Abstract
CD8+ T cells (TCD8+) differentiate into effector cells following recognition of specific peptide–major histocompatibility complex (MHC) class I complexes (pMHC-I) on the surface of professional APCs (pAPCs), such as dendritic cells. Antigenic pMHC-I can be generated from two spatially distinct sources. The direct presentation pathway involves generation of peptide from protein substrate synthesized within the cell that is presenting the pMHC-I. Alternatively, the cross presentation pathway involves presentation of antigen that is not synthesized within the presenting cell, but is derived from exogenous proteins synthesized within other donor cells. The mechanisms by which cross presentation of exogenous antigens occur in vivo remain controversial. The C-type lectin scavenger receptor A (SR-A) has been implicated in a number of potential cross presentation pathways, including the presentation of peptide bound to heat shock proteins, such as glycoprotein 96 (gp96), and the transfer of pMHC-I from a donor cell to the pAPC. We demonstrate here that initiation of TCD8+ responses is normal in mice lacking SR-A, and that the redundancy of ligand binding exhibited by the SR family is likely to be an important mechanism that ensures cross presentation in vivo. These observations emphasize the requirement to target multiple receptors and antigen-processing pathways during the rational design of vaccines aimed at eliciting protective TCD8+.
Keywords: calreticulin, dendritic cell, glycoprotein 96, scavenger receptor A
Introduction
CD8+ T cells (TCD8+) play a major role in the elimination of viruses, intracellular pathogens, and tumours. Antigen-specific TCD8+ recognize peptides of 8–11 amino acids bound to major histocompatibility class I (MHC-I) molecules. Activation of naïve TCD8+ to become effector TCD8+ requires that antigenic peptides be presented on MHC-I by professional antigen-presenting cells (pAPCs), in particular dendritic cells (DCs). Antigenic peptides can be generated in pAPCs by two spatially distinct routes. Direct presentation occurs when peptides are generated from endogenous antigen synthesized within the pAPC. Thus, direct presentation allows the sampling of the contents of a cell and clearance of infected cells by effector TCD8+. Alternatively, cross presentation involves the uptake by pAPCs of exogenously synthesized antigen derived from donor cells or pathogens. The endocytosed antigen is then processed and presented by the pAPC.1 The cross presentation pathway allows the generation of effector TCD8+ when antigen is expressed only within certain cell types (such as tumours of non-lymphoid origin or pathogens that do not infect pAPCs), or when a pathogen has evolved to block the direct presentation pathway in infected pAPCs.
The cellular and molecular mechanisms by which cross presentation occurs have been widely studied in vitro, but remain controversial in vivo. A number of studies indicate that the primary substrates for cross presentation in vivo are stable long-lived proteins.2–5 However, other studies of cross presentation in vivo implicate the transfer of antigenic peptides that are bound to molecular chaperones.6–9 Alternatively, minimal antigenic peptide may be transferred between cells in vitro via the relocation of peptide–MHC complexes from a donor cell to a pAPC.10,11
The C-type lectin family scavenger receptor A (SR-A) has been implicated in both the internalization of molecular chaperones5,12 and the transfer of peptide–MHC complexes between cells.11,13 SR-As are trimeric transmembrane glycoproteins14–16 that bind and mediate the internalization of modified low-density lipoprotein (LDL)17,18 and have been implicated in the development of atherosclerosis.19,20 SR-As are expressed by macrophages and DCs,13,21 the cells that are generally held to be responsible for mediating cross priming in vivo. SR-A has been implicated as having a role in the initiation of immune responses by binding, internalizing, and trafficking inflammatory microbial products such as lipopolysaccharide (LPS) and lipoteichoic acid (LTA)22,23 and as a phagocytic receptor for the internalization of particulates and microbes.22,24–27 Additionally, targeting of antigen to SR-A dramatically enhances cross presentation in vitro.28
Based on data implicating SR-A in the cross priming process, we sought to establish the requirement for SR-A in the induction of antiviral TCD8+ responses and the initiation of TCD8+ responses following immunization with molecular chaperone–peptide complexes. We find that, in contrast to in vitro studies, blocking of SR-A function in vivo affects neither the initiation of antiviral TCD8+ responses nor the cross priming of TCD8+ with cellular antigen or molecular chaperone–peptide complexes. However, blocking of the binding of all scavenger receptors with a competitive inhibitor significantly reduces the ability of DCs pulsed with molecular chaperone–peptide complexes to induce antigen-specific TCD8+, indicating that the redundancy in scavenger receptor ligands can compensate for a lack of SR-A. The presence of a number of redundant receptors reduces the ability of pathogens to specifically block the cross presentation pathway in vivo, indicating the importance of this pathway for induction of protective TCD8+.
Materials and methods
Animals
Female C57BL/6 mice were purchased from Charles River Laboratories (Wilmington, MA) or the National Cancer Institute (Frederick, MD). SR-A null (SR-A−/−) mice were a generous gift from M. W. Freeman (Massachusetts General Hospital, Boston, MA).29 OT-1 T-cell receptor (TCR) recombinase activation gene 1−/− (RAG1−/−) transgenic mice30,31 and F5 RAG1−/− mice32 were obtained from the National Institute of Allergy and Infectious Diseases Exchange Program. B6.SJL-Ptprca/BoAiTac mice were purchased from Taconic Farms (Germantown, NY) and bred to both OT-1 TCR and F5 TCR mice to produce OT-1.SJL and F5.SJL offspring, respectively. All mice were maintained under specific pathogen-free conditions at the M. S. Hershey Medical Center, and all studies were approved by the Penn State College of Medicine Institutional Animal Care and Use Committee.
Viruses
Vaccinia virus (VACV) and adenovirus (Ad) were a kind gift from Dr Jon Yewdell and Dr Jack Bennink (Laboratory of Viral Diseases, NIAID, Bethesda, MD). Influenza virus A/PR8 was obtained from SPAFAS Pathogen Free Egg Facility (Voluntown, CT).
Cell lines and cultures
All media and supplements were purchased from Invitrogen (Carlsbad, CA) except where noted. β2-microglobulin (β2m)−/− fibroblasts33 were maintained in Dulbecco’s modified Eagle’s minimal essential medium (DMEM) containing 10% fetal bovine serum (FBS) supplemented with penicillin/streptomycin and 2 mm l-glutamine. DCs and macrophages were grown from bone marrow as previously described.34,35
Peptides
TCD8+ responses were measured to the following peptides: ovalbumin OVA257–264 (SIINFEKL), influenza A/PR8 nucleoprotein NP366–374 (ASNENMETM), A/NT60 NP366–374 (ASNENMDAM), A/PR8 acid polymerase PA224–233 (SSLENFRAYV), VACV B8R20–27 (TSYKFESV), VACV A42R88–96 (YAPVSPIVI), VACV A19L47–55 (VSLDYINTM), A47L138–146 (AAFEFINSL) and K3L6–15 (YSLPNAGDVI). The responses to the OVA, B8R, A19L and A47L peptides are H2-Kb-restricted, and the responses to both NP peptides and the PA, A42L and K3L peptides are H2-Db-restricted.
Proteins
Calreticulin (CRT) was purified and depleted of endotoxin as previously described;36,37 endotoxin levels were assessed with the Cambrex (Walkersville, MD) QCL-1000 LAL assay. CRT was labelled with AlexaFluor 647-succinimidyl ester (Invitrogen), and labelled protein was subsequently isolated from unincorporated fluor by size exclusion chromatography.12,36,38 OVA20 peptide39 (contains SIINFEKL) was cross-linked to CRT using N-succinimidyl 3-(2-pyridyldithio)-propionate (Pierce, Rockford, IL) as previously described.8 CRT/OVA20 complexes were separated from free peptide by dialysis and size exclusion chromatography.
The N-terminal domain (NTD) of glycoprotein 96 (gp96; amino acid residues 22–337), in pET15b, was expressed in Escherichia coli strain BL21 cells and purified by nickel agarose affinity chromatography. Endotoxin was depleted from the affinity column-bound gp96 by washing with 100 column volumes of 1% Triton X-114 in phosphate-buffered saline (PBS) and subsequently with three column volumes of PBS. Endotoxin-depleted gp96 was eluted in 150 mm imidazole and dialysed against sterile PBS, and endotoxin levels were assessed as above. Where indicated, gp96–AlexaFluor conjugates were prepared according to the manufacturer’s instructions (Molecular Probes, Eugene, OR). Succinimidyl ester conjugates were used in all studies and unconjugated dye was removed by size exclusion chromatography (Sephadex G-25). Final dye coupling ratios varied from 1·5 to 2·5 mol dye:mol protein.
gp96 NTD–peptide complexes were prepared as follows. Peptide (OVA257–264 or influenza NT60 NP366–374) was incubated with gp96 (1 mg/ml) at 40° for 3 hr. Under these conditions, the gp96 undergoes a tertiary conformational change and is receptive to peptide association. Unbound peptide was subsequently removed by five cycles of centrifugal ultrafiltration, using a 10 000 MWCO VIVASPIN 2 column (Sartorius, Edgewood, NY) according to the manufacturer’s instructions.
Immunizations
C57BL/6 mice were immunized intraperitoneally with 1 mg of OVA257–264 peptide, 10 μg of LPS (both from Sigma, St Louis, MO) and 100 μg of FGK45 anti-CD40 antibody (from Dr R. Noelle, Dartmouth College, Lebanon, NH). CRT/OVA20 was injected into mice intraperitoneally along with either 10 μg of LPS or 100 μg of poly I:C (Sigma). Mice were immunized intradermally with amounts of gp96-OVA257–264 or gp96-NP366–374 indicated.
For influenza infections, mice were immunized with approx 600 haemagglutination units of A/Puerto Rico/8/34 virus intraperitoneally. For VACV infections, mice were immunized with 107 plaque-forming units (PFU) intravenously. To study cross priming, mice were immunized intraperitoneally with β2m−/− cells that were electroporated with antigen as described below or immunized intradermally in the ear pinnae with 106 PFU of the adenovirus rAdCMVNP, rAdK14NP or rAdSPCNP.
For cellular bone marrow-derived DC (BMDC) immunization, BMDCs were incubated with anti-CD11c beads and sorted using an AutoMACS magnetic sorter (Miltenyi Biotech, Auburn, CA). Where indicated, BMDCs were incubated with 75 μg/ml fucoidin (Sigma) to block SR-A binding and then with either gp96-OVA257–264 or gp96-NT60 NP366–374 at 4 or 37°. BMDCs were washed extensively to remove any free gp96 and injected intravenously into recipient mice.
Electroporation
Electroporation was performed as previously described.40 Briefly, approximately 4 × 106β2m−/− cells were suspended in PBS containing 1 mg/ml OVA with 10 mm MgCl2 and incubated on ice for 10 min. The cells were then electroporated in disposable cuvettes (Bio-Rad, Hercules, CA) on a Bio-Rad gene pulser at 0·25 kV with a capacitance of 250 μF. Following electroporation, cells were incubated on ice for an additional 10 min and washed three times with 10% Iscove’s modified Dulbecco’s medium (IMDM). Cells were irradiated at 20 000 rad prior to injection.
Adoptive transfer of T-cell receptor (TCR) transgenic cells
Spleens and lymph nodes were removed and homogenized to produce a single cell suspension, and mononuclear cells isolated either by centrifugation over a lymphocyte separation medium (LSM) cushion or by suspension in ACK lysing buffer (both from Cambrex) for 5 min. For in vivo killing (IVK) assays, cells were pulsed with 1 μg of OVA257–264 peptide, influenza NT60 NP366–374 peptide, or no peptide. Cells were washed twice and labelled with 0·5 μm (NP366–374 peptide pulsed) or 5 μm (OVA257–264 peptide pulsed) 5-(and 6-)carboxyfluorescein diacetate–succinimidyl ester (CFDA-SE) (Molecular Probes) for 10 min at 37°. Each recipient received 2 × 106 cells pulsed with each peptide intravenously or retro-orbitally 4 or 7 days post immunization. Effector function in IVK assays was measured as the percentage of targets killed by calculating the ratio of antigen-unpulsed, low-CFDA-SE cells to pulsed, high-CFDA-SE labelled cells. For proliferative studies, cells were washed twice, labelled with CFDA-SE, and washed once prior to intravenous injection.
T-cell culture
Cervical lymph nodes and the spleen were removed 30 days post immunization and homogenized, and red blood cells lysed using ACK lysing buffer. Splenocytes (3 × 107) were stimulated with 1/20–1/25 the number of PR8 NP366–374 peptide-pulsed APCs, in RPMI-1640 medium containing 10% FBS supplemented with penicillin/streptomycin, 2 mm l-glutamine, non-essential amino acids, sodium pyruvate, and 2-mercaptoethanol, and incubated at 37° for 6 days.
Cytokine production
Mononuclear cells isolated from splenocytes harvested 5 or 7 days post-immunization or plated TCD8+ that were harvested were washed twice after isolation over an LSM cushion as described above and plated in triplicate into individual wells of a 96-well plate (3 × 106 cells per well) for an intracellular cytokine secretion assay. Cells were stimulated with 1 μg of the relevant peptides for 2 hr at 37°. Brefeldin A (BFA; Sigma) at 10 μg/ml was added 2 hr after stimulation with peptide and cells were incubated for a further 4 hr. TCD8+ were then assayed for production of interferon (IFN)-γ by flow cytometry. An enzyme-linked immunosorbent assay (ELISA) was performed for the release of IFN-γ in the medium as per the manufacturer’s instructions (Biosource International, Camarillo, CA).
Flow cytometry
For all assays, cells were incubated on ice with Fc Block and 20% mouse serum (Sigma) for 20 min prior to staining for 40 min with the appropriate antibody. For cytokine production analysis, all antibodies were purchased from BD Biosciences (San Jose, CA) except where noted. Cells were stained with anti-CD8 phycoerythrin (PE)-Cy5 antibody (Clone 53-6·7), washed once with PBS, and fixed with 1% paraformaldehyde (PFA). Fixed cells were then stained with anti-IFN-γ- fluorescein isothiocyanate (FITC) antibody (Clone XMG1.2) in 0·5% saponin, washed, and analysed. For adoptive transfer and IVK readouts, anti-CD45.1-PE antibody (Clone A20; EBioscience, San Diego, CA) was used to identify OT-1.SJL, F5.SJL and B6.SJL cells. For SR-A expression by macrophages, anti-CD11b- allophycocyanin (APC) antibody (Clone M1/70) and anti-CD204-FITC antibody (Clone 2F8) (Serotec, Raleigh, NC) were used. For gp96 and CRT binding to BMDCs, anti-CD11c-PE antibody (Clone N418; EBioscience) was used.
Fluorescent staining and microscopy
To measure binding of gp96, wild-type or SR-A−/− BMDCs were plated on eight-well glass chamber slides (Nalge Nunc International, Rochester, NY). Uptake of gp96 was visualized following incubation of BMDCs with 25 μg/ml gp96-AlexaFluor 647 for 30 min at 4°. Following fixation with 4% PFA, BMDCs were stained with anti-CD11c-PE antibody (Clone N418) and slides were then overlaid with ProLong Gold antifade reagent (Molecular Probes).
Data analysis
For statistical analysis, the t-value of sample sets was determined using the unpaired Student’s t-test. The t-value and degrees of freedom from each sample set were then used to calculate P-values such that P-values less than 0·05 were considered to be statistically significant while those greater than 0·05 were considered to be not significant.
Results
Mice lacking SR-A mount normal TCD8+ responses following peptide immunization
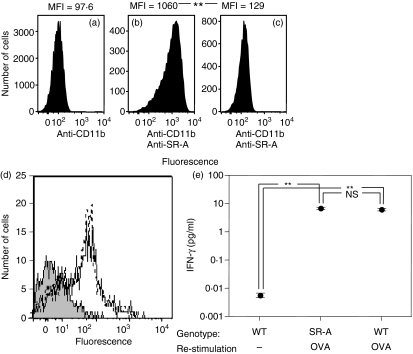
To examine the requirement for SR-A in the induction of TCD8+ responses, we obtained mice with a genetically targeted mutation of SR-A. To ensure that SR-A−/− mice lacked cell surface expression of the receptor, we generated bone marrow-derived macrophages and analysed expression by flow cytometry. As expected, SR-A−/− mice lacked detectable SR-A expression (Fig. 1a–c). However, equivalent levels of cell surface MHC class I were expressed by wild-type and SR-A−/− DCs (Fig. 1d). To examine whether SR-A−/− mice displayed an impaired ability to mount a TCD8+ response, we immunized with OVA257–264 peptide, an immunization strategy that does not require antigen processing and so should not be affected by a lack of SR-A. Equivalent levels of IFN-γ were produced in response to OVA257–264 peptide after immunization of SR-A−/− and wild-type mice (Fig. 1e).
Figure 1.
Scavenger receptor A (SR-A)−/− mice do not express SR-A, have normal levels of major histocompatibility complex class I (MHC-I) and mount normal primary peptide–specific CD8+ T-cell (TCD8+) immune responses. Macrophages from wild-type (WT) mice (a, b) or SR-A−/− mice (c) were stained with anti-Cd11b antibody alone (a) or anti-Cd11b antibody in tandem with anti-SR-A antibody (b, c) to measure SR-A expression levels via flow cytometry. MHC-I cell surface expression on wild-type (dotted line) or SR-A−/− (thin solid line) strains (d) by fluorescence-activated cell sorting (FACS) analysis was compared with background levels (shaded). Wild-type or SR-A−/− mice were immunized with ovalbumin (OVA)257–264 peptide + lipopolysaccharide (LPS) + anti-CD40 antibody and the ex vivo effector function of OVA-specific TCD8+ was measured via interferon (IFN)-γ production by enzyme-linked immunosorbent assay (ELISA) in response to OVA257–264 peptide 5 days post immunization (e). **P < 0·05; NS, not significant (P > 0·05). MFI, mean fluorescence intensity.
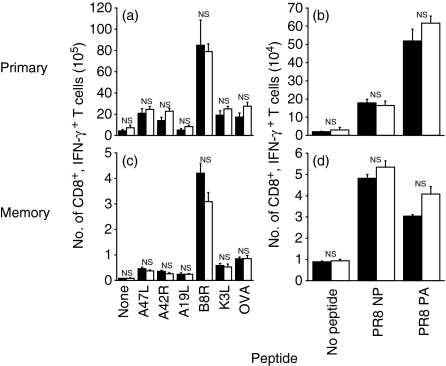
SR-A−/− mice develop normal primary and memory TCD8+ responses to viral challenge in vivo
SR-A−/− mice are more susceptible to bacterial infections than wild-type mice.29,41 To examine antiviral TCD8+ responses, we immunized SR-A−/− or wild-type mice with either recombinant VACV expressing OVA (rVACV-OVA; Fig. 2a,c) or influenza virus A/PR8 virus (Fig. 2b,d). We then examined the production of IFN-γ in response to incubation with antigenic peptide in either the primary (day 7; Fig. 2a,b) or memory (day 30; Fig. 2c,d) response. No significant differences were found in the number of IFN-γ-producing TCD8+ between SR-A−/− mice and wild-type mice in both the primary and memory TCD8+ responses, indicating that SR-A−/− mice are able to mount both normal primary and memory TCD8+ responses upon challenge with VACV and influenza. In addition, no obvious signs of distress, increased morbidity or mortality were observed in SR-A−/− mice challenged with either VACV or influenza when compared with wild-type mice.
Figure 2.
Scavenger receptor A (SR-A)−/− mice mount normal primary and memory CD8+ T-cell (TCD8+) immune responses upon viral challenge. SR-A−/− (filled bars) and wild-type (open bars) mice were immunized with recombinant vaccinia virus expressing ovalbumin (rVACV-OVA) (a, c) or influenza A/PR8 (b, d) to measure primary (a, b) and memory (c, d) TCD8+ responses. The ex vivo effector function of VACV or influenza-specific TCD8+ via interferon (IFN)-γ production in response to numerous viral peptides was examined 7 days post immunization for primary responses or 30 days post immunization for memory responses. The total number of viral-specific TCD8+ was measured. NS, not significant (P > 0·05). NP, nucleoprotein; PA, acid polymerase.
SR-A−/− mice cross present antigen efficiently in vivo
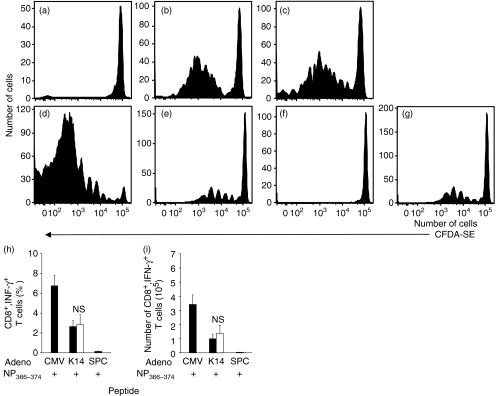
The relative contributions of the direct or cross presentation pathways following influenza virus or VACV challenge are currently unknown. To determine whether a defect in cross presentation exists in vivo in the absence of SR-A, SR-A−/− or wild-type mice were immunized with β2m−/− cells that are deficient in direct presentation,33 and so can only prime a TCD8+ response via the cross priming pathway. The β2m−/− cells were loaded with OVA under conditions in which antigen is limiting,40 and the proliferation of adoptively transferred OVA257–264-specific OT-1.SJL TCD8+ monitored following immunization. Adoptively transferred OT-1 proliferated in an antigen-specific manner following immunization of both SR-A−/− and wild-type mice (Fig. 3a–c).
Figure 3.
Scavenger receptor A (SR-A)−/− mice are efficient in the cross presentation of exogenous antigen to CD8+ T cells (TCD8+). 5-(and 6-) carboxyfluorescein diacetate–succinimidyl ester (CFDA-SE)-labelled OT-1.SJL (a–c) or F5.SJL (d–g) splenocytes were adoptively transferred into wild-type (a, b, d, e, f) or SR-A−/− (c, g) recipient mice. Recipient mice were immunized with β2-microglobulin (β2m)−/− cells loaded with ovalbumin (OVA) (a–c) or with adenovirus (Ad; Adeno) expressing nucleoprotein (NP) driven by the cytomegalovirus (CMV) (d), keratin 14 (K14) (e, g) or surfactant protein C (SPC) (f) promoters. Proliferation of adoptively transferred TCD8+ was determined 72 hr post immunization. The proportion of adoptively transferred cells proliferating was not significantly different (P > 0·05) between panels (e) and (g). Effector activity (h, i) of NT60 NP-specific TCD8+ in wild-type (closed bars) or SR-A−/− (open bars) mice was measured via production of interferon (IFN)-γ after recipient mice had been immunized intradermally with Ad expressing NP as described above. Spleens were harvested 6 days post immunization and TCD8+ grown for 7 days. The percentage (h) and number (i) of IFN-γ-producing TCD8+ were measured using peptide pulsation. NS, not significant (P > 0·05).
Although immunization with presentation-incompetent cells restricts presentation to the cross priming pathway, this strategy may not closely mimic mechanisms of cross priming used in vivo. In particular, the introduction of β2m−/− cells may not allow the transfer of antigen via gap junctions42 and the lack of stable cell surface MHC class I may prevent the transfer of peptide–MHC complexes via ‘nibbling’.10,11 To more closely examine these possibilities, SR-A−/− and wild-type mice were immunized with replication-deficient adenovirus expressing influenza NP driven by the tissue-targeted keratin 14 (K14; keratinocyte) or surfactant protein C (SPC; type II pneumocyte) promoters, or by the ubiquitous cytomegalovirus (CMV) promoter. We have previously demonstrated that intradermal immunization with K14 promoter-driven antigen results exclusively in induction of NP-specific TCD8+ via the cross presentation pathway.43 We monitored the proliferation of adoptively transferred NP-specific F5.SJL TCD8+ following immunization. As expected, intradermal infection with adenovirus expressing NP driven by the SPC lung promoter did not trigger TCD8+ proliferation (Fig. 3f), as virus did not reach the lung following immunization via this route. However, immunization with virus expressing antigen driven by the ubiquitous CMV promoter (Fig. 3d and data not shown) or the targeted K14 promoter (Fig. 3e,g) stimulated F5 proliferation in both wild-type (Fig. 3d,e) and SR-A−/− (Fig. 3g) mice.
To ensure that proliferating TCD8+ differentiated into effector cells, we re-stimulated splenocytes from wild-type or SR-A−/− mice immunized with adenovirus as above with NP366–374 peptide. Seven days later, the proportion (Fig. 3h) and absolute number (Fig. 3i) of TCD8+ producing IFN-γ in response to NP366–374 peptide was measured. As expected, ubiquitously expressed antigen stimulated robust effector function while lung promoter-driven antigen failed to stimulate effector cell function following intradermal immunization. However, there was no difference in the abilities of wild-type and SR-A−/− mice to mount an NP366–374-specific effector TCD8+ response following immunization with adenovirus expressing K14 promoter-driven antigen. Thus, no detectable difference was observed in the abilities of wild-type and SR-A−/− mice to cross-present virus-encoded antigen.
SR-A−/− mice mount normal responses following immunization with chaperone–peptide complexes
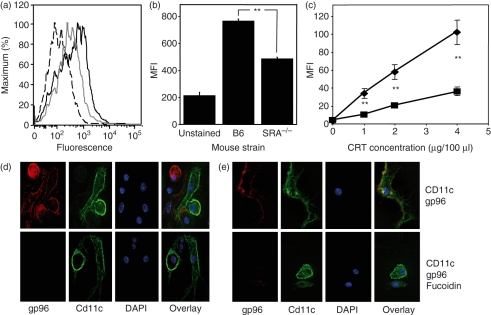
We measured the binding of molecular chaperones gp96 (Fig. 4a,b) and calreticulin (CRT) (Fig. 4c) by BMDCs. Binding was measured at 4° to prevent any contribution of fluid phase uptake to the signal measured by flow cytometry.41,44 Binding and internalization of fluorescently labelled gp96 was also visualized by deconvolution microscopy (Fig. 4d,e). SR-A−/− BMDCs exhibited a markedly reduced ability (68–78%) to bind both gp96 and CRT [Fig. 4a–c(▪)] as compared with wild-type BMDCs [Fig. 4a–c(♦)]. However, binding and internalization of chaperones could still be detected in SR-A−/− BMDCs, suggesting the presence of an additional chaperone receptor on these cells. To address this issue, BMDCs were incubated in the presence or absence of fucoidin, a competitive ligand for members of the scavenger receptor family. We have previously demonstrated that addition of fucoidin inhibits gp96 binding to pAPCs in vitro when measured by flow cytometry.12 The addition of fucoidin inhibited gp96 binding and internalization measured by confocal microscopy to background levels (Fig 4e), indicating a role for other scavenger receptors in the uptake of molecular chaperones in vitro.
Figure 4.
Scavenger receptor A (SR-A) is a receptor for glycoprotein 96 (gp96). Wild-type and SR-A−/− bone marrow-derived dendritic cells (BMDCs) were examined for their ability to bind fluorescently labelled gp96 (a, b, d, e) or calreticulin (CRT) (c). (a) Histograms of background fluorescence (dashed line), binding to SR-A−/− (solid grey line) or wild-type BMDCs (solid black line). (b) The mean fluorescence intensity (MFI) of these data. (c) Similar data after pulsing with CRT. **P < 0·05. Fluorescence microscopy was used to measure gp96 binding to wild-type (d) or SR-A−/− (e) BMDCs in the presence or absence of fucoidin, a competitive inhibitor of SR-A binding.
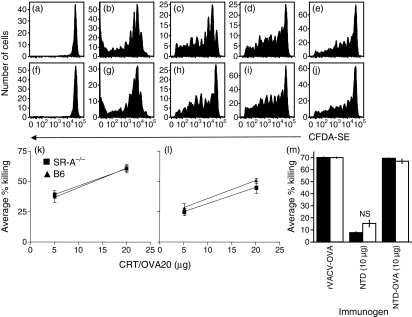
To examine the role of SR-A in the priming of TCD8+ by molecular chaperones, we adoptively transferred CFDA-SE-labelled OT-1.SJL (Fig. 5a–j) or F5.SJL (Supplementary material Fig. S1) TCD8+ into SR-A−/− (Fig. 5f–j, Fig. S1d–f) or wild-type mice (Fig. 5a–e, Fig. S1a–c). Recipient mice were then immunized intradermally with titrated amounts of gp96 coupled to OVA257–264 or influenza NT60 NP366–374 peptide. Proliferation of both OT-1.SJL and F5.SJL TCD8+, measured by CFDA-SE dilution, was equivalent in SR-A−/− (Fig. 5f–j, Fig. S1d–f) and wild-type (Fig. 5a–e, Fig. S1a–c) mice over a wide range of immunizing concentrations.
Figure 5.
Scavenger receptor A (SR-A)−/− mice are able to respond to glycoprotein 96 (gp96) and calreticulin (CRT) coupled to peptide as efficiently as wild-type mice. 5-(and 6-)carboxyfluorescein diacetate–succinimidyl ester (CFDA-SE)-labelled OT-1.SJL (a–j) or unlabelled OT-1 (m) splenocytes were adoptively transferred into wild-type [a–e, m (filled bars)] or SR-A−/− [f–j, m (open bars)] mice. Recipients were immunized intradermally with gp96 (a, f), or 10 μg (b, g), 2 μg (c, h), 0·4 μg (d, i), or 0·08 μg (e, j) of gp96 ovalbumin (OVA)257–264 or as shown (m). Proliferation of adoptively transferred OT-1.SJL CD8+ T cells (TCD8+) was determined 72 post immunization. The proportion of adoptively transferred cells proliferating was not significantly different (P > 0·05) between panels (b) and (g), or (c) and (h), or (d) and (i), or (e) and (j). Wild-type and SR-A−/− recipient mice were immunized with CRT-OVA20 (k, l) in the presence of lipopolysaccharide (LPS) (k) or poly I:C (l) or with recombinant vaccinia virus expressing ovalbumin (rVACV-OVA), 10 μg of gp96, or 10 μg of gp96 OVA257–264 (m). Four (k, l) or seven days (m) post immunization, CFDA-SE-labelled B6.SJL targets pulsed with relevant or irrelevant peptide were adoptively transferred into wild-type or SR-A−/− recipient mice and effector activity measured by comparing the clearance of cells pulsed with relevant peptide to clearance of cells pulsed with the irrelevant peptide. NS, not significant (P > 0·05); NTD, N-terminal domain.
To assess the effector function of TCD8+ triggered by chaperone–peptide complexes, we examined their ability to clear peptide-pulsed targets in vivo. We immunized SR-A−/− or wild-type mice with titrated amounts of CRT complexed to an OVA 20-mer peptide containing OVA257–264 (CRT-OVA20) in the presence of LPS (Fig. 5k) or poly I:C (Fig. 5l). Alternatively, SR-A−/− or wild-type mice were immunized with gp96 coupled to OVA257–264 (Fig. 5m) and the function of adoptively transferred OT-1 examined. Four days (Fig. 5k,l) or 7 days (Fig. 5m) post immunization, the antigen-specific cytolytic activity was compared with activity against cells pulsed with an irrelevant peptide. No difference in killing was found between SR-A−/− and wild-type mice when immunized with either CRT/OVA20 or gp96 coupled to OVA257–264.
Scavenger receptor blockade prevents induction of TCD8+ by gp96–peptide complexes
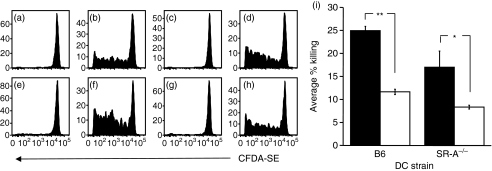
To further dissect the role of receptor-mediated endocytosis in the induction of TCD8+ by chaperone–peptide complexes, we immunized wild-type mice with either wild-type (Fig. 6a–d) or SR-A−/− (Fig. 6e–h) BMDCs pulsed with gp96 coupled to OVA257–264. Exposure to gp96 was at 37° (Fig. 6a,b,e,f) or 4° (Fig. 6c,d,g,h), a temperature at which internalization will not occur. The proliferation of adoptively transferred OT-1.SJL TCD8+ was measured 96 hr post immunization. As expected, there was no OT-1.SJL TCD8+ proliferation when BMDCs were pulsed with gp96 without peptide (Fig. 6a,c,e,g). However, proliferation of OT-1.SJL TCD8+ was equivalent in recipient mice immunized with SR-A−/− (Fig. 6e–h) or wild-type BMDCs (Fig. 6a–d) and when BMDCs were pulsed with gp96 at 37° (Fig. 6b,f) or 4° (Fig. 6d,h).
Figure 6.
Scavenger receptor A (SR-A)−/− bone marrow-derived dendritic cells (BMDCs) are efficient in the uptake of glycoprotein 96 (gp96) and the processing and presentation of gp96-bound peptides to CD8+ T cells (TCD8+). 5-(and 6-)carboxyfluorescein diacetate–succinimidyl ester (CFDA-SE)-labelled OT-1.SJL splenocytes were adoptively transferred into naïve recipient mice. Wild-type (a–d) and SR-A−/− (e–h) BMDCs were pulsed with 10 μg of gp96 (a, c, e, g) or gp96 ovalbumin (OVA)257–264 (b, d, f, h) at 4° (a, b, e, f) or 37° (c, d, g, h) for 30 min and transferred intravenously into recipient wild-type mice. Proliferation of adoptively transferred OT-1.SJL TCD8+ was determined via CFDA-SE dye dilution 72 hr post immunization. The proportion of adoptively transferred cells proliferating was not significantly different (P > 0·05) between panels (b) and (f), or (d) and (h). Mice were immunized with wild-type or SR-A−/− BMDCs pulsed with gp96-OVA257–264 (i) in the absence (closed bars) or presence (open bars) of fucoidin. Seven days post immunization, CFDA-SE-labelled B6.SJL targets pulsed with relevant or irrelevant peptide were adoptively transferred into wild-type or SR-A−/− mice. Effector function was measured as the percentage of targets killed. **P < 0·05; *P < 0·06; NS, not significant (P > 0·05).
To examine whether other scavenger receptors are involved in internalization of gp96–peptide complexes during initiation of TCD8+ responses, we pulsed SR-A−/− or wild-type BMDCs with gp96-OVA257–264 complexes at 4° in the presence or absence of fucoidin and immunized wild-type mice. Effector cytolytic activity displayed against OVA257–264 peptide-pulsed targets was compared to cytolysis of targets pulsed with an irrelevant peptide. Immunization with wild-type BMDCs elicited slightly higher levels of antigen-specific cytolysis than SR-A−/− BMDCs, but addition of fucoidin during the gp96 pulse reduced killing by approximately 50% with either population of BMDCs (Fig. 6i).
Discussion
SR-A has been repeatedly implicated as a receptor that plays a role in the priming of TCD8+ specific for exogenous antigens via a variety of mechanisms. Here we have utilized mice that are genetically deficient for SR-A to dissect the role of this receptor in the cross priming process. Our data demonstrate conclusively that SR-A is not required for the initiation of TCD8+ responses following viral challenge to antigen exclusively targeted to the cross priming pathway or following immunization with peptide–chaperone complexes. The implication is that cross presentation is an evolutionarily important means of generating protective TCD8+ that is protected by significant mechanistic redundancy.
The lack of a definitive, indispensable role for SR-A in vivo could be explained by a number of possibilities. The majority of investigations on the role of SR-A in cross presentation have been conducted in vitro and have used competitive inhibitors to examine the requirement for the receptor. In vitro studies have implicated many cell types and mechanisms in the cross priming pathway, but in vivo data substantiating the role of these cells and pathways are lacking. An example is the SR-A-dependent transfer of peptide–MHC complexes from one cell to another. A number of studies10,11,45 have described the transfer of peptide–MHC complexes between cells, particularly DCs, in vitro. However, studies examining the requirement for bone marrow-derived APCs during cross priming have utilized chimeric mice with MHC-mismatched or presentation-incompetent APCs.46–48 The published data indicate that MHC-mismatched or presentation-incompetent APCs cannot initiate a TCD8+ response in the majority of circumstances, strongly indicating that transfer of peptide–MHC complexes may not be involved in TCD8+ priming.
Our data do not rule out the possibility that SR-A can contribute to cross priming in vivo. Indeed, in vivo targeting of antigen to the SR-A family induced a strong TCD8+ response.28 Clearly, our data reinforce the previous observation12 that SR-A is a receptor for molecular chaperones, such as gp96 and CRT. However, the role of peptide–chaperone complexes in the transfer of antigen is controversial, as antigenic peptide does not bind to these molecules under physiological conditions.49,50 In addition, minimal antigenic peptide or other short-lived molecules are unlikely to be the substrate for cross priming in vivo.2–4 Nonetheless, immunization with molecular chaperones can elicit TCD8+,51 although this response may be the result of an adjuvant effect rather than via direct transfer of antigen.52 Other scavenger receptors, such as CD36, participate in recognition of pathogen components by toll-like receptors.53 However, CD36, like SR-A, is not required for cross priming in vivo.54
Despite a reduction in binding, SR-A−/− DCs readily stimulated antigen-specific TCD8+. The ability of fucoidin to partially inhibit this process indicates that other receptors, probably scavenger receptors, may play a role in cross priming of peptide–chaperone complexes. Lectin-like oxidized low density lipoprotein receptor 1 (LOX-1) mediates the cross presentation of heat shock protein 70 (hsp70)–peptide complexes, but is not a receptor for gp96.55 CD91 was first described as a master chaperone receptor.56,57 However, the inability of other CD91 ligands to competitively inhibit chaperone binding and subsequent cross presentation indicated that this was not the case,58,59 and subsequently the binding activity of SR-A was described.12 In addition, Schild et al. have described binding of gp96 to TLR2 and TLR4.60 However, this binding activity may participate in activation of APCs,60 rather than internalization of antigen, and may be facilitated by the role of gp96 as a ‘master chaperone’ of TLR folding.61
The most likely candidate to compensate for a lack of SR-A is scavenger receptor expressed by endothelial cell I (SREC-I), a receptor that can bind and endocytose gp96, CRT and hsp70.38,62 Our data currently do not distinguish whether SREC-I exclusively targets peptide–chaperone complexes to the MHC class I cross presentation pathway, or if it fulfils this role in addition to the contribution of SR-A. Inhibition of scavenger receptor binding at 4° with fucoidin reduced but did not ablate the ability of gp96-pulsed BMDCs to elicit TCD8+ responses. Thus, other receptors, such as TLR or other pattern recognition receptors, must be involved in the cross priming response.
Thus, despite strong in vitro evidence to support a role for SR-A in cross presentation, our data indicate that this receptor is not required for cross priming of TCD8+in vivo. The discrepancies between in vitro and in vivo observations may stem from at least two sources. First, in vitro studies may overestimate the importance of processes such as the transfer of peptide–MHC complexes between cells, a pathway that probably contributes minimally to the induction of antigen-specific TCD8+ during infection in vivo. Secondly, the complex systems studied in vivo probably contain compensatory mechanisms to overcome deficiencies. The redundancy that is incorporated into the cross presentation pathway in vivo prevents the development of strategies by pathogens to subvert presentation via this route. Thus, such redundancy indicates the importance of the cross priming pathway for induction of protective TCD8+ to pathogens and is likely to make therapeutic intervention to block cross presentation in situations such as induction of autoimmune TCD8+ an arduous task.
Acknowledgments
This work was supported by NIH grants AI070537 and AI056094 to CCN, a PA-DOH Commonwealth Tobacco Settlement Fund grant to CCN and EFT, NIH grant CA60395-11 to EFT, NIH grant CA-102392 to CVN, and NIH grant AI067405, COBRE P20RR016437, ACS award IRG-82-003-21, and a Hitchcock Foundation Research Fellowship to BLB. Animal experiments were conducted in a facility constructed with support from Research Facilities Improvement Grant Number C06 RR-15428-01 from the National Center for Research Resources, National Institutes of Health. We thank Irene Reider and Mel Epler for excellent technical assistance and Drs Jon Yewdell and Jack Bennink for many of the rVACV used. We also acknowledge the contributions of Nate Sheaffer of the Flow Cytometry Core Facility and Anne Stanley of the Macromolecular Core Facility of the Penn State College of Medicine and the NCCC Englert Cell Analysis Laboratory for FACS analysis.
Abbreviations
- Ad
adenovirus
- BMDCs
bone marrow-derived dendritic cells
- CFDA-SE
5-(and 6-)carboxyfluorescein diacetate–succinimidyl ester
- CMV
cytomegalovirus
- CRT
calreticulin
- ICS
intracellular cytokine staining
- IVK
in vivo killing
- K14
keratin 14
- MHC-I
major histocompatibility complex class I
- NP
nucleoprotein
- pAPC
professional antigen-presenting cell
- pMHC-I
peptide–MHC class I complexes
- SPC
surfactant protein C
- SR-A
scavenger receptor A
- TCD8+
cytotoxic T lymphocyte
- VACV
vaccinia virus
Supplementary material
The following supplementary material is available for this article online:
Scavenger receptor A (SR-A)−/− mice are able to respond to glycoprotein (gp96) coupled to peptide as efficiently as wild-type mice. 5-(and 6-)carboxyfluorescein diacetate-succinimidyl ester (CFDA-SE)-labeled F5.SJL (a–f) splenocytes were adoptively transferred into wild-type (a–c) or SR-A−/− (d–f) recipient mice. Recipient mice were immunized intradermally with gp96 (a, d), 10 μg (b, e) or 5 μg (c, f) gp96 NT60 NP366–374. Proliferation of adoptively transferred F5.SJL TCD8+ was determined via CFDA-SE dye dilution 72 hr post-immunization from the draining lymph nodes. The proportion of adoptively transferred cells proliferating was not significantly different (P > 0·05) between panels b and e, or c and f.
This material is available as part of the online article from http://www.blackwell-synergy.com/doi/abs/10.1111/j.1365-2567.2008.02861.x.
(This link will take you to the article abstract.)
Please note: Blackwell Publishing are not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Bevan MJ. Cross-priming for a secondary cytotoxic response to minor H antigens with H-2 congenic cells which do not cross-react in the cytotoxic assay. J Exp Med. 1976;143:1283–8. doi: 10.1084/jem.143.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Norbury CC, Basta S, Donohue KB, et al. CD8+ T cell cross-priming via transfer of proteasome substrates. Science. 2004;304:1318–21. doi: 10.1126/science.1096378. [DOI] [PubMed] [Google Scholar]
- 3.Wolkers MC, Brouwenstijn N, Bakker AH, Toebes M, Schumacher TN. Antigen bias in T cell cross-priming. Science. 2004;304:1314–7. doi: 10.1126/science.1096268. [DOI] [PubMed] [Google Scholar]
- 4.Shen L, Rock KL. Cellular protein is the source of cross-priming antigen in vivo. Proc Natl Acad Sci USA. 2004;101:3035–40. doi: 10.1073/pnas.0308345101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basta S, Stoessel R, Basler M, van den Broek M, Groettrup M. Cross-presentation of the long-lived lymphocytic choriomeningitis virus nucleoprotein does not require neosynthesis and is enhanced via heat shock proteins. J Immunol. 2005;175:796–805. doi: 10.4049/jimmunol.175.2.796. [DOI] [PubMed] [Google Scholar]
- 6.Suto R, Srivastava PK. A mechanism for the specific immunogenicity of heat shock protein- chaperoned peptides. Science. 1995;269:1585–8. doi: 10.1126/science.7545313. [DOI] [PubMed] [Google Scholar]
- 7.Singh-Jasuja H, Toes RE, Spee P, et al. Cross-presentation of glycoprotein 96-associated antigens on major histocompatibility complex class I molecules requires receptor-mediated endocytosis. J Exp Med. 2000;191:1965–74. doi: 10.1084/jem.191.11.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berwin B, Rosser MF, Brinker KG, Nicchitta CV. Transfer of GRP94 (Gp96)-associated peptides onto endosomal MHC class I molecules. Traffic. 2002;3:358–66. doi: 10.1034/j.1600-0854.2002.30505.x. [DOI] [PubMed] [Google Scholar]
- 9.Binder RJ, Blachere NE, Srivastava PK. Heat shock protein-chaperoned peptides but not free peptides introduced into the cytosol are presented efficiently by major histocompatibility complex I molecules. J Biol Chem. 2001;276:17163–71. doi: 10.1074/jbc.M011547200. [DOI] [PubMed] [Google Scholar]
- 10.Harshyne LA, Watkins SC, Gambotto A, Barratt-Boyes SM. Dendritic cells acquire antigens from live cells for cross-presentation to CTL. J Immunol. 2001;166:3717–23. doi: 10.4049/jimmunol.166.6.3717. [DOI] [PubMed] [Google Scholar]
- 11.Harshyne LA, Zimmer MI, Watkins SC, Barratt-Boyes SM. A role for class A scavenger receptor in dendritic cell nibbling from live cells. J Immunol. 2003;170:2302–9. doi: 10.4049/jimmunol.170.5.2302. [DOI] [PubMed] [Google Scholar]
- 12.Berwin B, Hart JP, Rice S, Gass C, Pizzo SV, Post SR, Nicchitta CV. Scavenger receptor-A mediates gp96/GRP94 and calreticulin internalization by antigen-presenting cells. EMBO J. 2003;22:6127–36. doi: 10.1093/emboj/cdg572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Becker M, Cotena A, Gordon S, Platt N. Expression of the class A macrophage scavenger receptor on specific subpopulations of murine dendritic cells limits their endotoxin response. Eur J Immunol. 2006;36:950–60. doi: 10.1002/eji.200535660. [DOI] [PubMed] [Google Scholar]
- 14.Freeman M, Ashkenas J, Rees DJ, Kingsley DM, Copeland NG, Jenkins NA, Krieger M. An ancient, highly conserved family of cysteine-rich protein domains revealed by cloning type I and type II murine macrophage scavenger receptors. Proc Natl Acad Sci U S A. 1990;87:8810–4. doi: 10.1073/pnas.87.22.8810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kodama T, Freeman M, Rohrer L, Zabrecky J, Matsudaira P, Krieger M. Type I macrophage scavenger receptor contains alpha-helical and collagen-like coiled coils. Nature. 1990;343:531–5. doi: 10.1038/343531a0. [DOI] [PubMed] [Google Scholar]
- 16.Rohrer L, Freeman M, Kodama T, Penman M, Krieger M. Coiled-coil fibrous domains mediate ligand binding by macrophage scavenger receptor type II. Nature. 1990;343:570–2. doi: 10.1038/343570a0. [DOI] [PubMed] [Google Scholar]
- 17.Krieger M, Herz J. Structures and functions of multiligand lipoprotein receptors: macrophage scavenger receptors and LDL receptor-related protein (LRP) Annu Rev Biochem. 1994;63:601–37. doi: 10.1146/annurev.bi.63.070194.003125. [DOI] [PubMed] [Google Scholar]
- 18.Platt N, Gordon S. Scavenger receptors: diverse activities and promiscuous binding of polyanionic ligands. Chem Biol. 1998;5:R193–203. doi: 10.1016/s1074-5521(98)90156-9. [DOI] [PubMed] [Google Scholar]
- 19.Gough PJ, Greaves DR, Suzuki H, et al. Analysis of macrophage scavenger receptor (SR-A) expression in human aortic atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 1999;19:461–71. doi: 10.1161/01.atv.19.3.461. [DOI] [PubMed] [Google Scholar]
- 20.Greaves DR, Gough PJ, Gordon S. Recent progress in defining the role of scavenger receptors in lipid transport, atherosclerosis and host defence. Curr Opin Lipidol. 1998;9:425–32. doi: 10.1097/00041433-199810000-00006. [DOI] [PubMed] [Google Scholar]
- 21.da Silva RP, Platt N, de Villiers JS, Gordon S. Membrane molecules and macrophage endocytosis: scavenger receptor and macrosialin as markers of plasma-membrane and vacuolar functions. Biochem Soc Trans. 1996;24:220–4. doi: 10.1042/bst0240220. [DOI] [PubMed] [Google Scholar]
- 22.Dunne DW, Resnick D, Greenberg J, Krieger M, Joiner KA. The type I macrophage scavenger receptor binds to gram-positive bacteria and recognizes lipoteichoic acid. Proc Natl Acad Sci USA. 1994;91:1863–7. doi: 10.1073/pnas.91.5.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hampton RY, Golenbock DT, Penman M, Krieger M, Raetz CR. Recognition and plasma clearance of endotoxin by scavenger receptors. Nature. 1991;352:342–4. doi: 10.1038/352342a0. [DOI] [PubMed] [Google Scholar]
- 24.Amiel E, Nicholson-Dykstra S, Walters JJ, Higgs H, Berwin B. Scavenger receptor-A functions in phagocytosis of E. coli by bone marrow dendritic cells. Exp Cell Res. 2007;313:1438–48. doi: 10.1016/j.yexcr.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peiser L, Gough PJ, Kodama T, Gordon S. Macrophage class A scavenger receptor-mediated phagocytosis of Escherichia coli: role of cell heterogeneity, microbial strain, and culture conditions in vitro. Infect Immun. 2000;68:1953–63. doi: 10.1128/iai.68.4.1953-1963.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haworth R, Platt N, Keshav S, et al. The macrophage scavenger receptor type A is expressed by activated macrophages and protects the host against lethal endotoxic shock. J Exp Med. 1997;186:1431–9. doi: 10.1084/jem.186.9.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jozefowski S, Kobzik L. Scavenger receptor A mediates H2O2 production and suppression of IL-12 release in murine macrophages. J Leukoc Biol. 2004;76:1066–74. doi: 10.1189/jlb.0504270. [DOI] [PubMed] [Google Scholar]
- 28.Abraham R, Singh N, Mukhopadhyay A, Basu SK, Bal V, Rath S. Modulation of immunogenicity and antigenicity of proteins by maleylation to target scavenger receptors on macrophages. J Immunol. 1995;154:1–8. [PubMed] [Google Scholar]
- 29.Suzuki H, Kurihara Y, Takeya M, et al. A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature. 1997;386:292–6. doi: 10.1038/386292a0. [DOI] [PubMed] [Google Scholar]
- 30.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 31.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–77. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 32.Mamalaki C, Elliott J, Norton T, Yannoutsos N, Townsend AR, Chandler P, Simpson E, Kioussis D. Positive and negative selection in transgenic mice expressing a T-cell receptor specific for influenza nucleoprotein and endogenous superantigen. Dev Immunol. 1993;3:159–74. doi: 10.1155/1993/98015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Norbury CC, Princiotta MF, Bacik I, Brutkiewicz RR, Wood P, Elliott T, Bennink JR, Yewdell JW. Multiple antigen-specific processing pathways for activating naive CD8+ T cells in vivo. J Immunol. 2001;166:4355–62. doi: 10.4049/jimmunol.166.7.4355. [DOI] [PubMed] [Google Scholar]
- 34.Norbury CC, Chambers BJ, Prescott AR, Ljunggren HG, Watts C. Constitutive macropinocytosis allows TAP-dependent major histocompatibility complex class I presentation of exogenous soluble antigen by bone marrow-derived dendritic cells. Eur J Immunol. 1997;27:280–8. doi: 10.1002/eji.1830270141. [DOI] [PubMed] [Google Scholar]
- 35.Norbury CC, Hewlett LJ, Prescott AR, Shastri N, Watts C. Class I MHC presentation of exogenous soluble antigen via macropinocytosis in bone marrow macrophages. Immunity. 1995;3:783–91. doi: 10.1016/1074-7613(95)90067-5. [DOI] [PubMed] [Google Scholar]
- 36.Walters JJ, Berwin B. Differential CD91 dependence for calreticulin and Pseudomonas exotoxin-A endocytosis. Traffic. 2005;6:1173–82. doi: 10.1111/j.1600-0854.2005.00351.x. [DOI] [PubMed] [Google Scholar]
- 37.Reed RC, Zheng T, Nicchitta CV. GRP94-associated Enzymatic Activities. Resolution by chromatographic fractionation. J Biol Chem. 2002;277:25082–9. doi: 10.1074/jbc.M203195200. [DOI] [PubMed] [Google Scholar]
- 38.Berwin B, Delneste Y, Lovingood RV, Post SR, Pizzo SV. SREC-I, a type F scavenger receptor, is an endocytic receptor for calreticulin. J Biol Chem. 2004;279:51250–7. doi: 10.1074/jbc.M406202200. [DOI] [PubMed] [Google Scholar]
- 39.Binder RJ, Srivastava PK. Essential role of CD91 in re-presentation of gp96-chaperoned peptides. Proc Natl Acad Sci USA. 2004;101:6128–33. doi: 10.1073/pnas.0308180101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Donohue KB, Grant JM, Tewalt EF, Palmer DC, Theoret MR, Restifo NP, Norbury CC. Cross-priming utilizes antigen not available to the direct presentation pathway. Immunology. 2006;119:63–73. doi: 10.1111/j.1365-2567.2006.02406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomas CA, Li Y, Kodama T, Suzuki H, Silverstein SC, El Khoury J. Protection from lethal gram-positive infection by macrophage scavenger receptor-dependent phagocytosis. J Exp Med. 2000;191:147–56. doi: 10.1084/jem.191.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neijssen J, Herberts C, Drijfhout JW, Reits E, Janssen L, Neefjes J. Cross-presentation by intercellular peptide transfer through gap junctions. Nature. 2005;434:83–8. doi: 10.1038/nature03290. [DOI] [PubMed] [Google Scholar]
- 43.Prasad SA, Norbury CC, Chen W, Bennink JR, Yewdell JW. Cutting edge: recombinant adenoviruses induce CD8 T cell responses to an inserted protein whose expression is limited to nonimmune cells. J Immunol. 2001;166:4809–12. doi: 10.4049/jimmunol.166.8.4809. [DOI] [PubMed] [Google Scholar]
- 44.Peiser L, Gordon S. The function of scavenger receptors expressed by macrophages and their role in the regulation of inflammation. Microbes Infect. 2001;3:149–59. doi: 10.1016/s1286-4579(00)01362-9. [DOI] [PubMed] [Google Scholar]
- 45.Dolan BP, Gibbs KD, Jr, Ostrand-Rosenberg S. Dendritic cells cross-dressed with peptide MHC class I complexes prime CD8+ T cells. J Immunol. 2006;177:6018–24. doi: 10.4049/jimmunol.177.9.6018. [DOI] [PubMed] [Google Scholar]
- 46.Sigal LJ, Crotty S, Andino R, Rock KL. Cytotoxic T-cell immunity to virus-infected non-haematopoietic cells requires presentation of exogenous antigen. Nature. 1999;398:77–80. doi: 10.1038/18038. [DOI] [PubMed] [Google Scholar]
- 47.Allan RS, Smith CM, Belz GT, van Lint AL, Wakim LM, Heath WR, Carbone FR. Epidermal viral immunity induced by CD8 alpha+ dendritic cells but not by Langerhans cells. Science. 2003;301:1925–8. doi: 10.1126/science.1087576. [DOI] [PubMed] [Google Scholar]
- 48.Huang AYC, Bruce AT, Pardoll DM, Levitsky HI. In vivo cross-priming of MHC class I-restricted antigens requires the TAP transporter. Immunity. 1996;4:349–55. doi: 10.1016/s1074-7613(00)80248-4. [DOI] [PubMed] [Google Scholar]
- 49.Baker-LePain JC, Reed RC, Nicchitta CV. ISO: a critical evaluation of the role of peptides in heat shock/chaperone protein-mediated tumor rejection. Curr Opin Immunol. 2003;15:89–94. doi: 10.1016/s0952791502000067. [DOI] [PubMed] [Google Scholar]
- 50.Nicchitta CV, Reed RC. The immunological properties of endoplasmic reticulum chaperones: a conflict of interest? Essays Biochem. 2000;36:15–25. doi: 10.1042/bse0360015. [DOI] [PubMed] [Google Scholar]
- 51.Blachere NE, Li Z, Chandawarkar RY, Suto R, Jaikaria NS, Basu S, Udono H, Srivastava PK. Heat shock protein-peptide complexes, reconstituted in vitro, elicit peptide-specific cytotoxic T lymphocyte response and tumor immunity. J Exp Med. 1997;186:1315–22. doi: 10.1084/jem.186.8.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baker-LePain JC, Sarzotti M, Fields TA, Li CY, Nicchitta CV. GRP94 (gp96) and GRP94 N-terminal geldanamycin binding domain elicit tissue nonrestricted tumor suppression. J Exp Med. 2002;196:1447–59. doi: 10.1084/jem.20020436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hoebe K, Georgel P, Rutschmann S, et al. CD36 is a sensor of diacylglycerides. Nature. 2005;433:523–7. doi: 10.1038/nature03253. [DOI] [PubMed] [Google Scholar]
- 54.Belz GT, Vremec D, Febbraio M, Corcoran L, Shortman K, Carbone FR, Heath WR. CD36 is differentially expressed by CD8+ splenic dendritic cells but is not required for cross-presentation in vivo. J Immunol. 2002;168:6066–70. doi: 10.4049/jimmunol.168.12.6066. [DOI] [PubMed] [Google Scholar]
- 55.Delneste Y, Magistrelli G, Gauchat J, et al. Involvement of LOX-1 in dendritic cell-mediated antigen cross-presentation. Immunity. 2002;17:353–62. doi: 10.1016/s1074-7613(02)00388-6. [DOI] [PubMed] [Google Scholar]
- 56.Binder RJ, Han DK, Srivastava PK. CD91: a receptor for heat shock protein gp96. Nat Immunol. 2000;1:151–5. doi: 10.1038/77835. [DOI] [PubMed] [Google Scholar]
- 57.Basu S, Binder RJ, Ramalingam T, Srivastava PK. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity. 2001;14:303–13. doi: 10.1016/s1074-7613(01)00111-x. [DOI] [PubMed] [Google Scholar]
- 58.Berwin B, Hart JP, Pizzo SV, Nicchitta CV. Cutting edge: CD91-independent cross-presentation of GRP94(gp96)-associated peptides. J Immunol. 2002;168:4282–6. doi: 10.4049/jimmunol.168.9.4282. [DOI] [PubMed] [Google Scholar]
- 59.Theriault JR, Mambula SS, Sawamura T, Stevenson MA, Calderwood SK. Extracellular HSP70 binding to surface receptors present on antigen presenting cells and endothelial/epithelial cells. FEBS Lett. 2005;579:1951–60. doi: 10.1016/j.febslet.2005.02.046. [DOI] [PubMed] [Google Scholar]
- 60.Vabulas RM, Braedel S, Hilf N, et al. The endoplasmic reticulum-resident heat shock protein Gp96 activates dendritic cells via the Toll-like receptor 2/4 pathway. J Biol Chem. 2002;277:20847–53. doi: 10.1074/jbc.M200425200. [DOI] [PubMed] [Google Scholar]
- 61.Yang Y, Liu B, Dai J, Srivastava PK, Zammit DJ, Lefrancois L, Li Z. Heat shock protein gp96 is a master chaperone for toll-like receptors and is important in the innate function of macrophages. Immunity. 2007;26:215–26. doi: 10.1016/j.immuni.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Theriault JR, Adachi H, Calderwood SK. Role of scavenger receptors in the binding and internalization of heat shock protein 70. J Immunol. 2006;177:8604–11. doi: 10.4049/jimmunol.177.12.8604. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Scavenger receptor A (SR-A)−/− mice are able to respond to glycoprotein (gp96) coupled to peptide as efficiently as wild-type mice. 5-(and 6-)carboxyfluorescein diacetate-succinimidyl ester (CFDA-SE)-labeled F5.SJL (a–f) splenocytes were adoptively transferred into wild-type (a–c) or SR-A−/− (d–f) recipient mice. Recipient mice were immunized intradermally with gp96 (a, d), 10 μg (b, e) or 5 μg (c, f) gp96 NT60 NP366–374. Proliferation of adoptively transferred F5.SJL TCD8+ was determined via CFDA-SE dye dilution 72 hr post-immunization from the draining lymph nodes. The proportion of adoptively transferred cells proliferating was not significantly different (P > 0·05) between panels b and e, or c and f.