Abstract
Interferon (IFN)-λ1 [interleukin (IL)-29] is a member of the interferon lambda family (also known as type III interferons), whose members are distantly related to both the type I interferons and members of the IL-10 family. While IFN-λ1 has significant antiviral activity, it is also becoming apparent that it has important immunoregulatory properties, especially with regard to the T helper type 2 (Th2) response. Previously, we have shown that IFN-λ1 is capable of down-regulating IL-13 production in an IFN-γ-independent manner and that this is mediated in part via monocyte-derived dendritic cells. Here, we have extended our knowledge of IFN-λ1 regulation of the human in vitro Th2 response by examining the regulation of three major Th2 cytokines, IL-4, IL-5 and IL-13, by IFN-λ1. Our results reveal that IFN-λ1 preferentially inhibits IL-13 production, compared with IL-4 or IL-5. Levels of IL-13 mRNA, the amount of secreted IL-13 protein and numbers of IL-13-positive CD3+ CD4+ cells were all significantly diminished by IFN-λ1. IFN-λ1 significantly decreased some aspects of IL-4 and IL-5 production, but its effects were not as consistent as those seen on IL-13. IFN-λ1 was also effective at decreasing IL-13 secretion under conditions designed to support the generation of Th2 cells. Irrespective of whether Concanavalin-A or T-cell-stimulatory microbeads were used, IFN-λ1 markedly diminished IL-13 secretion in cultures where IL-4 had been added. Thus, IFN-λ1 appears to be an inhibitor of human Th2 responses whose action is primarily directed towards IL-13 but which may also affect Th2 responses generally and does not invoke a complementary elevation of IFN-γ secretion.
Keywords: interferon-λ1, interleukin-13, interleukin-29, interleukin-4, interleukin-5, T helper type 2
Introduction
The discovery of the CRF2-12 receptor chain through its similarity with the external domain of the interleukin (IL)-22 receptor alpha chain (G. Gallagher, unpublished observations) and its pairing with an orphan ligand described as ‘similar to type-one interferon’,1 led to the discovery and characterization of the interferon (IFN) lambda family of ligands and their receptor, independently and in parallel, in two laboratories.2,3 This new family of four-helical-bundle interferons is distantly related to both the IL-10 family of ligands and the type I interferon family. Interestingly, although they share functional characteristics with members of the intronless IFN-α family, the IFN-λ genes have an identical intron/exon structure to members of the IL-10 family.
The family comprises three ligands (IFN-λ1/2/3) in humans and presumably other primates.2 In mice and perhaps other rodents, IFN-λ1 is disrupted.4 IFN-λ1/2/3 have been named ‘type III’ interferons and are also known as IL-29, IL-28A and IL-28B, respectively. Initial characterizations of the receptor demonstrated that it has a relatively wide tissue distribution, suggesting that many tissues contain IFN-λ1-responsive cells; this would be expected of a cytokine with antiviral activity.2,3 More recently, a bias towards action on epithelial cells has been suggested. Many investigators have studied the antiviral actions of the IFN-λ family5–9 and it has been demonstrated that IFN-λ1 has, broadly, a weaker protective action than IFN-α.
Peripheral T-cell subsets are often defined as ‘helper’ or ‘cytotoxic’ because of the mutually exclusive expression of the cell surface markers CD4 and CD8, respectively. It has become apparent that the T-helper response can itself be divided, according to whether it is polarized to support the development of hypersensitivity and/or antibody responses [so-called ‘T helper type 2’ (Th2) cells].10 These polarized T cells were defined according to certain signature cytokines produced upon stimulation.11,12 For example, Th1 cells produce IL-2 and IFN-γ, while Th2 cells produce IL-4, IL-5 and IL-13. More recently, an additional subset, the so-called ‘T-regulatory 1’ (Tr1) cells, was defined as able to secrete IL-10.13–16 The latest class of Th cell, the Th17 cell, is a vital player in inflammatory responses.17,18
The ability of IFN-λ1 to modulate human in vitro immune responses has been studied separately from its antiviral actions.19–21 It has been shown that IFN-λ1 is able to up-regulate the expression of a subset of chemokines (the so-called‘ELR’ chemokines: ITAC, IP-10 and MIG20); it can also closely regulate pro- and anti-inflammatory cytokine secretion in vitro, up-regulating IL-6, IL-8 and IL-10, while having no effect on IL-1β or tumour necrosis factor (TNF).19 Most interesting, however, have been its effects on cytokines central to the Th1/Th2 axis.
In our previous report,21 studies using Concanavalin-A (ConA) stimulation, mixed lymphocyte reactions and antigen-specific T-cell activation uniformly showed that IFN-λ1 was capable of down-regulating the Th2 response (as measured by IL-13 secretion), while leaving the Th1 response (as indicated by IFN-γ secretion) apparently intact. In the small proportion of donors where IFN-γ secretion was elevated with ConA, a 100× higher concentration of IFN-λ1 was required to effect this change than to reduce IL-13 secretion. Additionally, it was shown that the effect of IFN-λ1 on the Th2 response was mediated in part by apparently direct actions on the induction phase of dendritic cell differentiation from peripheral (CD14+) monocytes [maturing human monocyte-derived dendritic cells (MDDCs)].21
In the present experiments, we have extended these data to consider the effect of IFN-λ1 during ConA-driven T-cell activation on secretion, mRNA levels and numbers of cytokine-positive cells for the three defining Th2 cytokines: IL-4, IL-5 and IL-13. In addition, we have asked whether IFN-λ1 is active under Th2 polarizing conditions driven by ConA or T-cell stimulation beads coated with anti-CD2/3/28. Taken together with our previous data, our results demonstrate that one of the key specific immunoregulatory functions of IFN-λ1 is to diminish the Th2 response, with a particular emphasis on reducing IL-13.
Materials and methods
Cell culture conditions
Human peripheral blood mononuclear cells (PBMC) were isolated from anonymous buffy coats purchased from the Blood Center of New Jersey (West Orange, NJ). These buffy coats were completely anonymous and it was not possible to identify the donors. Fourteen donors were studied. PBMC were harvested by centrifugation over Histopaque 1077 (Sigma-Aldrich, St Louis, MO). After washing, cells were adjusted to a density of 4 × 105 cells/well in 96-well flat-bottom plates. Cells were stimulated with ConA (5 μg/ml) or MACS bead particles (Miltenyi Biotech, Auburn, CA) coated with anti-CD2/CD3/CD28 antibodies (‘beads’, one bead added per two cells) in the presence or absence of 100 ng/ml IFN-λ1 (Peprotech, Rocky Hill, NJ). In some cases, cultures were re-stimulated with phorbol 12-myristate 13-acetate (PMA; 20 ng/ml) + ionomycin (1 μm) for 6 hr prior to harvesting of supernatants. Experimental points were established in triplicate; supernatants were harvested after 24 hr, 3 days and 6 days for enzyme-linked immunosorbent assay (ELISA) (see text), while cells were stored at −80° in lysis buffer (Stratagene, La Jolla, CA) for RNA extraction. RPMI-1640 culture medium supplemented with 10% [volume/volume (v/v)] heat-inactivated fetal calf serum was used throughout. Unless otherwise stated, cell culture medium components were from GIBCO (Invitrogen, Carlsbad, CA).
Quantification of cytokine mRNA levels
Total RNA was extracted from cells (Stratagene) and cDNA prepared and subsequently assayed using a two-step procedure (‘AffinityScript’; Stratagene). Quantitative real-time reverse transcriptase–polymerase chain reaction (RT-PCR) was carried out using a Sybr-green method in a Stratagene MX-3000 instrument. cDNA samples were amplified thus: 10 min at 95° and then 40 cycles of 95° for 30 seconds, 55° for 60 seconds and 72° for 30 seconds. A melting curve analysis was carried out to verify that the cycle threshold (Ct) values were based upon a single PCR product. All primer concentrations were at 300 nm, except those for EF-1α (150 nm). Primer pairs for cytokine analysis were:
IL-4F, 5′-GCT GCC TCC AAG AAC ACA AC-3′;
IL-4R, 5′-CTG TAG AAC TGG CGG AGC AC-3′;
IL-5F, 5′-CTG CTG ATA GCC AAT GAG ACT C-3′;
IL-5R, 5′-CTT GCA CAG TTT GAC TCT CCA G-3′;
IL-13F, 5′-GAC AGC TGG CAT GTA CTG-3′;
IL-13R, 5′-CTC TGG GTC TTC TCG ATG-3′.
Relative levels of these cytokine cDNAs and the effect of IFN-λ1 were established using the ΔΔCt method against the housekeeping gene EF-1a:
EF-1aF, 5′-CTG AAC CAT CCA GGC CAA AT-3′;
EF-1aR, 5′-GCC GTG TGG CAA TCC AAT-3′.
The ΔCt for each cytokine was paired for each donor (absence or presence of IFN-λ1) and the data analysed as described above.
Quantification of secreted cytokine by ELISA
Levels of accumulated IL-4, IL-5 and IL-13 were determined by ELISA from 24-hr, 3-day and 6-day cultures. Antibody pairs for IL-4 and IL-5 were purchased from sBioscience (San Diego, CA) and those for IL-13 from R&D Systems (Minneapolis, MN). Manufacturers’ protocols were followed and all washes were performed with phosphate-buffered saline (PBS) containing 0·05% (v/v) Tween-20 (Sigma-Aldrich). Briefly, 96-well Nunesorb plates were coated with the appropriate capture antibody and incubated at 4° overnight (room temperature for the IL-13 ELISA). After washing, plates were blocked with 1% [weight/volume (w/v)] bovine serum albumin (BSA; Sigma-Aldrich) and then standards and culture supernatants were plated in triplicate. After incubation at 37° for 2 hr, plates were washed, exposed first to relevant biotinylated antibodies and then to streptavadin-conjugated horse-radish peroxidase and finally to the chromogen tetra-methyl benzidine (TMB). After 20 min, the reaction was halted by the addition of sulphuric acid and the optical density at 450 nm determined. Cytokine concentrations were calculated from the standard curve present on each plate.
Quantification of cytokine-containing cells by flow cytometry
Intracellular flow cytometry for Th1 and Th2 cytokines IFN-γ, IL-13, IL-4 and IL-5 was performed using direct cytoplasmic staining combined with cell-surface staining. Cells at a density of 1·5 × 106 cells/ml were stimulated in 6-well plates with ConA in the presence or absence of IFN-λ1 (100 ng/ml) at 37° in a 5% CO2 incubator. For detection of cytokine production, brefeldin-A (Sigma-Aldrich) was added at a final concentration of 5 μg/ml, and then the cells were incubated for an additional 2 hr. The cells were washed with cold 0·1% (w/v) BSA (Sigma-Aldrich) in PBS, blocked with 5% heat-inactivated human serum and stained with the appropriate fluorochrome-conjugated cell surface marker antibodies for 20 min at 4° (described below). Cells were then washed and fixed with 1% paraformaldehyde (Fisher Scientific, Hampton, NH) in PBS overnight at 4°. The cells were washed twice with 2% FCS-PBS and permeabilized with 0·5% saponin (Sigma-Aldrich) in 2% FCS-PBS for 15 min at room temperature. Cells were then incubated with fluorescein isothiocyanate (FITC)-anti-human-IL-4, phycoerythrin (PE)-anti-human-IL-13, allophycocyanin (APC)-anti-human-IL-5 (BD Biosciences, Franklin Lakes, NJ) or Alexa Fluor 647-anti-human-IFN-γ (eBioscience, San Diego, CA) for 30 min at room temperature. The cells were washed and fixed with 1% paraformaldehyde in PBS and analysed using a FACSCalibur instrument (Beckton Dickinson, Franklin Lakes, NJ) and flowjo software (TreeStar, Ashland, OR).
Statistical analyses
Data were considered to be non-parametrically distributed and analysed using the Mann–Whitney U-test or the Wilcoxon matched-pairs signed-rank test, as appropriate. A P-value of less than 0·05 was considered significant. Unless otherwise stated, where values are given, the median ± the semi-interquartile range is shown thus: a ± b.
Results
IFN-λ1 inhibits the production and secretion of IL-13
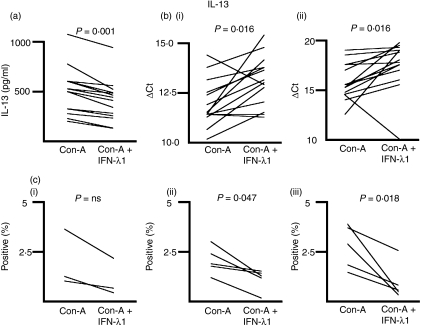
We measured the concentration of IL-13 in the medium of ConA-treated PBMC cultures in the presence or absence of IFN-λ1 at 24 hr and 6 days after initiation of the culture, by ELISA. Secreted IL-13 was not detected at 24 hr, presumably because sufficient levels had not yet accumulated in the medium, notwithstanding the presence of IL-13-positive cells (see below). Figure 1(a) shows the concentration of IL-13 in the absence and presence of IFN-λ1 for each of 14 donors at day 6. All 14 (14/14) showed an IFN-λ1-dependent decrease in IL-13 levels (P = 0·001; two-tailed Wilcoxon signed-rank test). The median reduction in IL-13 secretion in the presence of IFN-λ1 was 24·4 ± 6·85%, compared with ConA alone.
Figure 1.
Interferon (IFN)-λ1 inhibits the production and secretion of interleukin (IL)-13. (a) Cell culture supernatants were harvested and subjected to enzyme-linked immunosorbent assays (ELISAs) as described. Data for each donor are shown, with and without treatment with IFN-λ1. Supernatants were tested in triplicate and the median level of secreted cytokine determined. These median secreted cytokine levels for each donor were analysed using the Wilcoxon matched-pairs signed-rank test for paired data to reveal any effect of IFN-λ1. Fourteen of fourteen donors reduced their IL-13 secretion at day 6 in response to IFN-λ1. IL-13 was not detectable at 24 hr. (b) Total RNA was harvested from 24-hr (i) and 6-day (ii) cultures and the levels of IL-13 mRNA determined for each donor, and presented as the ΔCt. Higher ΔCt levels indicate lower mRNA levels. Eleven of fourteen donors had reduced IL-13 mRNA at 24 hr and 12 of 14 at 6 days, in response to IFN-λ1. (c) Cells were stimulated with Concanavalin-A (ConA) in the presence or absence of IFN-λ1 and the proportion of IL-13-positive CD3+ CD4+ cells determined at 6 hr, 24 hr and 6 days as shown in (i–iii). In each case, IL-13-positive cells were reduced by exposure to IFN-λ1. ns, not significant.
We next examined the effect of IFN-λ1 on IL-13 mRNA level, in the same cultures from which we had harvested the supernatant for ELISA. Figure 1(b) shows the levels of IL-13 mRNA in the presence or absence of IFN-λ1, as denoted by the ΔCt from the real-time RT-PCR assay. Fourteen donors were examined. At 24 hr (Fig. 1b(i)), 11 donors (11/14) showed a reduction in IL-13 mRNA of >10%, two donors showed an elevation of IL-13 mRNA of >10% and one showed no change. At 6 days [Fig. 1b(ii)], 12 donors (12/14) showed a reduction of IL-13 mRNA, one showed an elevation and one showed no change [P = 0·016 (24 hr); P = 0·016 (6 days), two-tailed Wilcoxon signed-rank test]. The median IL-13 mRNA level with IFN-λ1 present was 48·5 ± 21·5% at 24 hr and 38·5 ± 28·5% at 6 days, relative to ConA alone (51·5% and 61·5% reductions, respectively).
We further examined the effect of IFN-λ1 on the number of IL-13-bearing CD3+ CD4+ cells using intracellular flow cytometry and the corresponding mean fluorescence intensity (MFI); the data are shown in Fig. 1(c). Five donors were tested at 6 hr, 24 hr and 6 days [Fig. 1c(i–iii), respectively]. Only three donors had measurable IL-13-positive cells at 6 hr after stimulation. All donors with measurable IL-13-positive cells had the number of these cells reduced by IFN-λ1: 6 hr, 3/3; 24 hr, 5/5; 6 days, 5/5. This reduction was significant at 24 hr and 6 days (P = 0·047 and 0·018, respectively). The median reductions in IL-13-positive CD3+ CD4+ cells in the presence of IFN-λ1 were: 6 hr, 40·8 ± 11·3%; 24 hr, 38·1 ± 18·1% and 6 days, 73·3 ± 7·2%. The MFIs of the positive cells were neither reduced nor elevated by the IFN-λ1 treatment (data not shown). IL-13 was not observed in the CD3+ CD4− cell fraction at the time-points tested. Thus, the fluorescence-activated cell sorting (FACS) analysis supported our ELISA and PCR data.
IFN-λ1 inhibits the production and secretion of IL-4
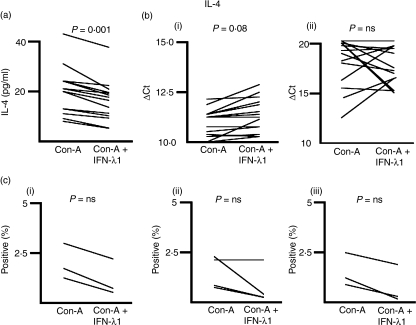
We measured the concentration of IL-4 in the medium of ConA-treated PBMC cultures in the presence or absence of IFN-λ1 at 24 hr and 6 days after initiation of the culture, by ELISA. IL-4 was not measurable at 6 days, presumably because it had been consumed in culture. Figure 2(a) shows the concentration of IL-4 in the presence and absence of IFN-λ1 for each of 14 donors at 24 hr. All 14 (14/14) showed an IFN-λ1-dependent decrease in IL-4 levels (P = 0·001, two-tailed Wilcoxon signed-rank test). The median reduction in IL-4 secretion in the presence of IFN-λ1 was 33·3 ± 6·75%.
Figure 2.
Interferon (IFN)-λ1 inhibits the production and secretion of interleukin (IL)-4. (a) Cell culture supernatants were harvested and subjected to enzyme-linked immunosorbent assays (ELISAs) as described. Data for each donor are shown with and without treatment with IFN-λ1. Supernatants were tested in triplicate and the median level of secreted cytokine determined. These median secreted cytokine levels for each donor were analysed using the Wilcoxon matched-pairs signed-rank test for paired data to reveal any effect of IFN-λ1. Fourteen of fourteen donors reduced their IL-4 secretion at 24 hr in response to IFN-λ1. IL-4 was not detectable at 6 days. (b) Total RNA was harvested from 24-hr (i) and 6-day (ii) cultures and the levels of IL-4 mRNA determined for each donor, and presented as the ΔCt. Higher ΔCt levels indicate lower mRNA levels. Eight of fourteen donors had reduced IL-4 mRNA at 24 hr and five of 14 at 6 days, in response to IFN-λ1. (c) Cells were stimulated with Concanavalin-A (ConA) in the presence or absence of IFN-λ1 and the proportion of IL-4-positive CD3+ CD4+ cells determined at 6 hr, 24 hr and 6 days as shown in (i–iii). In each case, IL-4-positive cells were reduced by exposure to IFN-λ1, but these reductions did not achieve significance. ns, not significant.
We next examined the effect of IFN-λ1 on IL-4 mRNA levels, in the same cultures from which we had harvested the supernatant for ELISA. Figure 2(b) shows the levels of IL-4 mRNA in the presence or absence of IFN-λ1, as denoted by the ΔCt from the real-time RT-PCR assay. Fourteen donors were examined. At 24 hr [Fig. 2b(i)], eight donors (8/14) showed a reduction in IL-4 mRNA of >10%, one donor showed an elevation of IL-4 mRNA of >10% and five showed no change. At 6 days [Fig. 2b(ii)], five donors (5/14) showed a reduction of IL-4 mRNA, seven showed an elevation and two showed no change [P = 0·027 (24 hr); P = 0·522 (not significant (NS), 6 days), two-tailed Wilcoxon signed-rank test]. The median IL-4 mRNA level in the presence of IFN-λ1 was 80·0 ± 18·0% at 24 hr and 111·5 ± 176·5% at 6 days, relative to ConA alone (20% reduction and 11·5% increase, respectively).
We further examined the effect of IFN-λ1 on the number of IL-4-bearing CD4+ T cells using intracellular flow cytometry and the corresponding MFI; the data are shown in Fig. 2(c). Five donors were tested at 6 hr, 24 hr and 6 days [Fig. 2c(i–iii), respectively]. One donor failed to generate measurable IL-4-positive cells. Only three donors had measurable IL-4-positive cells at 6 hr and 6 days after stimulation. All donors with measurable IL-4-positive cells had the number of these cells reduced by IFN-λ1, except one at 24 hr: 6 hr, 3/3; 24 hr, 3/4; 6 days, 3/3. The median reductions in IL-4-positive CD4+ T cells in the presence of IFN-λ1 were: 6 hr, 30·9 ± 21·3%; 24 hr, 35·7 ± 22·0% and 6 days, 38·9 ± 22·0%. These reductions were notable, but not to a significant degree at any time-point. The MFIs of the positive cells were neither reduced nor elevated by the IFN-λ1 treatment (data not shown). IL-4 was not observed in the CD3+ CD4− cell fraction at the time-points tested. Thus, the FACS analysis supported our ELISA and PCR data.
IFN-λ1 inhibits the production and secretion of IL-5
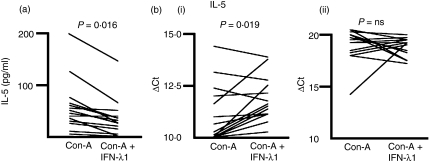
We measured the concentration of IL-5 in the medium of ConA-treated PBMC cultures in the presence or absence of IFN-λ1 at 24 hr and 6 days after initiation of the culture, by ELISA. IL-5 was not measurable at 24 hr, presumably because sufficient levels had not yet accumulated. Figure 3(a) shows the concentration of IL-5 in the presence and absence of IFN-λ1 for each of 14 donors at day 6. All but one donor (13/14) showed an IFN-λ1-dependent decrease in IL-5 levels (P = 0·0016, two-tailed Wilcoxon signed-rank test). The median reduction in IL-5 secretion in the presence of IFN-λ1 was 41·7 ± 11·5%.
Figure 3.
Interferon (IFN)-λ1 inhibits the production and secretion of interleukin (IL)-5. (a) Cell culture supernatants were harvested and subjected to enzyme-linked immunosorbent assays (ELISAs) as described. Data from each donor are shown with and without treatment with IFN-λ1. Supernatants were tested in triplicate and the median level of secreted cytokine determined. These median secreted cytokine levels for each donor were analysed using the Wilcoxon matched-pairs signed-rank test for paired data to reveal any effect of IFN-λ1. Thirteen of fourteen donors reduced their IL-5 secretion at day 6 in response to IFN-λ1. IL-5 was not detectable at 24 hr. (b) Total RNA was harvested from 24-hr (i) and 6-day (ii) cultures and the levels of IL-5 mRNA determined for each donor, and presented as the ΔCt. Higher ΔCt levels indicate lower mRNA levels. Eleven of fourteen donors had reduced IL-5 mRNA at 24 hr and six of 14 at 6 days, in response to IFN-λ1. ns, not significant.
We next examined the effect of IFN-λ1 on IL-5 mRNA levels, in the same cultures from which we had harvested the supernatant for ELISA. Figure 3(b) shows the levels of IL-5 mRNA in the presence or absence of IFN-λ1, as denoted by the ΔCt from the real-time RT-PCR assay. Fourteen donors were examined. At 24 hr [Fig. 3b(i)], 11 donors (11/14) showed a reduction in IL-5 mRNA of >10%, three donors showed an elevation of IL-5 mRNA of >10% and one showed no change. At 6 days [Fig. 3b(ii)], six donors (6/14) showed a reduction of IL-5 mRNA, seven showed an elevation and one showed no change [P = 0·019 (24 hr); P = 0·66 (NS 6 days), two-tailed Wilcoxon signed-rank test]. The median mRNA IL-5 levels in the presence of IFN-λ1 were 65·5 ± 26·5% at 24 hr and 109 ± 86·0% at 6 days (34·5% reduction and 9·0% elevation, respectively).
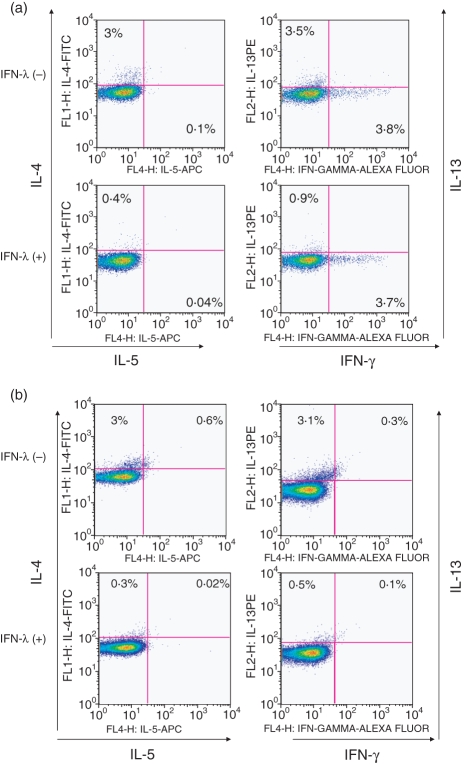
We further examined the effect of IFN-λ1 on the number of IL-5-bearing CD4+ T cells by intracellular flow cytometry and the corresponding MFI; very few IL-5-positive cells were found at the time-points examined. Typical flow cytometric analyses are shown in Fig. 4(a,b).
Figure 4.
Interferon (IFN)-λ1 lowers the numbers of interleukin (IL)-4, IL-5 and IL-13-positive CD4 T cells. Cells were harvested and prepared for flow cytometric analysis as described. (a, b) typical fluorescence-activated cell sorting (FACS) analysis plots obtained using flowjo software at 24 hr and 6 days, respectively. IL-5-positive CD4 T cells were very infrequent. No effect of IFN-λ1 on IFN-γ+ CD4+ cells was noted, as predicted from previous data. FITC, fluorescein isothiocyanate; PE, phycoerythrin.21
IFN-λ1 inhibits the secretion of IL-13 under Th2-promoting conditions
The results described above indicated that the most consistent effects of IFN-λ1 were on IL-13. We were curious to determine whether the inhibitory effect of IFN-λ1 on IL-13 secretion was apparent under conditions representing a Th2 environment. Accordingly, we stimulated PBMCs with ConA in the presence of IL-4, ± IFN-λ1. Furthermore, we established parallel experiments in which stimulation was achieved using beads coated with anti-CD2, anti-CD3 and anti-CD28 antibodies in order to preferentially drive T-cell activation. In some cases, as shown, cells were re-stimulated with PMA + ionomycin for 6 hr prior to harvesting the supernatant.
As shown in Fig. 5, while the magnitude of IL-13 secretion achieved under bead stimulation was lower than that with ConA, the pattern of response was identical for the two stimuli. As expected, inclusion of IL-4 resulted in an increase in IL-13 secretion, irrespective of whether ConA or beads were used to drive the experiment. Importantly, the presence of IFN-λ1 diminished these IL-4-magnified levels of IL-13, demonstrating that IFN-λ1 is effective at inhibiting IL-13 secretion in the context of a strong Th2-promoting environment.
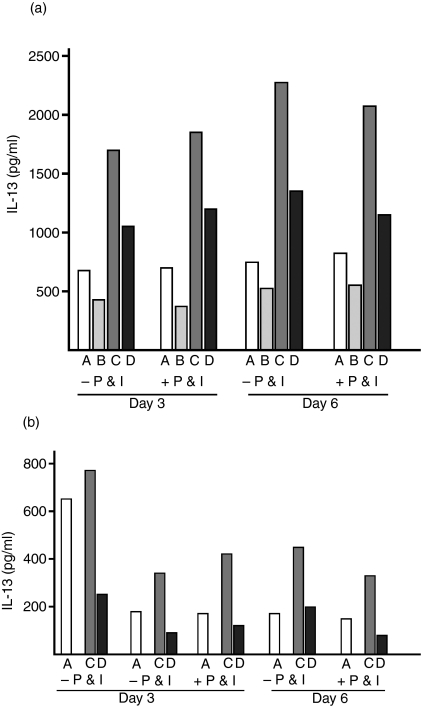
Figure 5.
Interferon (IFN)-λ1 inhibits interleukin (IL)-13 secretion under T helper type 2 (Th2)-promoting conditions. (a) Peripheral blood mononuclear cells (PBMC) were stimulated with Concanavalin-A (ConA) under the following conditions: A, ConA alone; B, ConA + IFN-λ1; C, ConA + IL-4; D, ConA + IL-4 + IFN-λ1. Cells were cultured for 3 or 6 days as shown and supernatants harvested for enzyme-linked immunosorbent assay (ELISA). In some cases, cultures were re-stimulated with phorbol 12-myristate 13-acetate (PMA) + ionomycin (P & I; see Materials and methods) before harvesting. The total time of culture was the same irrespective of re-stimulation. IFN-λ1 successfully inhibited IL-13 secretion under Th2-promoting conditions (presence of IL-4). (b) PMBC were stimulated as for (a), except that anti-CD2/CD3/CD28-conjugated beads (‘beads’) were used instead of ConA. Experimental condition B (beads + IFN-λ1) was not used. In each case (a and b), IL-13 secretion was reduced by the presence of IFN-λ1, irrespective of re-stimulation. IFN-λ1 successfully inhibited IL-13 secretion under Th2-promoting conditions (presence of IL-4). The results from three individual donors are presented. ELISA points were established in triplicate and the means are shown.
Using ConA, we found that IFN-λ1 reduced the IL-4-enhanced secretion of IL-13 by 36·1 or 37·1% and by 40·1 or 46·6% (in the absence or presence of PMA + ionomycin) on days 3 and 6, respectively. Using anti-CD2/CD3/CD28-coated beads, we found that IFN-λ1 reduced the IL-4-enhanced secretion of IL-13 by 73·2, 72·6 or 71·3% on day 3 (in the absence, absence or presence of PMA + ionomycin) and 55·5 or 74·0% on day 6 (in the absence or presence of PMA + ionomycin). Thus, IFN-λ1 was capable of down-regulating the IL-13 response even under culture conditions that supported and enhanced IL-13 production.
Discussion
The discovery of the CRF2-12 receptor chain through its similarity with the external domain of the IL-22 receptor alpha chain (G. Gallagher, unpublished observations) and its pairing with an orphan ligand described as ‘similar to type-one interferon’ led to the discovery and characterization of the interferon lambda family and their receptor in our laboratories and in parallel elsewhere.2,3 This is a new family of interferons, related to both the IL-10 family of ligands and the type I and type II interferon family. The receptor ‘alpha chain’ (CRF2-12/IL-28Ra) used by members of this new family is not used by any other known ligand, although the CRF2-4 (i.e. ‘beta’) chain is also part of the IL-10, IL-22 and IL-26 receptors.22–25
Binding of the IFN-λ1 ligand to its receptor initiates signalling reminiscent of that of the type I interferons. In particular, signal transducer and activator of transcription 2 (STAT2) is phosphorylated, ISGF3 is activated and OAS and MxA are induced.2 These observations, in conjunction with the induction of the IFN-λ1/2/3 ligands by a range of viral infections and their ability to rescue virally infected cells, have prompted a series of studies that have further investigated the antiviral properties of these cytokines. However, the IFN-λ ligands also induce the phosphorylation of STAT3, STAT4 and STAT5.2,26,27 Phosphorylation of STAT3 and STAT5 in particular suggests more complex properties for the IFN-λ ligand family; STAT3 is the signalling mechanism used by members of the IL-10 family (IL-10, IL-19, IL-20, etc.28–31) while STAT5 is used widely by cytokines such as IL-2, IL-3 and granulocyte–macrophage colony-stimulating factor (GM-CSF).32–36 STAT4 is the signature signalling molecule of the Th1 response,37–40 a process that is complemented by STAT1 signalling; thus, IFN-λ1 may be able to alter the Th1/Th2 balance. Notably, IFN-λ1 is not known to activate STAT6, ruling out an enhancing or inducing effect on the Th2 response.
We therefore decided to investigate T-cell regulation by IFN-λ1. Our previous data21 had demonstrated that IFN-λ1 is capable of down-regulating IL-13 secretion (the Th2 response). In the present report, we confirm and extend these observations in a new set of 14 donors. In our previous report,21 only five of 16 donors responded to IFN-λ1 by elevating IFN-γ secretion; in these present experiments, none did. Thus in only five of 30 donors did we observe a rise in IFN-γ with IFN-λ1 under ConA, while 29 of 30 decreased their IL-13 secretion. In the present work, we show that IFN-λ1 is capable of significantly down-regulating the secretion of three major Th2 and asthma-associated cytokines: IL-4, IL-5 and IL-13. In the present study, all donors responded with a reduction in IL-4 and IL-13 secretion, and all but one also reduced IL-5 secretion. These data strongly argue for a major role of IFN-λ1 in reducing the Th2 response overall, but, as our accompanying data suggest, its main effect may be on IL-13. As previously described, IFN-γ secretion was not affected (Fig. 4a).
This diminution in cytokine secretion was often accompanied by a reduction in mRNA levels. This reduction was significant for IL-13 at both 24 hr and 6 days, with a majority of donors responding (11/14 and 12/14, respectively), suggesting that IFN-λ1 was continuously repressing IL-13 transcription. The effect was not so prolonged for IL-4 or IL-5. A significant reduction in IL-4 mRNA levels at 24 hr was found for only eight of 14 donors, but the five (of 14) responding donors at 6 days did not generate a significant reduction overall. A similar picture emerged for IL-5, with a significant overall reduction in mRNA levels at 24 hr being derived from the individual reductions of 11 of 14 donors, while the six responders at 6 days did not produce an overall reduction in IL-5 mRNA. The reasons for these differences are unclear, but may reflect individual genetic variation or the choice of time-points examined. Overall, our data show that IFN-λ1 is acting in part by reducing Th2 cytokine transcription in activated cells; indeed, mRNA for all three cytokines was diminished at 24 hr and the effect of IFN-λ1 on IL-13 transcription was maintained even as far as 6 days after the initiation of the experiment.
In separate experiments, we examined the ability of IFN-λ1 to alter the number of cytokine-positive CD3+ CD4+ cells following ConA stimulation. All donors tested at three time-points showed a reduction of IL-13-positive cells and only one did not show a concomitant reduction in IL-4-positive CD3+ CD4+ cells, indicating that the decrease in cytokine secretion was very likely mirrored by a decrease in secreting cells; we found very few IL-5-secreting cells in these cultures at the time-points described. Thus, the FACS analysis was strongly supportive of the ELISA and mRNA data from 14 donors. In addition, it is possible that cells other than CD3+ contributed to the overall levels of secreted cytokine and mRNA (CD3+ CD4− cells did not; data not shown). These questions are both currently under investigation.
Cognisant of the fact that in allergic, atopic or asthmatic individuals an immunological environment may exist that actively supports Th2 responses, and that these responses are mediated by T cells, we investigated the role of IFN-λ1 under Th2-supporting conditions. As shown in Fig. 5, IFN-λ1 strongly diminished the secretion of IL-13 even against a background of elevated IL-13 induced by IL-4 added to the culture. This was true irrespective of whether ConA stimulation was used or the stimulation was by CD2/CD3/CD28 beads (normally considered to be a T-cell-stimulating agent). Thus, we interpret our data as showing a role for IFN-λ1 in the diminution of IL-13 production with weaker, or perhaps secondary, reductions in two other key Th2 cytokines, IL-4 and IL-5.
Previously,19–21 we have shown cytokine- and chemokine-modulating effects of IFN-λ1 on human PBMC. Chemokine up-regulation occurred prior to IFN-γ elevation and cytokine up-regulation was highly selective. Interestingly, our previous experiments on MDDC expanded in vitro suggested that IFN-λ1 may up-regulate IL-12 secretion (Fig. 5a) 21. Furthermore, the presence of IFN-λ1 in mixed lymphocyte reactions diminished IL-13 but also elevated IFN-γ, suggesting that certain stimuli may cause an elevation of the Th1 response to accompany the diminution of the Th2 response described here. The apparent stimulus-dependent action of IFN-λ1 on the Th1 response does not, however, detract from its strong Th2-reducing role described here and previously with ConA stimulation and allo-stimulation on IFN-λ1-treated MDDCs.21 Indeed, in certain circumstances it may be advantageous if a pathological Th2 response were actively diverted to be more Th1 in nature.
A greater understanding of how Th2 responses are developed and controlled may be of use in the diagnosis and treatment of human disease. In bacterial and viral pathogenesis, TOLL-like receptors recognize cell wall, capsid or nucleic acid signatures, triggering the innate immune system to drive the development of Th1 responses. Little evidence has yet been presented to suggest a parallel route to the initiation of Th2 responses; indeed, it has been shown that Th2 responses can arise almost ‘by default’ in the absence of Th1 triggering.41 Furthermore, elevated superoxide and other forms of oxidative stress can promote human Th2 responses.42 In addition, there is good evidence that helminths, for example, can act as Th2 adjuvants (e.g.43,44). The development of competent Th2 responses and the production of IL-4-secreting T cells, in particular, are very important in protecting the host from helminth infection. IL-4 is directly required for the amplification of immunoglobulin E (IgE) secretion45 and the secretion of other Th2-derived cytokines such as IL-5,46 which is important for the activation of anti-helminth defences, such as eosinophils and mast cells (e.g.47). Understanding how IL-4-secreting T cells are developed and amplified is therefore very important in the understanding of how protective immunity to helminths is elaborated.
Better knowledge generally of how to regulate Th2 responses may also be of use in the control of asthmatic and allergic responses. IL-13 and the Th2 response are recognized as key elements in the pathogenesis of bronchial asthma;48,49 their mechanisms of action and means of control are of interest as new therapeutic targets. In experimental asthma models, airway hyperreactivity accompanied by high levels of IL-4 and IL-5 secretion can be induced in intact but not IL-13−/− mice. Sensitized animals can be protected by treatment with IL-13Rα-chain constructs, although the IL-4Rα chain may be involved50–52 and unbalanced Th2 responses can lead to asthma-like phenomena.53 Recently, it was reported that asthma patients undergoing viral challenge fail to produce the levels of IFN-λ1 found in healthy individuals.54 This has led directly to speculation that IFN-λ1 may be used to treat viral exacerbation of asthma. Here, for the first time, we demonstrate that IFN-λ1 is able to diminish the secretion of all three key Th2 cytokines, IL-4, IL-5 and IL-13, the principal cytokine mediators of asthma and associated Th2 mechanisms in healthy individuals.
Elucidation of the genetics of asthma susceptibility and severity is a continuously evolving field.55–57 Cytokine polymorphisms, known to be associated with a number of autoimmune disorders,58 are regularly surveyed for their role in asthma, allergy and atopy, but more recently adhesion molecules,59,60 antimicrobial peptides,61 complement62 and the chemokine system63 have all come to be considered. Of great interest to the present study is a recent report describing associations of the human type I interferon locus on chromosome 964 with asthma. The status of IFN-λ1 in this regard is presently under investigation.
Our present data on IFN-λ1 complement recent work on IL-19. IL-19 has been shown to up-regulate human Th2 responses.65,66 While this may be a result in part of actions on developing myeloid dendritic cells,67 it is also partly a result of direct actions on naïve T cells.66 Interestingly, elevated IL-19 levels have been reported in the serum of asthma patients and administration of IL-19 up-regulated IL-13 levels in experimental animals;68 furthermore, IL-19 can induce TNF secretion from human monocyte cell lines in vitro.69 The discovery of two cytokines whose activity profile is directed towards Th2-cell regulation opens new doors for the manipulation of these responses in experimental systems and human disease.
Acknowledgments
This work was funded intramurally by HUMIGEN. All authors are employees of HUMIGEN. We are grateful to Dr M. Tevfik Dorak for statistical discussions.
References
- 1.Sheppard P, Presnell SR, Fox B, Gilbert T, Haldeman B, Grant FJ. Interferon-like protein, Zcyto21. United States Patent Application No. 002003963 April 4, 2002.
- 2.Kotenko SV, Gallagher G, Baurin VV, et al. IFN-lambdas mediate antiviral protection through a distinct class II cytokine receptor complex. Nat Immunol. 2003;4:69–77. doi: 10.1038/ni875. [DOI] [PubMed] [Google Scholar]
- 3.Sheppard P, Kindsvogel W, Xu W, et al. IL-28, IL-29 and their class II cytokine receptor IL-28R. Nat Immunol. 2003;4:63–8. doi: 10.1038/ni873. [DOI] [PubMed] [Google Scholar]
- 4.Lasfar A, Lewis-Antes A, Smirnov SV, et al. Characterization of the mouse IFN-lambda ligand-receptor system: IFN-lambdas exhibit antitumor activity against B16 melanoma. Cancer Res. 2006;66:4468–77. doi: 10.1158/0008-5472.CAN-05-3653. [DOI] [PubMed] [Google Scholar]
- 5.Bartlett NW, Buttigieg K, Kotenko SV, Smith GL. Murine interferon lambdas (type III interferons) exhibit potent antiviral activity in vivo in a poxvirus infection model. J Gen Virol. 2005;86:1589–96. doi: 10.1099/vir.0.80904-0. [DOI] [PubMed] [Google Scholar]
- 6.Meager A, Visvalingam K, Dilger P, Bryan D, Wadhwa M. Biological activity of interleukins-28 and -29: Comparison with type I interferons. Cytokine. 2005;31:109–18. doi: 10.1016/j.cyto.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Osterlund P, Veckman V, Siren J, Klucher KM, Hiscott J, Matikainen S, Julkunen I. Gene expression and antiviral activity of alpha/beta interferons and interleukin-29 in virus-infected human myeloid dendritic cells. J Virol. 2005;79:9608–17. doi: 10.1128/JVI.79.15.9608-9617.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robek MD, Boyd BS, Chisari FV. Lambda interferon inhibits Hepatitis B and C virus replication. J Virol. 2005;79:3851–4. doi: 10.1128/JVI.79.6.3851-3854.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ank N, West H, Bartholdy C, Eriksson K, Thomsen AR, Paludan SR. Lambda interferon (IFN-lambda), a type III IFN, is induced by viruses and IFNs and displays potent antiviral activity against select virus infections in vivo. J Virol. 2006;80:4501–9. doi: 10.1128/JVI.80.9.4501-4509.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 11.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–57. [PubMed] [Google Scholar]
- 12.Seder RA, Paul WE. Acquisition of lymphokine-producing phenotype by CD4+ T cells. Annu Rev Immunol. 1994;12:635–73. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- 13.Groux H, O’Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–42. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 14.Roncarolo MG, Bacchetta R, Bordignon C, Narula S, Levings MK. Type 1 T regulatory cells. Immunol Rev. 2001;182:68–79. doi: 10.1034/j.1600-065x.2001.1820105.x. [DOI] [PubMed] [Google Scholar]
- 15.Levings MK, Sangregorio R, Sartirana C, Moschin AL, Battaglia M, Orban PC, Roncarolo MG. Human CD25+ CD4+ T suppressor cell clones produce transforming growth factor beta, but not interleukin 10, and are distinct from type 1 T regulatory cells. J Exp Med. 2002;196:1335–46. doi: 10.1084/jem.20021139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levings MK, Gregori S, Tresoldi E, Cazzaniga S, Bonini C, Roncarolo MG. Differentiation of Tr1 cells by immature dendritic cells requires IL-10 but not CD25+ CD4+ Tr cells. Blood. 2005;105:1162–9. doi: 10.1182/blood-2004-03-1211. [DOI] [PubMed] [Google Scholar]
- 17.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–32. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 18.Park H, Li Z, Yang XO, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–41. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jordan WJ, Eskdale J, Boniotto M, Rodia M, Kellner D, Gallagher G. Modulation of the human cytokine response by interferon lambda-1 (IFN-lambda1/IL-29) Genes Immun. 2007;8:13–20. doi: 10.1038/sj.gene.6364348. [DOI] [PubMed] [Google Scholar]
- 20.Pekarek V, Srinivas S, Eskdale J, Gallagher G. Interferon lambda-1 (IFN-lambda1/IL-29) induces ELR(-) CXC chemokine mRNA in human peripheral blood mononuclear cells, in an IFN-gamma-independent manner. Genes Immun. 2007;8:177–80. doi: 10.1038/sj.gene.6364372. [DOI] [PubMed] [Google Scholar]
- 21.Jordan WJ, Eskdale J, Srinivas S, Pekarek V, Kelner D, Rodia M, Gallagher G. Human interferon lambda-1 (IFN-lambda1/IL-29) modulates the Th1/Th2 response. Genes Immun. 2007;8:254–61. doi: 10.1038/sj.gene.6364382. [DOI] [PubMed] [Google Scholar]
- 22.Kotenko SV, Krause CD, Izotova LS, Pollack BP, Wu W, Pestka S. Identification and functional characterization of a second chain of the interleukin-10 receptor complex. EMBO J. 1997;16:5894–903. doi: 10.1093/emboj/16.19.5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kotenko SV, Izotova LS, Mirochnitchenko OV, Esterova E, Dickensheets H, Donnelly RP, Pestka S. Identification of the functional interleukin-22 (IL-22) receptor complex: the IL-10R2 chain (IL-10Rbeta) is a common chain of both the IL-10 and IL-22 (IL-10-related T cell-derived inducible factor, IL-TIF) receptor complexes. J Biol Chem. 2001;276:2725–32. doi: 10.1074/jbc.M007837200. [DOI] [PubMed] [Google Scholar]
- 24.Donnelly RP, Sheikh F, Kotenko SV, Dickensheets H. The expanded family of class II cytokines that share the IL-10 receptor-2 (IL-10R2) chain. J Leukoc Biol. 2004;76:314–21. doi: 10.1189/jlb.0204117. [DOI] [PubMed] [Google Scholar]
- 25.Sheikh F, Baurin VV, Lewis-Antes A, et al. Cutting edge: IL-26 signals through a novel receptor complex composed of IL-20 receptor 1 and IL-10 receptor 2. J Immunol. 2004;172:2006–10. doi: 10.4049/jimmunol.172.4.2006. [DOI] [PubMed] [Google Scholar]
- 26.Dumoutier L, Lejeune D, Hor S, Fickenscher H, Renauld JC. Cloning of a new type II cytokine receptor activating signal transducer and activator of transcription (STAT)1, STAT2 and STAT3. Biochem J. 2003;370:391–6. doi: 10.1042/BJ20021935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dumoutier L, Tounsi A, Michiels T, Sommereyns C, Kotenko SV, Renauld JC. Role of the interleukin (IL)-28 receptor tyrosine residues for antiviral and antiproliferative activity of IL-29/interferon-lambda 1: similarities with type I interferon signalling. J Biol Chem. 2004;279:32269–74. doi: 10.1074/jbc.M404789200. [DOI] [PubMed] [Google Scholar]
- 28.Blumberg H, Conklin D, Xu WF, et al. Interleukin 20: discovery, receptor identification, and role in epidermal function. Cell. 2001;104:9–19. doi: 10.1016/s0092-8674(01)00187-8. [DOI] [PubMed] [Google Scholar]
- 29.Dumoutier L, Leemans C, Lejeune D, Kotenko SV, Renauld JC. Cutting edge: STAT activation by IL-19, IL-20 and mda-7 through IL-20 receptor complexes of two types. J Immunol. 2001;167:3545–9. doi: 10.4049/jimmunol.167.7.3545. [DOI] [PubMed] [Google Scholar]
- 30.Parrish-Novak J, Xu W, Brender T, et al. Interleukins 19, 20, and 24 signal through two distinct receptor complexes. Differences in receptor-ligand interactions mediate unique biological functions. J Biol Chem. 2002;277:47517–23. doi: 10.1074/jbc.M205114200. [DOI] [PubMed] [Google Scholar]
- 31.Wang M, Tan Z, Zhang R, Kotenko SV, Liang P. Interleukin 24 (MDA-7/MOB-5) signals through two heterodimeric receptors, IL-22R1/IL-20R2 and IL-20R1/IL-20R2. J Biol Chem. 2002;277:7341–7. doi: 10.1074/jbc.M106043200. [DOI] [PubMed] [Google Scholar]
- 32.Beadling C, Guschin D, Witthuhn BA, Ziemiecki A, Ihle JN, Kerr IM, Cantrell DA. Activation of JAK kinases and STAT proteins by interleukin-2 and interferon alpha, but not the T cell antigen receptor, in human T lymphocytes. EMBO J. 1994;13:5605–15. doi: 10.1002/j.1460-2075.1994.tb06898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Azam M, Erdjument-Bromage H, Kreider BL, et al. Interleukin-3 signals through multiple isoforms of Stat5. EMBO J. 1995;14:1402–11. doi: 10.1002/j.1460-2075.1995.tb07126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gouilleux F, Pallard C, Dusanter-Fourt I, et al. Prolactin, growth hormone, erythropoietin and granulocyte-macrophage colony stimulating factor induce MGF-Stat5 DNA binding activity. EMBO J. 1995;14:2005–13. doi: 10.1002/j.1460-2075.1995.tb07192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hou J, Schindler U, Henzel WJ, Wong SC, McKnight SL. Identification and purification of human Stat proteins activated in response to interleukin-2. Immunity. 1995;2:321–9. doi: 10.1016/1074-7613(95)90140-x. [DOI] [PubMed] [Google Scholar]
- 36.Mui AL, Wakao H, O’Farrell AM, Harada N, Miyajima A. Interleukin-3, granulocyte-macrophage colony stimulating factor and interleukin-5 transduce signals through two STAT5 homologs. EMBO J. 1995;14:1166–75. doi: 10.1002/j.1460-2075.1995.tb07100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jacobson NG, Szabo SJ, Weber-Nordt RM, Zhong Z, Schreiber RD, Darnell JE, Jr, Murphy KM. Interleukin 12 signalling in T helper type 1 (Th1) cells involves tyrosine phosphorylation of signal transducer and activator of transcription (Stat)3 and Stat4. J Exp Med. 1995;181:1755–62. doi: 10.1084/jem.181.5.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cho SS, Bacon CM, Sudarshan C, Rees RC, Finbloom D, Pine R, O’Shea JJ. Activation of STAT4 by IL-12 and IFN-alpha: evidence for the involvement of ligand-induced tyrosine and serine phosphorylation. J Immunol. 1996;157:4781–9. [PubMed] [Google Scholar]
- 39.Thierfelder WE, van Deursen JM, Yamamoto K, et al. Requirement for Stat4 in interleukin-12-mediated responses of natural killer and T cells. Nature. 1996;382:171–4. doi: 10.1038/382171a0. [DOI] [PubMed] [Google Scholar]
- 40.Murphy KM, Ouyang W, Szabo SJ, Jacobson NG, Guler ML, Gorham JD, Gubler U, Murphy TL. T helper differentiation proceeds through Stat1-dependent, Stat4-dependent and Stat4-independent phases. Curr Top Microbiol Immunol. 1999;238:13–26. doi: 10.1007/978-3-662-09709-0_2. [DOI] [PubMed] [Google Scholar]
- 41.Jankovic D, Kullberg MC, Hieny S, Caspar P, Collazo CM, Sher A. In the absence of IL-12, CD4(+) T cell responses to intracellular pathogens fail to default to a Th2 pattern and are host protective in an IL-10(-/-) setting. Immunity. 2002;16:429–39. doi: 10.1016/s1074-7613(02)00278-9. [DOI] [PubMed] [Google Scholar]
- 42.King MR, Ismail AS, Davis LS, Karp DR. Oxidative stress promotes polarization of human T cell differentiation toward a T helper 2 phenotype. J Immunol. 2006;176:2765–72. doi: 10.4049/jimmunol.176.5.2765. [DOI] [PubMed] [Google Scholar]
- 43.Holland MJ, Harcus YM, Riches PL, Maizels RM. Proteins secreted by the parasitic nematode Nippostrongylus brasiliensis act as adjuvants for Th2 responses. Eur J Immunol. 2000;30:1977–87. doi: 10.1002/1521-4141(200007)30:7<1977::AID-IMMU1977>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 44.Liu Z, Liu Q, Pesce J, et al. Nippostrongylus brasiliensis can induce B7-independent antigen-specific development of IL-4-producing T cells from naive CD4 T cells in vivo. J Immunol. 2002;169:6959–68. doi: 10.4049/jimmunol.169.12.6959. [DOI] [PubMed] [Google Scholar]
- 45.Lebman DA, Coffman RL. Interleukin 4 causes isotype switching to IgE in T cell-stimulated clonal B cell cultures. J Exp Med. 1988;168:853–62. doi: 10.1084/jem.168.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu Y, Chen L, Huang Z, Alkan S, Bunting KD, Wen R, Wang D, Huang H. Cutting edge: IL-5 primes Th2 cytokine-producing capacity in eosinophils through a STAT5-dependent mechanism. J Immunol. 2004;173:2918–22. doi: 10.4049/jimmunol.173.5.2918. [DOI] [PubMed] [Google Scholar]
- 47.Ustun S, Turgay N, Delibas SB, Ertabaklar H. Interleukin (IL) 5 levels and eosinophilia in patients with intestinal parasitic diseases. World J Gastroenterol. 2004;10:3643–6. doi: 10.3748/wjg.v10.i24.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang SK, Xiao HQ, Kleine-Tebbe J, Paciotti G, Marsh DG, Lichtenstein LM, Liu C. IL-13 expression at the sites of allergen challenge in patients with asthma. J Immunol. 1995;155:2688–94. [PubMed] [Google Scholar]
- 49.Humbert M, Durham SR, Kimmitt P, et al. Elevated expression of messenger ribonucleic acid encoding IL-13 in the bronchial mucosa of atopic and nonatopic subjects with asthma. J Allergy Clin Immunol. 1997;99:657–65. doi: 10.1016/s0091-6749(97)70028-9. [DOI] [PubMed] [Google Scholar]
- 50.Wills-Karp M, Chiaramonte M. Interleukin-13 in asthma. Curr Opin Pulm Med. 2003;9:21–7. doi: 10.1097/00063198-200301000-00004. [DOI] [PubMed] [Google Scholar]
- 51.Ray A, Cohn L. Th2 cells and GATA-3 in asthma: new insights into the regulation of airway inflammation. J Clin Invest. 1999;104:985–93. doi: 10.1172/JCI8204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mattes J, Yang M, Siqueira A, et al. IL-13 induces airways hyperreactivity independently of the IL-4R alpha chain in the allergic lung. J Immunol. 2001;167:1683–92. doi: 10.4049/jimmunol.167.3.1683. [DOI] [PubMed] [Google Scholar]
- 53.Finotto S, Hausding M, Doganci A, Maxeiner JH, Lehr HA, Luft C, Galle PR, Glimcher LH. Asthmatic changes in mice lacking T-bet are mediated by IL-13. Int Immunol. 2005;17:993–1007. doi: 10.1093/intimm/dxh281. [DOI] [PubMed] [Google Scholar]
- 54.Contoli M, Message SD, Laza-Stanca V, et al. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat Med. 2006;12:1023–6. doi: 10.1038/nm1462. [DOI] [PubMed] [Google Scholar]
- 55.Ober C, Hoffjan S. Asthma genetics 2006: the long and winding road to gene discovery. Genes Immun. 2006;7:95–100. doi: 10.1038/sj.gene.6364284. [DOI] [PubMed] [Google Scholar]
- 56.Piavaux B, Jeurink PV, Groot PC, Hofman GA, Demant P, Van Oosterhout AJ. Mouse genetic model for antigen-induced airway manifestations of asthma. Genes Immun. 2007;8:28–34. doi: 10.1038/sj.gene.6364354. [DOI] [PubMed] [Google Scholar]
- 57.Benson M, Carlsson L, Guillot G, Jernås M, Langston MA, Rudemo M, Andersson B. A network-based analysis of allergen-challenged CD4+ T cells from patients with allergic rhinitis. Genes Immun. 2006;7:514–21. doi: 10.1038/sj.gene.6364322. [DOI] [PubMed] [Google Scholar]
- 58.Hollegaard MV, Bidwell JL. Cytokine gene polymorphism in human disease: on-line databases, Supplement 3. Genes Immun. 2006;7:269–76. doi: 10.1038/sj.gene.6364301. [DOI] [PubMed] [Google Scholar]
- 59.Zhu G, Carlsen K, Carlsen KH, et al. Polymorphisms in the endothelin-1 (EDN1) are associated with asthma in two populations. Genes Immun. 2008;9:23–9. doi: 10.1038/sj.gene.6364441. [DOI] [PubMed] [Google Scholar]
- 60.Puthothu B, Krueger M, Bernhardt M, Heinzmann A. ICAM1 amino-acid variant K469E is associated with paediatric bronchial asthma and elevated sICAM1 levels. Genes Immun. 2006;7:322–6. doi: 10.1038/sj.gene.6364302. [DOI] [PubMed] [Google Scholar]
- 61.Leung TF, Li CY, Liu EK, Tang NL, Chan IH, Yung E, Wong GW, Lam CW. Asthma and atopy are associated with DEFB1 polymorphisms in Chinese children. Genes Immun. 2006;7:59–64. doi: 10.1038/sj.gene.6364279. [DOI] [PubMed] [Google Scholar]
- 62.Barnes KC, Grant AV, Baltadzhieva D, et al. Variants in the gene encoding C3 are associated with asthma and related phenotypes among African Caribbean families. Genes Immun. 2006;7:27–35. doi: 10.1038/sj.gene.6364267. [DOI] [PubMed] [Google Scholar]
- 63.Tremblay K, Lemire M, Provost V, et al. Association study between the CX3CR1 gene and asthma. Genes Immun. 2006;7:632–9. doi: 10.1038/sj.gene.6364340. [DOI] [PubMed] [Google Scholar]
- 64.Chan A, Newman DL, Shon AM, Schneider DH, Kuldanek S, Ober C. Variation in the type I interferon gene cluster on 9p21 influences susceptibility to asthma and atopy. Genes Immun. 2006;7:169–78. doi: 10.1038/sj.gene.6364287. [DOI] [PubMed] [Google Scholar]
- 65.Gallagher G, Dickensheets H, Eskdale J, et al. Cloning, expression and initial characterization of interleukin-19 (IL-19), a novel homologue of human interleukin-10 (IL-10) Genes Immun. 2000;1:442–50. doi: 10.1038/sj.gene.6363714. [DOI] [PubMed] [Google Scholar]
- 66.Oral HB, Kotenko SV, Yilmaz M, Mani O, Zumkehr J, Blaser K, Akdis CA, Akdis M. Regulation of T cells and cytokines by the interleukin-10 (IL-10)-family cytokines IL-19, IL-20, IL-22, IL-24 and IL-26. Eur J Immunol. 2006;36:380–8. doi: 10.1002/eji.200425523. [DOI] [PubMed] [Google Scholar]
- 67.Gallagher G, Eskdale J, Jordan W, et al. Human interleukin-19 and its receptor: a potential role in the induction of Th2 responses. Int Immunopharmacol. 2004;4:615–26. doi: 10.1016/j.intimp.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 68.Liao SC, Cheng YC, Wang YC, et al. IL-19 Th2 cytokines and was up-regulated in asthma patients. J Immunol. 2004;173:6712–8. doi: 10.4049/jimmunol.173.11.6712. [DOI] [PubMed] [Google Scholar]
- 69.Zhong H, Wu Y, Berlardinelli L, Zeng D. A2B adenosine receptors induce IL-19 from bronchial epithelial cells, resulting in TNF-alpha increase. Am J Respir Cell Mol Biol. 2006;35:587–92. doi: 10.1165/rcmb.2005-0476OC. [DOI] [PubMed] [Google Scholar]