Participants (counselors and administrators, N=84) were assessed prior to and six months following the intervention. Results revealed that the intervention significantly improved naltrexone knowledge, and participants who received the intervention reported greater satisfaction with the education they received, as well as greater utilization of the information. The effect of the intervention on attitudes about naltrexone was encouraging, but failed to reach statistical significance. The present study is the first reported attempt to develop and test an intervention specifically to improve acceptance of adjunctive medications for alcoholism among non-medical addiction professionals.
Keywords: technology transfer, training, pharmacotherapy, naltrexone, education
1. INTRODUCTION
Despite FDA approval of several medications for the treatment of alcohol dependence and the accumulating evidence that medications should play a more central role in the treatment of alcoholism (Foxhall, 2005), few alcohol dependent individuals have been treated with pharmacotherapies (Petrakis, Leslie & Rosenheck, 2003; Thomas, Wallack, Lee, McCarty & Swift, 2003). This disconnect is due in part to the fact that addiction treatment agencies, which deliver the majority of alcoholism treatment in the United States, are staffed primarily by non-medical addiction counselors, who rarely discuss alcoholism pharmacotherapies with their clients (Forman, Bovasso & Woody, 2001; McLellan, 2002; Thomas et al., 2003).
It is likely that alcoholism treatment outcomes would be improved if counselors educate their clients about the value of adjunctive medications, assist interested clients in obtaining a prescription, and support medication compliance. This contention is supported by the strong evidence for that exists regarding the incremental benefit of naltrexone in the treatment of alcohol dependence. Meta-analyses of controlled clinical trials have shown that when naltrexone is added to psychosocial counseling, clients have a lower percentage of drinking days, fewer drinks per drinking episode, longer times to relapse, more days of abstinence, and lower total alcohol consumption during treatment (Bouza, Magro, Munoz & Amate, 2004). In addition, clients receiving adjunctive naltrexone have fewer cravings than placebo patients during active treatment and have a 28% lower risk of treatment termination (Srisurapanont & Jarusuraisin, 2005).
Unfortunately, educational interventions to improve counselors’ knowledge of and confidence in the value of alcohol medications are lacking. The present study was designed to address this void.
Recent investigations have examined factors associated with the clinical endorsement of adjunctive medications by non-medical community-based alcoholism counselors. Thomas and colleagues (2003) found that a primary reason counselors did not recommend naltrexone was a lack of knowledge about its effectiveness. Indeed, most alcoholism practitioners have little knowledge of adjunctive medications, and they question their utility in clinical practice (Meza, Cunningham, el-Gyebaly & Couper, 2001). Others have found that support for medication use is higher among counselors with more advanced degrees and those with more experience in the alcoholism field (Forman et al., 2001; Roman & Johnson, 2002). Data from our work supports these findings: years of experience in the addiction field, education level, and knowledge about naltrexone were each positively correlated with addiction professionals’ valuation of the importance of alcohol pharmacotherapy (Thomas & Miller, 2007).
Lack of knowledge of pharmacotherapy by addictions counselors is not surprising given that they receive little training in this area, either formally in their degree programs or informally by pharmaceutical representatives. In addition, alcoholism pharmacotherapy research is typically reported in specialty scientific journals that are not widely read by addiction counselors. Even if these scientific reports were well known among counselors, adoption rates are poor for treatment innovations reported only in the research literature (Hall, Sorensen & Loeb, 1988; Sorensen, Hall, Loeb, Allen, Glaser & Greenberg, 1988). Manuals written for clinicians by researchers typically lead to similarly low adoption rates (Hall, et al. 1988).
Brown (2000) has aptly noted that the adoption of new treatment technologies depends on the clarity and relevance of the educational format within which the information about that technology is presented. Although recent efforts such as SAMHSA’s Blending Initiative exist to translate research information into formats relevant to practitioners (Clay, 2006), evidence-based educational interventions to disseminate alcoholism pharmacotherapy information to community-based counselors are lacking.
The present study was designed to develop and test a prototype intervention to disseminate alcoholism pharmacotherapy information to non-medical addiction counselors. For this initial effort, we limited the focus of training to naltrexone. The development of the intervention was unique in that it involved a collaborative effort between academic researchers and community-based counselors, as recommended in the Institute of Medicine report (Lamb, Greenlick & McCarty, 1998). The intervention was pilot tested with a group of community-based addiction counselors and administrators not involved in the development of the intervention. The intervention purposely included (1) clear and concise didactic information that was both relevant to and easily understood by counselors and (2) an academic detailing (i.e., onsite educational discussion) booster session. Relevant, clear information and academic detailing sessions have both been shown to produce more effective and lasting educational experiences than traditional lectures or manuals alone (Brown, 2000; Gomel, Saunders, Wutzke, Hardcastle & Carnegie 1996).
The hypotheses tested were that counselors and administrators who received the educational intervention (vs. control condition) would (1) have greater improvements in knowledge and attitudes about the value of naltrexone and (2) be more satisfied and report higher utilization of the information they received. The project was conducted in two phases: development of the intervention (year 1) and pilot testing the intervention (year 2).
2. MATERIALS AND METHODS
2.1 Development Phase
Participants
Phase 1 participants (N=7) were a collaborative, multidisciplinary team of academic researchers and community-based treatment practitioners assembled to develop the educational intervention. The team was comprised of an experimental psychologist (SET), a clinical psychologist (PMM), a psychiatrist boarded in addiction medicine (SWB), an expert consultant with experience in translating naltrexone information to practitioners in the community (R. Swift, MD, Ph.D.), the director of a local community-based addiction treatment center, and two certified addictions counselors at the center. The goal was to design an intervention that would convey accurate and useful information about naltrexone treatment for alcoholism in a way that would be most acceptable and useful to addiction counselors and administrators.
Procedures
Brainstorming sessions were conducted with the first three members of team listed above (the investigators for the study) to develop the elements of the intervention. These sessions were guided by the extant research literature on diffusion of information and innovations. Both written handouts and didactic instruction (via PowerPoint presentation) were considered as basic training tools. In addition, based on the results of the NIAAA-supported Researcher-in-Residence program (National Institute on Alcohol Abuse and Alcoholism, 2002), an on-site academic detailing/booster session was considered a critical element of the intervention. Through an iterative process, investigators devised a draft intervention which was presented to other Phase I participants (listed above), who provided feedback and recommendations. Examples of specific feedback were recommendations to include a client information brochure as well as specific information on the medication costs particularly as compared to the cost of alcoholic beverages.
At the end of this discussion and feedback process, the core team of three investigators revised the draft intervention to incorporate suggestions. The final versions of the experimental intervention and control condition is described in detail below.
Intervention
The intervention consisted of seven elements (see Table 1), under three general categories—printed information, didactic instruction, and academic detailing. The control condition contained only printed information; specifically, the Frequently Asked Questions handout about naltrexone for alcoholism and the pharmaceutical package insert for naltrexone. The FAQ handout was based on information from the extant literature and the Naltrexone and Alcoholism Treatment Improvement Protocol (O’Malley, 1998). The package insert for naltrexone (ReVia) was provided by DuPont Pharma, (Wilmington, DE).
Table 1.
Result of the intervention development phase and the elements of the intervention that were delivered to each group.
| Control group | Intervention group | |
|---|---|---|
| Didactic instruction (Powerpoint presentation) | X | |
| Empirical research findings | ||
| How it works | ||
| Benefits to the counseling process | ||
| Information about cost | ||
| Suggested ways to talk to clients about naltrexone | ||
| Written information | ||
| Frequently Asked Questions about naltrexone | X | X |
| Brochures to provide to clients about naltrexone | X | |
| Medication referral cards to provide to clients | X | |
| Package insert from naltrexone | X | X |
| Notes from Powerpoint presentation | X | |
| Academic detailing (3 months after initial visit) | X | |
| In-person visit | ||
| Review of information | ||
| Q & A | ||
| Group brainstorming to overcome barriers to naltrexone use |
Participants assigned to the intervention condition received materials provided to the control group, plus didactic instruction (a PowerPoint presentation delivered by Drs. Thomas and Miller, approximately 40 minutes in length) and discussion time afterwards (20 minutes). In addition, each counselor received 100 informative brochures to provide to clients regarding natlrexone, and 100 referral cards that clients could take to a physician to initiate a discussion about naltrexone. These cards were the size of a credit card, so that the client could easily transport the card in a wallet or purse for a future meeting with a prescribing physician.
2.2 Pilot Testing
Participants
Prior to initiation of the project, directors of seven community-based addiction treatment agencies in South Carolina were contacted and informed about the research. The agencies included in the study were partially self-selecting—they expressed interest in being involved in research projects with university investigators and all had a staff of at least 10 full-time counselors who treated adults with alcohol dependence. The agency that provided expertise for the development phase of the project (above) was not included in the pilot testing phase.
The remaining six agencies were all similar in size (based on the number of full-time counselors) and had low turnover rates for counseling staff (< 10% annually). None employed an onsite physician to treat outpatient clients. All participating agencies were public treatment centers supported in part by federal block grants. Three agencies each were randomly assigned to the intervention vs. control condition. Directors of these participating agencies agreed to allow project research staff to conduct on-site training at their agencies to inform staff of the opportunity to participate in the study.
Individuals employed in these six treatment centers were invited to participate in the pilot testing phase of the study during an in-person visit to each center. Inclusion criteria were (1) the individual must be employed full-time at the center as a counselor or administrator, and (2) if a counselor, the individual must be involved in treating adult alcoholic clients. Counselors who only treated adolescents were not included in the study. Participation was voluntary, and before data were collected, all willing participants signed an informed consent agreement approved by their agencies and by the Institutional Review Board of the Medical University of South Carolina. Counselors and administrators who participated in the project received a certificate of participation, which provided two hours of continuing education credit. All but four eligible individuals chose to participate; these individuals declined because they were leaving their agency for another position (n=2) or about to retire (n=2). Total number of participants (across all agencies) was 84 (control group n=47; intervention group n=37). The average number of participants within each agency was 15 (SD=.58) for agencies in the control group and 12 (SD=4.5) for agencies in the intervention group, t(4)=1.27, p=.27.
Procedures
Visits to each treatment center were conducted during a staff retreat or monthly staff meeting, so as not to interfere with delivery of clinical services and to ensure that the maximum number of participants would be available for assessment and delivery of the intervention (or control condition). At the first visit, informed consent agreements were collected and confirmation of eligibility was established. Before the intervention was delivered, baseline assessments were conducted.
The first visit to each agency required approximately 1.5 hours. This included obtaining informed consent, collecting baseline data, and delivering either the experimental intervention (approximately one hour in length) or control condition. Sites randomized to the experimental condition (n=3) received an additional visit— academic detailing (clinic-based educational activity aimed at individual practitioners)—three months after the initial visit (described in the Results section). All agencies were visited six months after the baseline visit to collect outcome data. The experimental group received three visits (each 3 months apart) and the control group received two visits (6 months apart).
Assessments
Except for a demographic questionnaire and the treatment satisfaction/utilization survey, all assessment instruments were administered at baseline and again six months later. The demographic questionnaire was administered only at baseline and included queries about years of experience in the addiction treatment field, years working at the current treatment center, and recovery status regarding substance use problems. The treatment satisfaction/utilization survey was administered only at the six-month follow-up period.
Naltrexone Knowledge Test
Knowledge about naltrexone was assessed with a study-specific, 10-item questionnaire (Thomas & Miller, 2007). Response options were “true”, “false”, and “don’t know” (both “don’t know” and incorrect responses were considered incorrect for test scoring). Items included statements generated from NIAAA-produced reports on the utility of naltrexone and from the pharmaceutical package insert for naltrexone. Sample statements include “Naltrexone is approved by the FDA for the treatment of alcohol dependence” (true); “Naltrexone has abuse potential, so it must be administered to the patient daily by a healthcare provider” (false); and “Naltrexone makes a drinker sick if s/he drinks alcohol while taking it, which helps the person stay motivated not to drink” (false). Participants’ test scores reflected the percentage of items answered correctly (0–100%).
Attitudes about Naltrexone and Adjunctive Pharmacotherapies
These constructs were measured with items from the “What Works in Treating Alcohol Problems” questionnaire. This questionnaire contains statements derived from a study conducted by Morgenstern and McCrady (1992), with additional statements relating specifically to the use of pharmacotherapies for alcoholism reported by Meza and colleagues (Meza et al., 2001). Respondents are instructed to consider their experiences in treating alcohol dependent clients in providing their opinions about the value of different treatment approaches using a 7-point Likert-type scale. Two items from the instrument were of primary interest for the present study; these items measured the respondent’s valuation of naltrexone and adjunctive pharmacotherapies, in general. For each of these statements, higher scores reflected more positive valuation.
Treatment Satisfaction and Utilization of Information
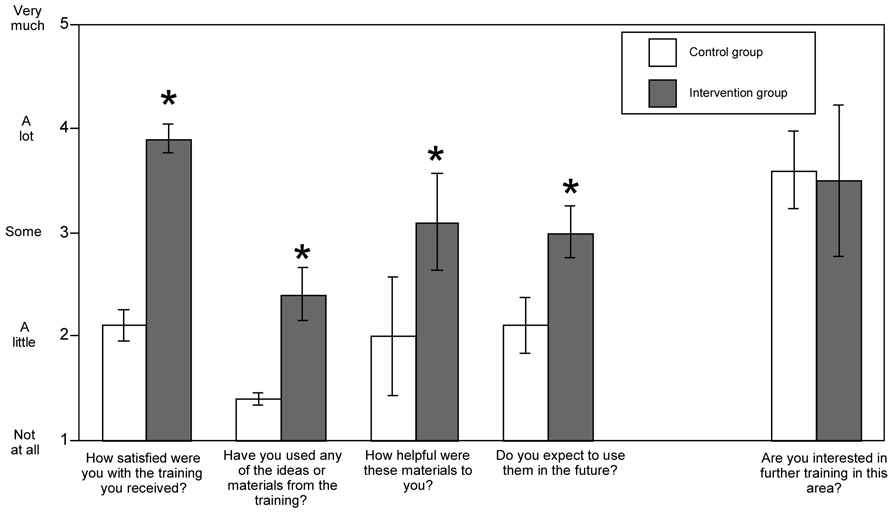
Satisfaction with the intervention or control condition and self-reported utilization of the information presented was assessed at the 6-month follow-up only. These constructs were assessed with a study-specific survey, and participants were asked to rate their responses on a 5-point scale from 1 (not at all) to 5 (very much). This survey included four statements about the value of the intervention: (1) How satisfied were you with the training provided?; (2) Have you used any of the ideas or materials from the training?; (3) How helpful were these materials to you? and (4) Do you expect to use them in the future? It also included one statement about regarding interest in further, more specialized training in the area of alcohol medications that was analyzed separately.
Analytic Plan
The primary hypotheses were that (1) the educational intervention vs. control condition would improve naltrexone knowledge and attitudes about the value of adjunctive alcohol pharmacotherapies, and (2) participants who received the educational intervention would be more satisfied with the intervention than control participants, and they would report greater utilization of the information learned.
Treatment centers, rather than individual counselors, were randomized to the two treatment conditions. Thus, the data collected were nested, i.e. participants were nested within center. These data were analyzed as a hierarchical linear model with participants (level 1) nested within centers (level 2) using HLM 6.0 (Raudenbush, Bryk & Congdon, 2004). The group assignment (intervention vs. control) is a variable that distinguishes centers and is a level 2 variable; variables that differed among individual participants within centers (e.g. counselor age or years of experience) are level 1 variables. Treatment was represented as an indicator-coded (1=intervention, 0=control) dummy variable and pre- and post- intervention scores on knowledge and attitude instruments were the level 1 variables in this analysis. Baseline (pre-intervention) scores were mean-centered for each agency and then employed as level-1 covariates (the multi-level analog of an analysis of covariance of post-treatment scores with pre-treatment scores as the covariate).
3. RESULTS
3.1 Participants
Table 2 shows the characteristics of the participants in the pilot study. The majority of participants (79%) were counselors; 21% were administrators. Most (57%) had some graduate school experience, and all but four had a four-year college degree. On average, participants had worked in the addiction field for approximately ten years, and most had been employed at their present agency for at least five years. There were no differences between groups on any measure, except for age, where the participants in agencies randomized to the experimental group were significantly older, t(82)=2.01, p=.05. Inclusion of age as a covariate in the outcome analyses did not alter results.
Table 2.
Demographic information for participants. The only ethnic minority group with substantial representation was African American. Groups were significantly different (p ≤ .05) only on age of participants.
| Control group | Intervention group | |
|---|---|---|
| N agencies | 3 | 3 |
| Total number of participants | 47 | 37 |
| Counselors | 82% | 73% |
| Female | 66% | 73% |
| Caucasian | 77% | 70% |
| Age, M (SD) | 42 (11.1) | 47 (10.4) |
| Post-graduate degree/licensure | 61% | 54% |
| In recovery for substance use disorder | 26% | 16% |
| Years working in addiction field | 9.0 (8.9) | 11.0 (8.8) |
| Years working in present agency | 5.2 (6.6) | 7.7 (7.5) |
At the follow-up interview, 69% of the original 84 participants provided outcome data. The rate of attrition did not differ between the control and intervention groups. While all participants were still employed at their respective agencies, 26 participants (15 in the control agencies; 11 in the experimental agencies) were not present at their agencies on the day that outcome data were collected. Participants who did vs. did not provide outcome data did not differ on any demographic variable, except ethnicity, X2(1)=4.40, p=.04. Among Caucasian participants, 77% provided outcome data; for African American participants, the rate was lower (53%). For all analyses, missing data were treated as missing; no imputation techniques were used.
3.2 Naltrexone Knowledge
Detailed information about participants’ knowledge about naltrexone at baseline (range=0 to 100) has been published previously (Thomas & Miller, 2007), including responses on each of the ten items that comprise the questionnaire. The items in the questionnaire were highly related (Cronbach’s alpha = .82) and were analyzed as a single scale.
Groups did not differ at baseline on knowledge scores (control group M=31.1, SD=25.0; intervention group M=38.9, SD=30.6), t(82)=1.30, p=.20. Baseline knowledge scores reflected chance performance (33% correct, given that three response options were available for each item).
Knowledge increased substantially in both groups at post-treatment. The difference in improvement was examined by comparing the difference between group means (means of agency means) relative to the variation of agency means. The agencies assigned to the intervention group improved in knowledge significantly more than control agencies, t(4)=3.66 p=.034, where the post-test mean knowledge score for the intervention agencies was 78.4 (95% CI 78.2, 78.60) vs. 63.9 (95% CI 63.16, 64.64) for the control agencies (means reflect baseline-adjusted scores).
We also examined the probability of answering at least 8 of 10 questions correctly at follow-up (equivalent to a grade of A or B). This analysis was performed as a hierarchical logistic regression with structure similar to the primary analysis above except that the outcome variable was binary rather than continuous. Adjusting for baseline knowledge, participants in the intervention group were significantly more likely (p=.042) to score 80 or above [odds ratio = 4.24 (1.54–11.7)]. The intervention group had 65% of participants scoring at least 80% of questions correct at post-test, whereas only 34% of the control group achieved this goal. Taken together, these results support that the educational intervention significantly improved participants’ knowledge about naltrexone.
3.3 Attitudes about Naltrexone
Participants were asked to rate the value of encouraging alcoholic clients to talk to a physician about naltrexone. On a scale from −3 to +3, mean agreement ratings at baseline were around zero for each group [control mean (SD)=0.68 (0.91); intervention mean (SD)=0.73 (0.84)], reflecting the belief that this approach with client would have no effect (i.e., neither positive nor negative). A rating of “no effect” (as indexed by a score of −1, 0 or 1 on the item) was provided by 82% of controls and 84% of the intervention group at baseline. Only 15% of controls and 16% of the intervention group rated this approach as essential (score of 2 or 3). Groups did not differ in their ratings of this item at baseline, either by the continuous measure, t(4)=.259, p=.81 or with a binary (essential vs not essential), t(4)=.26, p=.81.
Post-treatment means were 2.25 (95% CI 2.07, 2.43) and 2.5 (95% CI 2.27, 2.74) for the control and intervention groups, respectively. Further, at follow-up, only 29% of the control group rated the approach as “essential” vs 50% of the intervention group. Superiority of the intervention group approached but did not reach significance with the dependent variable analyzed as a continuous variable (t(3)=2.08, p=.12) or as a binary variable (t(3)=2.0, p=.13). In the latter case, it is notable that the treatment doubled the odds of participants rating the advice as essential, OR=2.47 (.92–6.7).
3.4 Attitudes about Pharmacotherapies in General
At baseline, ratings on the item regarding the value of adjunctive alcohol pharmacotherapies (“Pharmacologic interventions have convincingly been shown to be useful in the treatment of alcohol dependence”) were low, similar to the ratings about the value of naltrexone (above). This item was scored on a 1–7 scale, where 1 reflects strong disagreement, 4 reflects neither agreement nor disagreement, and 7 reflects strong agreement. Average rating for the control groups was 4.28 (SD=2.13), and average rating for the intervention group was 4.4 (SD=1.34), which were not significantly different, t(4)=.324, p=.76. Average ratings for each group corresponded with the mode: the majority of each group (83% of the control group and 89% of the intervention group) rated this item with a neutral response (as defined by a rating of 3,4, or 5) at baseline.
Group means at follow-up were 4.55 (95% CI 4.16, 4.94) in the control and 5.0 (95% CI 4.51, 5.49) in the intervention group, which again approached but did not reach significance, t(3)=2.1, p=.12. Data on rates of agreement were also examined, where the percent of each group that rated the item 6 or 7 was compared using hierarchical logistic regression (post-test controlling for pre-test). At follow-up, rate of agreement in the intervention group (35%) was twice that observed in the control group (16%). Also, the intervention group showed marked change (from 6% to 35% of the group agreeing with the statement), whereas the control group results remained flat (from 13% to 16% agreement). Despite this difference, this effect failed to reach significance, t(3)=2.1, p=.12; odds ratio = 2.8 (.952-8.3), likely due to the small sample size.
3.5 Satisfaction with the intervention and self-reported utilization of information
The four ratings on utilization and satisfaction were highly related (Cronbach’s alpha = .85) and were analyzed initially as a single scale. The intervention group ratings were significantly higher (M=12.5, 95% CI 11.5, 13.5) than those of the control group (8.0, 95% CI 6.69, 9.31), t(4)=7.5, p <.001. Post hoc analysis of the four individual items indicated that the participants in the intervention group responded more positively to the intervention than did participants in the control group. Specifically, intervention group members were more likely to be satisfied with the training they received t(4)=14.4, p<.001, to find the material useful t(4)=2.8 p<.05, to report using the ideas/material provided t(4)=7.8 p<.001, and to expect to use the information/materials in the future t(4)=4.3, p=.016. Means and 95% CIs are shown in Figure 1. Though not part of the primary hypotheses, we also asked participants whether they were interested in receiving more specialized training regarding alcohol medications. Both groups responded similarly and positively to this item, t(4)=.23 p=.83 (see Figure 1).
Figure 1.
Ratings of satisfaction and utilization of information received provided by both groups at follow-up. Group means and 95% CIs are shown.
4. DISCUSSION
The present project was conducted to develop and pilot test an intervention designed to improve knowledge and attitudes among non-medical community-based addiction treatment counselors and administrators about the use of naltrexone as an adjunctive treatment for alcoholism. While other barriers exist to adopting pharmacotherapies in addiction treatment settings (Ducharme, Knudsen & Roman, 2006; Fuller et al., 2005; Miller et al., 2006; Thomas et al., 2003), the one that has received the most empirical support is also the one that can be most readily addressed—counselor knowledge and confidence in the value of these medications.
The close collaboration of clinicians and researchers was a considerable strength in the development of the intervention. The multi-disciplinary team—physicians, clinical and experimental psychologists, an administrator, and addiction counselors—provided perspectives in the development process that might not otherwise have been considered. For example, the first draft of the intervention failed to include information that the addiction counselors felt was needed, such as the monthly cost of the medication and practical issues related to starting medication. Even though counselors would not be the ones to prescribe naltrexone, the two counselors on the development team indicated that having this information in hand was critical for them to consider whether naltrexone might be helpful for a client and whether they would feel comfortable in presenting this option to the client. Because of their input, this information was added to the final product. In addition, the counselors reviewed the handout materials to be provided to clients and made valuable suggestions regarding their content and wording, resulting in a more client-friendly brochure. The convergence of these different perspectives was essential in developing the final product.
The intervention presented information with a multi-modal approach, including a didactic presentation and corresponding lecture notes, relevant reading materials, client brochures, and referral/information cards about naltrexone, to be provided by clients to their physicians. The intervention was designed not only to teach, but also to facilitate the counselor’s quality of client care. While the value of the client-focused handouts was not explicitly assessed, counselors and administrators in the intervention group reported that this literature was very helpful. The brochures reminded the counselor to consider whether a client was a possible candidate for naltrexone, and to discuss it with him/her.
The intervention was evaluated for its effect on changing knowledge, attitudes, and satisfaction and self-reported utilization among counselors and administrators. Results showed that the intervention significantly improved naltrexone knowledge, and participants who received the intervention were more satisfied with their learning experience and reported greater likelihood of using acquired knowledge in the future. The intervention showed positive, though not statistically significant, effects on improving attitudes about naltrexone and adjunctive pharmacotherapies, in general. As discussed in greater detail below, the lack of significance might be due to the small number of agencies included in this pilot study.
Both groups improved in their ratings over time. It may be that even the minimal information provided to the control group was sufficient to provide participants with the requisite knowledge, or it could be that this information stimulated counselors to seek out more information about naltrexone on their own. Future studies will be valuable in determining both the nature of this process and differential characteristics of counselors who are influenced by different intensities of educational interventions. Both groups reported an interest in receiving additional information about alcoholism pharmacotherapies, an encouraging result in itself. Because of the limited timeframe and resources of this pilot study, we could not assess whether the intervention resulted in actual (vs. self-reported) behavior change (i.e., whether counselors in the intervention vs. control group differentially shared naltrexone information with their clients). Determining with both extensive self-reports as well as with objective behavioral measures whether the intervention changes clinical practice is a goal of future research.
Because of the pilot nature of this study, several limitations, including the small sample size, should be noted. Because agencies, not individual participants, were randomized to conditions, the appropriate analytic approach for this data structure is one that accounts for the nested design. While a hierarchical analysis reduces the sample size to six (3 agencies per group), this is a necessary consideration since counselors within agencies resemble each other more than do counselors between agencies (e.g. intracluster correlation coefficients for baseline naltrexone knowledge was .145, a value that suggests significant clustering, cf. Hox, 2002). All hypotheses were tested regarding whether agencies in the intervention group differed from those in the control group, relative to expected variation among agencies, not among individuals. Although univariate tests such as analysis of covariance increase power by utilizing each individual’s data singly (so that the sample size is 84 for this study), such tests introduce a positive bias given this study design. An example of the disparity in power can be seen in the analysis of the item regarding the usefulness of pharmacological intervention. A traditional analysis (ANCOVA of follow-up score with baseline score as a covariate) reveals significant superiority of the intervention group at p=.03; results from hierarchical analysis, however, only approached significance (p=.12). The difference between the two analyses is that the first uses as a standard of comparison the variability of individual subjects while the second uses the variability among agencies. We opted to use the more conservative (and arguably, more accurate) analytic approach. To increase power, future studies should include more agencies, even if the number of employees within each agency is small.
Additional limitations include the fact that the single-item questions used to determine changes in attitudes limited a full assessment of this construct. In future studies, we will be able to examine attitudes with more comprehensive measures. Also, generalizability of these results is undermined by two factors. First, African American participants were less likely to provide outcome data compared to White participants; second, agencies in this study may not represent addiction treatment centers nationally, particularly regarding stability/tenure for treatment staff. The state-based addiction treatment system in South Carolina is comparatively more stable than similar agencies nationwide, reflected in part by the fact that counselor turnover rates at the agencies participating in this study were less than 10% per year, whereas annual turnover rates of 20%–50% are typical, according to the National Treatment Center Study (see Knudsen, Johnson & Roman, 2003) and the National Survey of Substance Abuse Treatment Services (see McLellan, Carise & Kleber, 2003). Because of these limitations, the results of this pilot study are most valuable in directing future large-scale studies, which should include a wider range of treatment facilities and more intensive efforts to collect outcome data from all participants.
Finally, to focus the scope of this initial project, only information about naltrexone was presented in the intervention. It is not possible to determine with the present study whether addiction counselors will be similarly influenced by information about the utility of other alcohol medications.
In conclusion, this multi-faceted training program—developed collaboratively by addictions counselors, administrators, and researchers—improved knowledge about the use of naltrexone among non-medical personnel in community addiction treatment centers. Participants in the intervention group reported increased likelihood of recommending naltrexone to their clients. In addition, participants in the intervention group were highly satisfied with the training they received, and the results suggested a benefit in improving attitudes about naltrexone and pharmacotherapies in general. Participants in both groups expressed an interest in learning more about alcoholism pharmacotherapy, indicating that there is a real need for continued dissemination efforts.
Future investigations are needed to further evaluate this training program with a broader and larger sample of agencies, and to include information about other alcohol FDA-approved pharmacotherapies. In addition, studies are needed to evaluate the extent to which improvements in knowledge and attitudes lead to changes in actual practice behavior. Given the focus of this research on attitudes and on intention to change behavior, future studies may be well served by formulating hypotheses within such models as the theory of planned behavior or the theory of reasoned action (Ajzen & Fishbein, 1980; Fishbein & Ajzen, 1975). Within these frameworks, intention to change behavior is a key and intention, in turn, is a joint function of attitude toward the behavior and perceived norms to perform the behavior. Recently, such theoretical formulations have proved useful in examining client and counselor attitudes toward the use of medications for the treatment of opioid dependence (Rieckmann, Daley, Fuller, Thomas, & McCarty, 2007).
The present study represents a practical first step toward helping community-based addiction counselors adopt adjunctive pharmacotherapies into their treatment repertoire, which ultimately will translate into improved outcomes for clients.
ACKNOWLEDGMENTS
This study was supported by a grant from NIAAA (AA015065). The authors thank Elaine Ramsey and Abigail Shealy for their technical assistance in conducting the study. Thanks also to the following staff at the Dorchester Alcohol and Drug Commission (DADC), Summerville, South Carolina, for their involvement in Phase 1 of the project (the development of the intervention): Mr. Samuel Miller (agency Director), and Ms. Kristen Campbell and Mr. Kevin Shea (addiction counselors at DADC). The authors also thank the counselors and administrators of the following treatment centers in the state who participated in the pilot study: The Kennedy Center, Moncks Corner; The Aiken Center, Aiken; Greenville County Commission on Alcohol and Drug Abuse, Greenville; Spartanburg Alcohol and Drug Abuse Commission, Spartanburg; Keystone Substance Abuse Services, Rock Hill; Shoreline Behavioral Health Services, Conway. Finally, the authors appreciate Dr. Robert Swift, who participated in the development of the intervention and provided invaluable expertise as a consultant for this project. A limited portion of the manuscript was presented in a symposium at the 2006 meeting of the Research Society on Alcoholism (Baltimore, MD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ajzen I, Fishbein M. Understanding attitudes and predicting social behavior. Englewood Cliffs, NJ: Prentice-Hall; 1980. [Google Scholar]
- Brown BS. From research to practice: The bridge is out and the water's rising. In: Levy JA, Stephens RC, McBride DC, editors. Emergent issues in the field of drug abuse (Vol 7, pp 345–365) in Advances in Medical Sociology series. New York: Elsevier; 2000. [Google Scholar]
- Bouza C, Magro A, Munoz A, Amate JM. Efficacy and safety of naltrexone and acamprosate in the treatment of alcohol dependence: A systematic review. Addiction. 2004;99:811–828. doi: 10.1111/j.1360-0443.2004.00763.x. [DOI] [PubMed] [Google Scholar]
- Clay RA. Initiative blends research and practice. SAMHSA News. 2006;14(5):1–3. [Google Scholar]
- Ducharme LJ, Knudsen HK, Roman PM. Trends in the adoption of medications for alcohol dependence. Journal of Clinical Psychopharmacology. 2006;26 Supp1:13–19. doi: 10.1097/01.jcp.0000246209.18777.14. [DOI] [PubMed] [Google Scholar]
- Fishbein M, Ajzen I. Belief, attitude, intention and behavior: An introduction to theory and research. Reading, MA: Addison-Wesley; 1975. [Google Scholar]
- Forman RF, Bovasso G, Woody G. Staff beliefs about addiction treatment. Journal of Substance Abuse Treatment. 2001;21(1):1–9. doi: 10.1016/s0740-5472(01)00173-8. [DOI] [PubMed] [Google Scholar]
- Foxhall K. In alcohol treatment, a medication surge may be near. Addiction Professional. 2005 January;:14–20. [Google Scholar]
- Fuller B, Rieckmann T, McCarty D, Smith KW, Levine H. Adoption of naltrexone to treat alcohol dependence. Journal of Substance Abuse Treatment. 2005;28:273–280. doi: 10.1016/j.jsat.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Gomel MK, Saunders JB, Wutzke SE, Hardcastle DM, Carnegie MA. Sydney, Australia: Department of Health Services and Health; Implementation of early intervention for hazardous alcohol consumption in general-practice (Report prepared for the Research and Drug Program) 1996
- Hall SM, Sorensen JL, Loeb PC. Development and diffusion of a skills-training intervention. In: Baker TB, Cannon DS, editors. Assessment and Treatment of Addictive Disorders. New York: Praeger; 1988. pp. 180–204. [Google Scholar]
- Hox J. Multilevel analysis: Techniques and applications. Mahwah, NJ: Lawrence Erlbaum; 2002. [Google Scholar]
- Knudsen HK, Johnson JA, Roman PM. Retaining counseling staff at substance abuse treatment centers: effects of management practices. Journal of Substance Abuse Treatment. 2003;24:129–135. doi: 10.1016/s0740-5472(02)00357-4. [DOI] [PubMed] [Google Scholar]
- Lamb S, Greenlick M, McCarty D. Bridging the gap: Forging new partnerships in community-based drug abuse treatment. Washington DC: National Academy Press; 1998. [PubMed] [Google Scholar]
- McLellan AT. Technology transfer and the treatment of addiction: What can research offer practice? Journal of Substance Abuse Treatment. 2002;22(4):169–170. doi: 10.1016/s0740-5472(02)00240-4. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Carise D, Kleber HD. Can the national addiction treatment infrastructure support the public's demand for quality care? Journal of Substance Abuse Treatment. 2003;25:117–121. [PubMed] [Google Scholar]
- Meza EF, Cunningham JA, el-Guebaly N, Couper L. Alcoholism: Beliefs and attitudes among Canadian alcoholism treatment practitioners. Canadian Journal of Psychiatry. 2001;46:167–172. doi: 10.1177/070674370104600209. [DOI] [PubMed] [Google Scholar]
- Miller WR, Sorensen JL, Selzer JA, Brigham GS. Disseminating evidence-based practices in substance abuse treatment: A review with suggestions. Journal of Substance Abuse Treatment. 2006;31:25–39. doi: 10.1016/j.jsat.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Morgenstern J, McCrady BS. Curative factors in alcohol and drug treatment: Behavioral and disease model perspectives. British Journal of Addiction. 1992;87(6):901–912. doi: 10.1111/j.1360-0443.1992.tb01985.x. [DOI] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. Rockville, MD: National Institute on Alcohol Abuse and Alcoholism and Center for Substance Abuse Treatment; Researcher in Residence Program: Improving Treatment Practice in North Carolina. 2002
- O’Malley S. Treatment Improvement Protocol (TIP) Series 28 (DHHS Publication No. SMA 98-3206) Rockville, MD: Substance Abuse and Mental Health Services Administration, Center for Substance Abuse Treatment; 1998. Naltrexone and alcoholism treatment. [PubMed] [Google Scholar]
- Petrakis IL, Leslie D, Rosenheck R. Use of naltrexone in the treatment of alcoholism nationally in the Department of Veterans Affairs. Alcoholism: Clinical and Experimental Research. 2003;27:1780–1784. doi: 10.1097/01.ALC.0000095861.43232.19. [DOI] [PubMed] [Google Scholar]
- Raudenbush W, Bryk AS, Congdon R. HLM: Hierarchical Linear and Nonlinear Modeling (Version 6.0) Licolnwood, Ill: Scientific Software International, Inc.; 2004. [Google Scholar]
- Riecknamm T, Daley M, Fuller BE, Thomas CP, McCarty D. Client and counselor attitudes toward the use of medications for treatment of opioid dependence. Journal of Substance Abuse Treatment. 2007;32:207–215. doi: 10.1016/j.jsat.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman PM, Johnson J. Adoption and implementation of new technologies in substance abuse treatment. Journal of Substance Abuse Treatment. 2002;22(4):210–218. doi: 10.1016/s0740-5472(02)00241-6. [DOI] [PubMed] [Google Scholar]
- Sorensen JL, Hall SM, Loeb P, Allen T, Glaser EM, Greenberg PD. Dissemination of a job seekers' workshop to drug treatment programs. Behavior Therapy. 1988;19(2):143–155. [Google Scholar]
- Srisurapanont M, Jarusuraisin N. Opioid antagonists for alcohol dependence. Cochrane Database of Systematic Reviews. 2005;25 doi: 10.1002/14651858.CD001867.pub2. CD001867. [DOI] [PubMed] [Google Scholar]
- Thomas C, Wallack S, Lee S, McCarty D, Swift RM. Research to practice: Adoption of naltrexone in alcoholism treatment. Journal of Substance Abuse Treatment. 2003;24:1–11. [PubMed] [Google Scholar]
- Thomas SE, Miller PM. Knowledge and attitudes about pharmacotherapy for alcoholism: A survey of counselors and administrators in community-based addiction treatment centres. Alcohol and Alcoholism. 2007;42:113–118. doi: 10.1093/alcalc/agl100. [DOI] [PubMed] [Google Scholar]