Abstract
Background
The severe acute respiratory syndrome (SARS) virus is a member of the Coronaviridae (CoV) family that first appeared in the Guangdong Province of China in 2002 and was recognized as an emerging infectious disease in March 2003. Over 8000 cases and 900 deaths occurred during the epidemic. We report the safety and immunogenicity of a SARS DNA vaccine in a Phase I human study.
Methods
A single-plasmid DNA vaccine encoding the Spike (S) glycoprotein was evaluated in 10 healthy adults. Nine subjects completed the 3 dose vaccination schedule and were evaluated for vaccine safety and immune responses. Immune response was assessed by intracellular cytokine staining (ICS), ELISpot, ELISA, and neutralization assays.
Results
The vaccine was well tolerated. SARS-CoV-specific antibody was detected by ELISA in 8 of 10 subjects and neutralizing antibody was detected in all subjects who received 3 doses of vaccine. SARS-CoV-specific CD4+ T-cell responses were detected in all vaccinees, and CD8+ T-cell responses in ∼20% of individuals.
Conclusions
The VRC SARS DNA vaccine was well tolerated and produced cellular immune responses and neutralizing antibody in healthy adults.
Keywords: T-cell vaccine, Emerging infectious disease, Vaccine clinical trial
1. Background
The first cases of an atypical pneumonia appeared in Fosham, Guangdong Province, China in November 2002. By February 2003, over 700 cases of severe acute respiratory syndrome (SARS) were reported in the Guangdong Province of China. Before it was contained by public health isolation and quarantine measures, the epidemic spread to 25 countries over 5 continents, and affected 8422 people [1], [2].
SARS infection causes respiratory disease and other organ systems, including the gastrointestinal tract, are also severely affected. The elderly and immunocompromised are more severely affected and suffer greater morbidity and mortality. By July 2003, 916 deaths had been attributed to the infection by this virus [2]. Based on serologic data from samples collected prior to the outbreak and retrospectively analyzed, up to 40% of individuals working in the animal trade were seropositive but had no history of illness [3], indicating that SARS may be either extremely mild or asymptomatic in some cases. Severe acute respiratory syndrome virus (SARS-CoV) is an enveloped RNA virus and a member of the Coronaviridae family that also includes other human pathogens which typically cause mild upper respiratory infections.
Coronaviruses are enveloped viruses with a positive-sense, single-stranded RNA genome. SARS-coronavirus (SARS-CoV) was unknown prior to the 2003 outbreak of disease and may be a mutant human coronavirus that acquired new virulence factors allowing for infection of the human population [4]. The genomic RNA is encased in nucleocapsid (N) protein, which is surrounded by a lipid membrane containing the Spike glycoprotein (S), membrane glycoprotein (M), and envelope (E) proteins. Oligomers of the S-glycoprotein form a characteristic spike that protrudes from the membrane [4], [5]. Viral entry into host target cells appears to be mediated by SARS-CoV Spike (S) glycoprotein and is dependent on angiotensin-converting enzyme 2 (ACE2) as the functional receptor [6]. In addition to being responsible for attachment to the cellular receptor, S contains epitopes for viral neutralization and T-cell responses [7]. Studies performed by VRC investigators and colleagues have shown the importance of S-glycoprotein for coronavirus assembly and trafficking [8]. Other studies have demonstrated neutralization of pseudovirions expressing this protein by serum from convalescent SARS patients and the ability of the DNA plasmid vaccine described here to induce protective immunity by eliciting cellular and humoral immunity to SARS-CoV in animal models, including the generation of neutralizing antibodies (NAbs) measured in a plaque-reduction assay [8], [9]. Studies performed in Beijing, China with serum from patients with SARS using a neutralization assay against a pseudotyped lentiviral vector bearing the S protein indicated that NAbs were first detected 5–10 days after onset of symptoms, peaked at 20–30 days and were sustained for more than 150 days [10].
The nature of the spread and the severity of illness prompted widespread attempts to identify and understand the disease. The cause of SARS was determined to be a novel coronavirus and the virus was fully sequenced by May 2003 [11], [12]. Rapid identification and sequencing of the virus allowed scientists to begin developing candidate vaccines quickly. Currently, there are no licensed human SARS vaccines, and only one other vaccine clinical trial has been reported evaluating a whole-inactivated SARS vaccine candidate developed by Sinovac Biotech Co. Ltd. in China [13]. The current report describes the results of a candidate SARS DNA vaccine evaluated in a Phase I clinical trial in healthy adults initiated within 19 months after the sequence of the virus was initially published.
2. Methods
2.1. Study design
The VRC 301 protocol was a Phase I open-label study of the safety, tolerability, and immunogenicity of a SARS recombinant plasmid DNA vaccine encoding SARS Spike glycoprotein in healthy adult subjects. This single-site study was conducted at the Vaccine Research Center (VRC), National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH) in Bethesda, Maryland. Experimental guidelines of The U.S. Department of Health and Human Services were followed in the conduct of clinical research, and the protocol was approved by the NIAID Institutional Review Board. Ten subjects, ages 21–49, were enrolled in the study from December 13, 2004 through May 2, 2005. Three injections of vaccine, at a dose of 4 mg each, were administered, on study days 0, 28, and 56 at a 4 mg dose in the lateral deltoid muscle via the Biojector 2000® Needle-Free Injection Management System™. The dose and route studied in this trial were based on preclinical data and data from clinical trials of VRC DNA vaccines for other pathogens [14], [15], [16]. Subject safety was monitored by evaluating laboratory and clinical findings and adverse reactions at study visits and adverse events were coded with the Medical Dictionary for Regulatory Activities (MedDRA). Solicited symptoms of local and systemic reactogenicity, including pain, redness, swelling, myalgia, malaise, headache, chills, nausea and temperature, were collected by subject self report on 5-day diary cards following each vaccination. Subjects were followed for a total of 32 weeks and study visits were completed in December 2005.
2.2. Vaccine
The vaccine, VRC-SRSDNA015-00-VP, is composed of a single closed circular plasmid DNA macromolecule (VRC-8318) that has been produced in bacterial cell cultures containing a kanamycin selection medium. Bacterial cell growth is dependent upon the expression of the kanamycin resistance protein encoded by a portion of the plasmid DNA. Following growth of bacterial cells harboring the plasmid, the plasmid DNA is purified and the vaccine does not contain cellular or viral components. The plasmid encodes for a single protein cloned into the expression vector CMV/R, that has been previously described [17] and evaluated in clinical trials for candidate HIV and Ebola DNA vaccines [14], [16]. The plasmid was constructed to produce a deletion mutant of the SARS Spike glycoprotein (Urbani strain, GenBank AY278741) with the cytoplasmic domain truncated (SΔCD) [9]. The VRC-SRSDNA015-00-VP vaccine is based upon cDNA expression of SARS Spike glycoprotein (Urbani strain) with codon-modification to optimize expression in human cells. It expresses the full sequence except for deletion of the last 13 COOH-terminal amino acids. Vaccine for the clinical trial was prepared under current Good Manufacturing Practice (cGMP) conditions by Vical, Inc. (La Jolla, CA). The vaccine met a lot of release specifications prior to administration. The DNA vaccine was manufactured at a 4 mg/ml concentration in phosphate-buffered saline (PBS).
2.3. Measurement of antibody responses by ELISA
VRC plasmid 8318 was expressed in 293 cells and purified for the major protein product. Duplicate wells of serial dilutions of the volunteer sera were incubated for 2 h at 37 °C on SARS spike antigen-coated/blocked plates, followed by biotin-conjugated antibody (60 min at room temperature), Streptavidine-horseradish peroxidase (30 min at room temperature) and tetramethylbenzidine (TMB) substrate (10 min at room temperature). Color development was stopped by addition of 0.9 M sulfuric acid and plates were read within 30 min at 450 nm on the Molecular Devices Spectramax 384-plus ELISA Plate reader (Sunnyvale, CA). Mean optical density (OD) for each dilution was corrected for the mean OD of the same dilution of the pre-immunization sample. Endpoint titers for each volunteer were established as the last dilution with a pre-immunization corrected OD >0.2.
2.4. Measurement of neutralizing antibody responses
Subject samples were assessed for the presence of vaccine-induced neutralizing antibody by two assays. First, in a luciferase reporter pseudotyped lentiviral-based assay serial dilutions of sera were incubated with HIV-1 based luciferase reporter virus particles expressing SARS Spike glycoprotein that were produced in 293 T cells as previously described [18]. Target cells were human renal adenocarcinoma cell line 786-O obtained from ATCC. The inhibitory concentration 80% (IC80) is reported as a reciprocal dilution, and the analysis was performed using previously described methods [19]. Secondly, two-fold dilutions of heat-inactivated serum were assayed in quadruplicate wells of a 96-well plate using a starting dilution of 1:4. This microneutralization assay measured antibodies that neutralized the infectivity of 100 TCID50 of SARS-CoV in Vero cell monolayers. The presence of viral cytopathic effect was read on days 3 and 4 and the dilution of serum that completely prevented cytopathic effect in 50% of the wells was calculated by the Reed–Muench formula [20].
2.5. Measurement of T-cell responses by ELISpot
ELISpot was performed on subject samples at baseline and at weeks 2, 6, 8, 10, 12, and 32 as previously described [16]. Cells were stimulated overnight with vaccine insert specific peptide pools at 2 × 105 cells per well. Results are expressed as mean spot-forming cells (SFC) per million PBMC.
2.6. Measurement of T-cell responses by intracellular cytokine staining (ICS)
CD4 and CD8 T-cell responses were measured by ICS at baseline and at weeks 2, 6, 8, 10, 12, and 32 as previously described [16]. Cells were stimulated with vaccine insert specific peptide pools. Cells were permeabilized, washed, and stained with directly conjugated anti-human CD3, CD4, CD8, IFN-γ and IL-2 antibodies and were assessed for CD3, CD8, CD4, and IFN-γ/IL-2 expression on a FACSCalibur flow cytometer (BDIS).
2.7. Statistical methods
All assays are treated as binary (responders/non-responders). We use the usual 95% central exact confidence intervals for binomial rates. We are 97.5% confident that the true response rates in the antibody assays are larger than the lower limit. Calculations were done in R version 2.3.1. A positive T-cell response for ICS and ELISpot data was based on composite criteria as previously described in four published studies of candidate vaccines [14], [15], [16]. SAS (Version 9.0; SAS Institute) and S-plus (Version 6.2; Insightful) were used for analyses.
3. Results
3.1. Study population demographics
Ten healthy adult subjects ages 21–49 years (mean age 35.5) were enrolled in the study. The subject population consisted of 70% male, predominantly white (90%) and non-Hispanic/Latino (80%) subjects. The mean BMI was 24.6 (range 19.7–33.9). All subjects had an educational level of college or higher with 30% having an advanced degree. The demographic data regarding subject gender, age, race/ethnicity, body mass index (BMI) and educational level at the time of enrollment are shown (Table 1 ).
Table 1.
Demographic characteristics.
| Category | Sub-category | All (n = 10) |
|---|---|---|
| Gender | Male | 7 (70.0%) |
| Female | 3 (30.0%) | |
| Age | 18–20 | 0 (0.0%) |
| 21–30 | 3 (30.0%) | |
| 31–40 | 4 (40.0%) | |
| 41–50 | 3 (30.0%) | |
| Mean (S.D.) | 35.5 (9.0) | |
| Range | [21, 49] | |
| Race | American Indian/Alaskan Native | 0 (0.0%) |
| Asian | 1 (10.0%) | |
| Black or African American | 0 (0.0%) | |
| Native Hawaiian or other Pacific Islander | 0 (0.0%) | |
| White | 9 (90.0%) | |
| Multiracial | 0 (0.0%) | |
| Ethnicity | Non-Hispanic/Latino | 8 (80.0%) |
| Hispanic/Latino | 2 (20.0%) | |
| BMI | Under 18.5 | 0 (0.0%) |
| 18.5–24.9 | 6 (60.0%) | |
| 25.0–29.9 | 3 (30.0%) | |
| 30.0 or over | 1 (10.0%) | |
| Mean (S.D.) | 24.6 (4.1) | |
| Range | [19.7, 33.9] | |
| Education | Less than high school graduate | 0 (0.0%) |
| High school graduate/GED | 0 (0.0%) | |
| College/University | 7 (70.0%) | |
| Advanced degree | 3 (30.0%) | |
A summary of demographic characteristics at enrollment including gender, age, race/ethnicity, body mass index, and education level. Age represents age at enrollment day.
3.2. Vaccine safety
Data collected from subject diary cards show that all 10 subjects (100%) experienced at least one mild injection site symptom following a vaccination (pain/tenderness, swelling and redness) with pain/tenderness at the injection site being the most common complaint. Five of the ten (50%) subjects reported at least one mild systemic symptom (myalgia, malaise, headache, chills, or fever) following vaccination. Among the solicited symptoms, none of the subjects reported nausea. None of the subjects reported moderate or severe symptoms following vaccination (Table 2 ).
Table 2.
Summary of local and systemic reactogenicity.
| Symptoms intensity | All vaccines (n = 10) |
|---|---|
| A. | |
| Pain/Tenderness | |
| None | 0 |
| Mild | 10 (100%) |
| Moderate | 0 |
| Swelling | |
| None | 8 (80%) |
| Mild | 2 (20%) |
| Moderate | 0 |
| Redness | |
| None | 6 (60%) |
| Mild | 4 (40%) |
| Moderate | 0 |
| Any local symptom | |
| None | 0 |
| Mild | 10 (100%) |
| Moderate | 0 |
| B. | |
| Malaise | |
| None | 6 (60%) |
| Mild | 4 (40%) |
| Moderate | 0 |
| Myalgia | |
| None | 7 (70%) |
| Mild | 3 (30%) |
| Moderate | 0 |
| Headache | |
| None | 9 (90%) |
| Mild | 1 (10%) |
| Moderate | 0 |
| Chills | |
| None | 9 (90%) |
| Mild | 1 (10%) |
| Moderate | 0 |
| Nausea | |
| None | 10 (100%) |
| Mild | 0 |
| Moderate | 0 |
| Temperature | |
| None | 9 (90%) |
| Mild | 1 (10%) |
| Moderate | 0 |
| Any systemic symptom | |
| None | 5 (50%) |
| Mild | 5 (50%) |
| Moderate | 0 |
Maximum local (A.) and systemic (B.) reactogenicity. The local injection site reactions were recorded by clinicians at 30–45 min post-injection and were then recorded as self-assessments at home by subjects on a 5-day diary card. Systemic reactions were recorded as self-assessments at home by subjects on a 5-day diary card following each injection. There were no reports of severe local symptoms following vaccination (A). There were no reports of severe systemic symptoms following vaccination (B).
All subjects were followed for 32 weeks for safety and immune response. Nine of 10 subjects completed the 3 dose vaccination schedule. One subject (Subject “G”) received an oral glucocorticoid to treat poison ivy contact dermatitis after the second vaccination and consequently was withdrawn from the vaccination schedule. There were no Serious Adverse Events and there were no grade 3 or 4 (severe or life-threatening) adverse events. Overall, study vaccinations were well tolerated and found to be safe in healthy subjects, ages 21–49 years.
3.3. Antibody responses
SARS Spike glycoprotein specific antibody was detected by ELISA in 8 of 10 (80%) of subjects at one or more timepoints (Table 3 ). Neutralization was not detected in the microneutralization plaque-reduction assay. SARS specific neutralizing antibody as assessed by pseudotyped lentiviral vector reporter neutralization assay was detected in 8 of 10 (80%) of subjects at one or more timepoints (Fig. 1 ). The neutralizing antibody response peaked between week 8 and 12 with 6 subjects remaining positive at week 32. Pseudovirus neutralization assays are highly sensitive, and this is a possible reason for the discrepancy in the results between the two neutralization assays.
Table 3.
SARS specific antibody titer assessed by ELISA.
| Subject | Week 0 | Week 8 | Week 12 | Week 32 |
|---|---|---|---|---|
| A | <30 | ND | <30 | ND |
| B | <30 | ND | <30 | ND |
| C | <30 | 810 | 810 | 90 |
| D | <30 | <30 | 90 | <30 |
| E | <30 | 90 | 270 | 30 |
| F | <30 | 90 | 270 | 90 |
| G | <30 | 270 | 270 | <30 |
| H | <30 | <30 | 270 | 30 |
| I | <30 | <30 | 270 | 30 |
| J | <30 | 30 | 90 | 90 |
Not done, ND. If a subject was negative by ELISA at Week 12, ELISA was not performed at other timepoints. Subject “G” received 2 of 3 vaccinations.
Fig. 1.
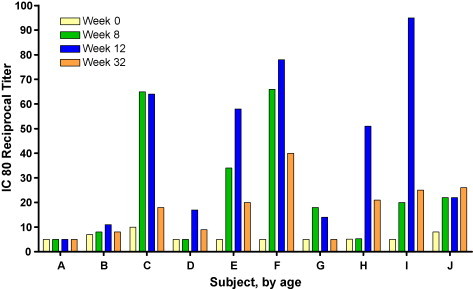
Magnitude and frequency of neutralizing antibody response. Individual subjects are designated by letters A–J, sorted by ascending age on the x-axis. The IC80 (inhibitory concentration 80%) reciprocal titer is represented on the y-axis. The time course of the study is shown for each subject: week 0 (yellow bars), week 8 (green bars), week 12 (blue bars), and week 32 (orange bars). Vaccinations were administered at weeks 0, 4, and 8. Subject “G” received 2 of 3 vaccinations.
3.4. T-cell responses
SARS Spike glycoprotein specific, CD4+ and CD8+ T-cell responses induced by the vaccine were assessed by ICS and ELISpot. CD4+ T-cell responses to SARS CoV Spike antigen were detected by ICS in all subjects (10/10) between week 2 and 32. CD8+ T-cell responses were detected in 2 of 10 (20%) of subjects. CD4+ T-cell responses were of greater frequency and magnitude than CD8+ responses (Fig. 2 ). CD4 and CD8+ T-cell responses as assessed by ELISpot were detected in 7 of 10 (70%) of subjects during the course of the trial, and all 7 were positive by week 6 (2 weeks after the 2nd dose of vaccine). The peak T-cell response occurred between week 8 and 12 and when present, was sustained throughout the 32-week trial.
Fig. 2.
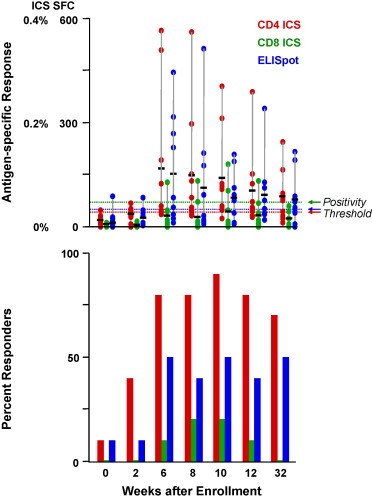
Magnitude and frequency of CD4 and CD8 T-cell responses by ICS and ELISpot analysis at specific timepoints throughout the study. Magnitude of response is represented on the upper graph, percent positive CD4 (red bars) or CD8 cells (green bars) for ICS or spot-forming colonies (SFC) for ELISpot (blue bars). The horizontal black bars represent the mean. A sample was considered positive if it was above the thresholds indicated by the dashed lines. Separate positivity criteria for CD4 and CD8 ICS and ELISpot were developed and validated for overlapping peptide-based stimulations. The frequency of response is represented by percent responders on the lower graph. Weeks after enrollment is shown on the x-axis, applicable for upper and lower graphs. Vaccinations were administered at weeks 0, 4, and 8.
4. Discussion
SARS represents a recently emergent infectious disease that has caused severe illness, global panic, and economic disruption. The rapid response to the 2003 SARS outbreak defines the quintessential response by public health and biomedical communities for a newly emerging infectious disease. The ability to quickly identify, describe, characterize and develop countermeasures, including vaccines, against future emerging infectious pathogens is critical to maintain public health and economic stability. The global response to the SARS epidemic provided insight and education for public health experts and scientists, which can now be utilized to more optimally respond to future emerging infections, including viruses such as avian influenza.
In response to the 2003 SARS infections, several laboratories rapidly developed vaccine candidates including the SARS candidate DNA vaccine described in this report. After preclinical evaluation for safety and demonstration of efficacy in a lethal murine challenge model [9], a Phase I human trial reported here was initiated within 17 months. Similar candidate DNA vaccines for HIV, Ebola virus, and West Nile virus (WNV) have previously been evaluated as safe and shown to elicit vaccine-induced cellular and humoral immune responses, including neutralizing antibody responses to the WNV vaccine [21]. Like the previously evaluated VRC DNA vaccines, the SARS vaccine induced vaccine-specific T-cell and antibody responses, including neutralizing antibody against SARS Spike glycoprotein in a sensitive pseudotyped lentiviral reporter assay. In studies of SARS patients, antibodies to SARS CoV spike, membrane, envelope and nucleocapsid proteins are present as assessed by ELISA, but neutralizing antibody is only elicited by the Spike glycoprotein [22], [23], [24].
In this open-label Phase I clinical trial, the VRC SARS DNA vaccine was evaluated as safe and well tolerated. The vaccine was immunogenic with SARS spike glycoprotein-specific T-cell responses induced in all subjects and neutralizing antibody responses detected in 8 of 10 subjects. SARS spike protein-specific cellular responses were primarily CD4+ T cells, and a minority of subjects had detectable SARS spike protein-specific CD8+ T-cell responses. The CD8+ T-cell response is an important effector mechanism for viral clearance and induction of this population is a goal for gene-based vaccines. In prior VRC clinical trials of DNA vaccines against HIV, Ebola, and West Nile virus, vaccine-specific CD4+ T-cell responses were detected in nearly all subjects, while the frequency of measurable CD8+ T-cell responses varied from 7% to 64% [14], [15], [16], [21]. This aspect of DNA vaccine-induced immunity will require additional development.
An investigational inactivated SARS vaccine candidate developed by Sinovac Biotech Co. Ltd. was found to be immunogenic in a Phase 1 study conducted in China [13]. Thirty-six healthy subjects received 2 doses of the inactivated SARS-CoV vaccine (either 16 or 32 SARS-CoV units) or placebo control, and all vaccine recipients seroconverted by day 42 post-vaccination. The geometric mean titer of NAb (measured in a plaque-reduction format) peaked 2 weeks following the second immunization, and decreased after 4 weeks, similar to the kinetics observed in the SARS DNA vaccinated subjects described in this report. Vaccine-induced cellular immune responses were not reported. Studies of recovered SARS patients have demonstrated long-lived effector/central memory T-cell responses to SARS S-protein [25], as well as to the other SARS viral proteins [26], [27], [28], and CD8+ T cells are thought to play an important role in SARS immunity [24].
These safety data and immune responses along with data from previously reported trials evaluating similar DNA vaccines against other pathogens, including Ebola, WNV and HIV, indicate that this SARS DNA vaccine should be further considered in expanded clinical evaluations for potential future SARS outbreaks [14], [15], [16], [21], alone or in prime-boost combination with other vectors. The SARS DNA vaccine induced neutralizing antibodies which are strongly associated with recovery from natural SARS infection [13] as well as cellular immune responses that may be an important component of SARS immunity. The neutralization activity was detected by a sensitive pseudotyped lentiviral assay, but not by a less sensitive microneutralization plaque-reduction assay. Because the SARS CoV has been contained by careful surveillance and other public health measures, licensure of such a vaccine is likely to require use of the animal rule in models that reflect the pathogenesis in humans, and the identification and validation of immune correlates. This vaccine also demonstrates the feasibility of rapid manufacturing and regulatory review and provides additional safety and immunogenicity data to support the concept of DNA vaccination as a potential vaccine platform for future emerging infectious diseases.
Acknowledgements
We thank the study volunteers who graciously gave their time and understand the importance of finding a safe and effective SARS vaccine. We also thank NIH Clinical Center staff, NIAID staff, RCHSPB staff, PRPL and OCPL staff, EMMES Corporation (Phyllis Zaia, Lihan Yan and others), Vical Incorporated, Biojector, Inc. (Richard Stout and others) and other supporting staff (Richard Jones, Kathy Rhone-Reed, Theodora White, Mario Carranza and Monique Young) who made this work possible. We are grateful as well for the advice and important preclinical contributions of NIAID investigators and key staff, including Kanta Subbarao, Kimberlee Wallace, Daniel Douek, Wing-Pui Kong, Peter Kwong, Abraham Mittelman, Steve Perfetto, Srini Rao, Robert Seder, Richard Wyatt.
The work was funded by the National Institute of Allergy and Infectious Diseases Intramural Research Program.
References
- 1.Peiris J.S., Guan Y., Yuen K.Y. Severe acute respiratory syndrome. Nat Med. 2004;10(12 Suppl.):S88–S97. doi: 10.1038/nm1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cumulative number of reported probable cases of SARS. 2003.
- 3.Guan Y., Zheng B.J., He Y.Q., Liu X.L., Zhuang Z.X., Cheung C.L. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science. 2003;302(5643):276–278. doi: 10.1126/science.1087139. [DOI] [PubMed] [Google Scholar]
- 4.Holmes K.V. SARS-associated coronavirus. N Engl J Med. 2003;348(20):1948–1951. doi: 10.1056/NEJMp030078. [DOI] [PubMed] [Google Scholar]
- 5.Navas-Martin S.R., Weiss S. Coronavirus replication and pathogenesis: implications for the recent outbreak of severe acute respiratory syndrome (SARS), and the challenge for vaccine development. J Neurovirol. 2004;10(2):75–85. doi: 10.1080/13550280490280292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong S.K., Li W., Moore M.J., Choe H., Farzan M. A 193-amino acid fragment of the SARS coronavirus S protein efficiently binds angiotensin-converting enzyme 2. J Biol Chem. 2004;279(5):3197–3201. doi: 10.1074/jbc.C300520200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gallagher T.M. Murine coronavirus spike glycoprotein. Receptor binding and membrane fusion activities. Adv Exp Med Biol. 2001;494:183–192. [PubMed] [Google Scholar]
- 8.Yang Z.Y., Huang Y., Ganesh L., Leung K., Kong W.P., Schwartz O. pH-dependent entry of severe acute respiratory syndrome coronavirus is mediated by the spike glycoprotein and enhanced by dendritic cell transfer through DC-SIGN. J Virol. 2004;78(11):5642–5650. doi: 10.1128/JVI.78.11.5642-5650.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang Z.Y., Kong W.P., Huang Y., Roberts A., Murphy B.R., Subbarao K. A DNA vaccine induces SARS coronavirus neutralization and protective immunity in mice. Nature. 2004;428(6982):561–564. doi: 10.1038/nature02463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nie Y., Wang G., Shi X., Zhang H., Qiu Y., He Z. Neutralizing antibodies in patients with severe acute respiratory syndrome-associated coronavirus infection. J Infect Dis. 2004;190(6):1119–1126. doi: 10.1086/423286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marra M.A., Jones S.J., Astell C.R., Holt R.A., Brooks-Wilson A., Butterfield Y.S. The genome sequence of the SARS-associated coronavirus. Science. 2003;300(5624):1399–1404. doi: 10.1126/science.1085953. [DOI] [PubMed] [Google Scholar]
- 12.Rota P.A., Oberste M.S., Monroe S.S., Nix W.A., Campagnoli R., Icenogle J.P. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300(5624):1394–1399. doi: 10.1126/science.1085952. [DOI] [PubMed] [Google Scholar]
- 13.Lin J.T., Zhang J.S., Su N., Xu J.G., Wang N., Chen J.T. Safety and immunogenicity from a phase I trial of inactivated severe acute respiratory syndrome coronavirus vaccine. Antivir Ther. 2007;12(7):1107–1113. [PubMed] [Google Scholar]
- 14.Catanzaro A.T., Roederer M., Koup R.A., Bailer R.T., Enama M.E., Nason M.C. Phase I clinical evaluation of a six-plasmid multiclade HIV-1 DNA candidate vaccine. Vaccine. 2007;25(20):4085–4092. doi: 10.1016/j.vaccine.2007.02.050. [DOI] [PubMed] [Google Scholar]
- 15.Graham B.S., Koup R.A., Roederer M., Bailer R.T., Enama M.E., Moodie Z. Phase 1 safety and immunogenicity evaluation of a multiclade HIV-1 DNA candidate vaccine. J Infect Dis. 2006;194(12):1650–1660. doi: 10.1086/509259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin J.E., Sullivan N.J., Enama M.E., Gordon I.J., Roederer M., Koup R.A. A DNA vaccine for Ebola virus is safe and immunogenic in a phase I clinical trial. Clin Vaccine Immunol. 2006;13(11):1267–1277. doi: 10.1128/CVI.00162-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barouch D.H., Yang Z.Y., Kong W.P., Korioth-Schmitz B., Sumida S.M., Truitt D.M. A human T-cell leukemia virus type 1 regulatory element enhances the immunogenicity of human immunodeficiency virus type 1 DNA vaccines in mice and nonhuman primates. J Virol. 2005;79(14):8828–8834. doi: 10.1128/JVI.79.14.8828-8834.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang Y., Yang Z.Y., Kong W.P., Nabel G.J. Generation of synthetic severe acute respiratory syndrome coronavirus pseudoparticles: implications for assembly and vaccine production. J Virol. 2004;78(22):12557–12565. doi: 10.1128/JVI.78.22.12557-12565.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shu Y., Winfrey S., Yang Z.Y., Xu L., Rao S.S., Srivastava I. Efficient protein boosting after plasmid DNA or recombinant adenovirus immunization with HIV-1 vaccine constructs. Vaccine. 2007;25(8):1398–1408. doi: 10.1016/j.vaccine.2006.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Subbarao K., McAuliffe J., Vogel L., Fahle G., Fischer S., Tatti K. Prior infection and passive transfer of neutralizing antibody prevent replication of severe acute respiratory syndrome coronavirus in the respiratory tract of mice. J Virol. 2004;78(7):3572–3577. doi: 10.1128/JVI.78.7.3572-3577.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin J.E., Pierson T.C., Hubka S., Rucker S., Gordon I.J., Enama M.E. A West Nile virus DNA vaccine induces neutralizing antibody in healthy adults during a phase 1 clinical trial. J Infect Dis. 2007;196(12):1732–1740. doi: 10.1086/523650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buchholz U.J., Bukreyev A., Yang L., Lamirande E.W., Murphy B.R., Subbarao K. Contributions of the structural proteins of severe acute respiratory syndrome coronavirus to protective immunity. Proc Natl Acad Sci USA. 2004;101(26):9804–9809. doi: 10.1073/pnas.0403492101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu L., Manopo I., Leung B.P., Chng H.H., Ling A.E., Chee L.L. Immunological characterization of the spike protein of the severe acute respiratory syndrome coronavirus. J Clin Microbiol. 2004;42(4):1570–1576. doi: 10.1128/JCM.42.4.1570-1576.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen J., Subbarao K. The immunobiology of SARS*. Annu Rev Immunol. 2007;25:443–472. doi: 10.1146/annurev.immunol.25.022106.141706. [DOI] [PubMed] [Google Scholar]
- 25.Yang L.T., Peng H., Zhu Z.L., Li G., Huang Z.T., Zhao Z.X. Long-lived effector/central memory T-cell responses to severe acute respiratory syndrome coronavirus (SARS-CoV) S antigen in recovered SARS patients. Clin Immunol. 2006;120(2):171–178. doi: 10.1016/j.clim.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peng H., Yang L.T., Li J., Lu Z.Q., Wang L.Y., Koup R.A. Human memory T cell responses to SARS-CoV E protein. Microbes Infect. 2006;8(9–10):2424–2431. doi: 10.1016/j.micinf.2006.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peng H., Yang L.T., Wang L.Y., Li J., Huang J., Lu Z.Q. Long-lived memory T lymphocyte responses against SARS coronavirus nucleocapsid protein in SARS-recovered patients. Virology. 2006;351(2):466–475. doi: 10.1016/j.virol.2006.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang L., Peng H., Zhu Z., Li G., Huang Z., Zhao Z. Persistent memory CD4+ and CD8+ T-cell responses in recovered severe acute respiratory syndrome (SARS) patients to SARS coronavirus M antigen. J Gen Virol. 2007;88(Pt 10):2740–2748. doi: 10.1099/vir.0.82839-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


