Abstract
In normal conditions intestinal epithelial cells (IECs) constitutively stimulate regulatory CD4+ T cells. However, in Crohn's disease (CD), this major histocompatibility complex (MHC) class II-restricted antigen presentation results in stimulation of proinflammatory CD4+ T cells. We hypothesized that these alternative functions might be mediated by differential sorting and processing of antigens into distinct MHC II-enriched compartments (MIICs). Accordingly, we analysed the endocytic pathways of lumenally applied ovalbumin (OVA) in IECs of the jejunum and ileum of wild-type (WT) and TNFΔARE/WT mice that develop a CD-resembling ileitis. Using quantitative reverse transcription polymerase chain reaction, we found that messenger RNA levels of interferon-γ, tumour necrosis factor-α, interleukin-17 and interleukin-10 were significantly up-regulated in the inflamed ileum of TNFΔARE/WT mice, confirming CD-like inflammation. Fluorescence and immunoelectron microscopy revealed the presence of MHC II and invariant chain throughout the late endocytic compartments, with most molecules concentrated in the multivesicular bodies (MVB). OVA was targeted into MVB and, in contrast to other MIICs, accumulated in these structures within 120 min of exposure. The IEC-specific A33 antigen localized to internal vesicles of MVB and A33/class II-bearing exosomes were identified in intercellular spaces. Remarkably, the expression pattern of MHC II/invariant chain molecules and the trafficking of OVA were independent of mucosal inflammation and the specific region in the small intestine. MVB seem to be principally responsible for class II-associated antigen processing in IECs and to constitute the origin of MHC II-loaded exosomes. The distinctive functions of IECs in antigen presentation to CD4+ T cells might arise as a result of differential processing within the MVB identified here.
Keywords: antigen traffic, exosomes, inflammatory bowel disease, intestinal epithelial cells, major histocompatibility complex molecules
Introduction
Increasing evidence suggests an active role of intestinal epithelial cells (IECs) in sampling lumenal antigens and regulating associated T-cell responses as non-professional antigen-presenting cells (APCs).1 The functional outcome of antigen presentation by IECs differs depending on the influence of inflammatory stimuli. Recent in vivo data showed IEC-mediated induction of CD4+ T cells with a regulatory phenotype under constitutive conditions.2 In contrast, inflammatory stimuli enable IECs to activate CD4+ T cells that proliferate and secrete a panel of proinflammatory cytokines.3,4 In this context, IECs might be involved in the perpetuation/aggravation of mucosal inflammation in Crohn's disease (CD), which is critically driven by an aberrant activation of CD4+ T cells.5 However, the underlying mechanisms that determine the pathophysiologically relevant consequences of major histocompatibility complex class II (MHC II) -related antigen presentation by IECs to CD4+ T cells remain unclear.
Numerous studies on professional APCs, especially dendritic cells, have yielded detailed insights into the mechanisms and regulation of antigen processing and MHC II/peptide loading.6–8 These processes are proposed to occur in specialized compartments of the endocytic tract, referred to as MHC II-enriched compartments (MIICs). The current data indicate that MIICs represent a heterogeneous set of structures, evident by morphology and also by functional criteria such as processing of endocytic tracers. The different morphology of MIICs corresponds to multivesicular bodies (MVB), multilamellar bodies (MLB) and electron-dense bodies (EDB). They accumulate MHC II proteins and related molecules such as invariant chain (Ii) and are characterized as late endocytic structures by an acidic pH, the presence of proteases and the expression of lysosome-associated membrane proteins (LAMPs). The IECs constitutively express MHC II along with molecules necessary for antigen loading, e.g. Ii.9,10 Elevated levels of MHC II are observed in the context of mucosal inflammation such as CD. In line with professional APCs, MHC II-restricted antigen presentation to CD4+ T cells by IECs was suggested to require antigen sorting into acidic compartments and the activity of acidic hydrolases, basic features of MIICs.4 In previous studies, we demonstrated the passage of orally fed ovalbumin (OVA) into class II-containing late endocytic structures in the non-inflamed jejunum of mice.11 Remarkably, late endocytic targeting of OVA in jejunal IECs was not detected in germ-free mice but could be reconstituted by low doses of interferon-γ (IFN-γ), providing the first hint that trafficking of exogenous antigens in IECs might be influenced by cytokines.12 Despite these initial results, a comprehensive, detailed characterization of MIICs in the epithelial cells of the small intestine, particularly in the context of inflammatory pathophysiology, is lacking.
Although the functional consequences remain inconclusive, recent data indicate that class II-restricted antigen presentation by IECs may not be confined to direct cell–cell contact with CD4+ T cells.13–15 MHC II/peptide-loaded exosomes have been shown to be released by IECs and to exert immunogenic properties, for example, via dendritic cell-mediated presentation.16 Tolerogenic effects of in vitro and in vivo obtained exosomes were demonstrated as well as the priming for an immunogenic response in the context of a systemic challenge. The generation and subcellular origin of exosomes derived from IECs remains to be defined. Class-II bearing exosomes shed by professional APCs such as dendritic cells were found to originate intracellularly from MVB.17 Carrying MHC II–peptide complexes these were capable of directly stimulating CD4+ T-cell proliferation in vitro.18 These data further strengthen the pivotal role of MIICs in MHC II-associated antigen presentation.
We assumed that differences in the nature of MIICs or their accessibility to antigens might contribute to the different competences of IECs to act as APCs. The present study provides a systematic analysis of MIICs within IECs by light and electron microscopy. In addition to the morphological characterization, we focused on their intersections with endocytic routes of a lumenal tracer protein and possible function as a source of epithelial derived exosomes in the gut. To unravel potential inflammation-related differences in these processes in vivo, we used TNFΔARE mice, which spontaneously develop a transmural ileitis resembling the inflammation in CD.19 Both proximal and distal areas of the small intestine were investigated and compared to unravel region-specific differences that might predispose to the typical presence of CD in the terminal ileum. Our results yield insights into the nature of MIICs in IECs and their antigen uptake, the early steps in antigen presentation, in both the healthy and diseased states.
Materials and methods
Murine ileitis model – TNFΔARE/WT mice
Heterozygous TNFΔARE/WT and control wild-type (WT; C57/129SvEv) mice were a generous gift from G. Kollias (Biomedical Sciences Research Center Alexander Fleming, Vari, Athens, Greece). These mice are characterized by a deletion of the tumour necrosis factor (TNF) AU-rich elements that affect TNF messenger RNA (mRNA) destabilization and translational repression in haemopoietic and stromal cells.19 As a consequence, mice develop a transmural ileal inflammation with characteristic features of CD detectable from around 8 weeks. TNFΔARE/WT and WT mice used for the experiments were 12 weeks old. Mice were kept in a specific pathogen-free environment and maintained on a OVA-free diet. The animal studies were conducted according to the Animal Welfare Act and received approval from the local authorities (23/p/04).
RNA extraction and reverse transcription
Total RNA was extracted from at least six identical samples per group (jejunum versus ileum; TNFΔARE/WT and controls) using the RNeasy Minikit (Qiagen, Hilden, Germany) following the manufacturer’s instructions. 1 μg of extracted RNA was digested using DNAse 1 (Sigma, Taufkirchen, Germany). Reverse transcription was performed using Superscript II reverse transcriptase (Invitrogen, Karlsruhe, Germany) and random hexamers according to the manufacturer’s instructions.
Real-time quantitative polymerase chain reaction
Taq-man assays for different cytokines and the housekeeping gene MLN 51 were designed using primerexpress software (Applied Biosystems, Foster City, CA) and synthesized by MWG Biotech AG (Ebersberg, Germany). Taq-man probes were labelled with FAM as reporter and TAMRA as quencher. Optimized runs were performed on an ABI 7000 sequence detection system (Applied Biosystems) and started with 10 min at 95° followed by 50 cycles with 15 seconds at 95° and 1 min at 60°. Polymerase chain reactions (PCRs) were performed under the following conditions: 900 nm of each primer, 200 nm of the respective probe, 2 μl of sample cDNA, 12·5 μl of PCR master mix (Eurogentec, Seraing, Belgium) and 10·9 μl diethylpyrocarbonate-treated water to give a final volume of 25 μl. Samples and no template controls were included in each run as duplexes and each run was performed twice. Ten-fold dilutions of cDNA were subjected to PCR and standard curves were generated based on these results using PCR software (Applied Biosystems) to monitor the kinetics of the reactions.
Relative gene expression of cytokines was calculated on the base of Ct values of two independently performed PCR runs (ratio = 2Ct EF−1a−Ct vimentin). Ratios represent relative gene expressions and are displayed as copies per 100 copies of MLN 51. For statistical evaluation data from groups of six mice were compared using the Mann–Whitney–Wilcoxon rank sum test. Statistical significance was assumed at P ≤ 0·05 level.
In vivo administration of antigen into jejunal or ileal loops
Mice were anaesthetized for up to 2 hr with repeated intraperitoneal applications of ketamin (Pfizer, Karlsruhe, Germany) and xylazin (Bayer, Leverkusen, Germany). After laparotomy, small bowel loops were prepared in either the jejunum or the terminal ileum by placing proximal and distal ligatures about 2 cm apart with the mesenteric circulation left intact. Solution of OVA (10 mg/ml; 0·1 ml in saline, fraction V, Sigma) was injected into the loops through a loose ligature, which was tightened as the needle was withdrawn. After allowing OVA uptake to proceed for 10, 30, 60 or 120 min, specimens were obtained from antigen-incubated tissue, washed in saline and fixed in formaldehyde for immunoelectron processing. Loops filled with saline alone served as controls. Mice were killed at the end of the experiment under dense anaesthesia.
Antibodies
The primary antibodies used were: affinity-purified polyclonal rabbit antibodies against OVA,11,12 Ii (gift from N. Barois, Department of Molecular Cell Biology, University of Oslo, Norway)20 and murine A33 (gift from J. Heath, Ludwig Institute for Cancer Research, Melbourne, Australia);21 monoclonal rat antibodies against LAMP-1 (clone 1D4B; BD Bioscience PharMingen, Hamburg, Germany) and MHC II (clone CD311; gift from D. Kaiserlian, Inserm, Lyon, France).22 The specificity of our polyclonal OVA antibodies has been demonstrated by preincubation of OVA antibodies with OVA in a previous project.12 Gold-conjugated goat antisera against rabbit and rat immunoglobulin G (IgG; 6 or 12 nm; Dianova, Hamburg, Germany) were used to visualize the binding sites of the primary antibodies in immunoelectron microscopy. For immunofluorescence microscopy, Alexa-Fluor 488- and 555-conjugated goat antisera against rabbit and rat IgG (Molecular Probes, Eugene, OR) were used.
Light microscopy
For histopathological examination jejunal and ileal biopsies from TNFΔARE/WT and WT mice were embedded in paraffin. Sections were stained with haematoxylin and eosin and analysed using a Zeiss Axiophot microscope (Jena, Germany). For immunofluorescence analysis, specimens were frozen in liquid nitrogen. Air-dried sections (6 μm) were fixed in acetone/methanol (1 : 1) and successively incubated with primary antibodies, appropriate Alexa Fluor-conjugates and bis-benzimide (Sigma) for 60 min each. Double-labelling was performed simultaneously, using primary antibodies derived from different species coupled with the appropriate fluorochrome-conjugates. Cross-reactivity of antibodies applied in double-labelling experiments was excluded. Analysis of fluorochrome-labelled sections was carried out using a Zeiss LSM 510 Meta confocal microscope.
Immunoelectron microscopy
Cryosectioning and immunogold-labelling of ultrathin sections was carried out according to the post-embedding technique of Griffiths.23 Jejunal and ileal specimens from TNFΔARE/WT and WT mice were fixed in 5% formaldehyde, cryoprotected in 0·03 m polyvinylpyrrolidone/1.6 m sucrose and frozen in liquid nitrogen. Ultrathin cryosections (60 nm) were incubated with primary and appropriate gold-conjugated secondary antibodies for 45 min each. Labelled sections were contrasted with 4% uranyl acetate, embedded in 2% methylcellulose and examined using a Philips EM 400 T transmission electron microscope (Kassel, Germany).
Quantification of MHC II and invariant chain labelling in the ileal epithelium
The distribution of the subcellular labelling for MHC II and Ii in IECs was determined in ileal specimens of three TNFΔARE/WT and WT mice, respectively. Quantification was performed according to the technique of Lucocq et al.24 by an observer unaware of any information regarding the mice. Labelling was analysed on ultrathin sections of two grids per mouse, double-labelled for MHC II and Ii. On each grid, 100 gold particles per antigen were counted and assigned to the different subcellular compartments in IECs. The gold counts assessed were used to construct a ranking of the labelling distribution. These results were presented as mean percentage (± SD) of gold particles for each compartment.
Results
Transmural ileitis resembling Crohn's disease ileitis in TNFΔARE/WT mice
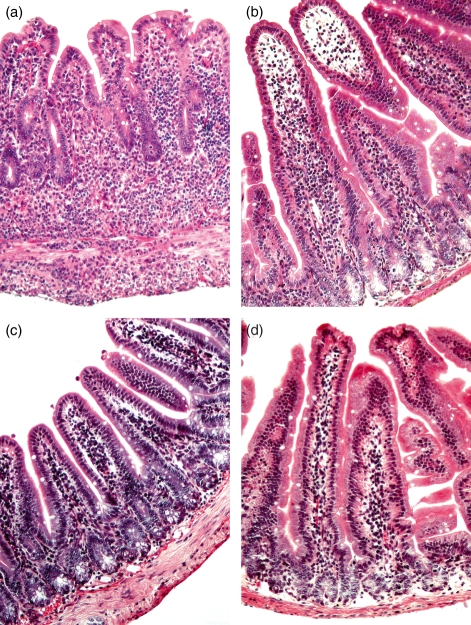
Histopathological examination of jejunal and ileal specimens was carried out in all the mice studied (data not shown). In contrast to WT mice, ileal specimens of TNFΔARE/WT mice showed signs of transmural inflammation (Fig. 1a): villi were blunted, broadened and distorted. Marked inflammatory infiltrates were seen in all layers of the bowel wall. Infiltrates were composed of mononuclear leucocytes, plasma cells and scattered neutrophils. Non-caseating granulomas were occasionally detected. In contrast, the jejunum of TNFΔARE/WT mice, as well as ileal or jejunal tissue of WT mice displayed a regular morphology without any signs of pathological inflammation (Fig. 1b–d).
Figure 1.
TNFΔARE/wild-type (WT) mice spontaneously develop a transmural ileitis. Sections were made of jejunal and ileal specimens from TNFΔARE/WT and WT mice and stained with haematoxylin & eosin. (a) The ileum of TNFΔARE/WT mice showed signs of severe inflammation affecting all layers of the bowel wall. In contrast jejunal biopsies of TNFΔARE/WT (b) and the ileum (c) and jejunum (d) of WT mice revealed a regular histology.
Expression levels of proinflammatory cytokines are up-regulated in the ileum of TNFΔARE/WT mice
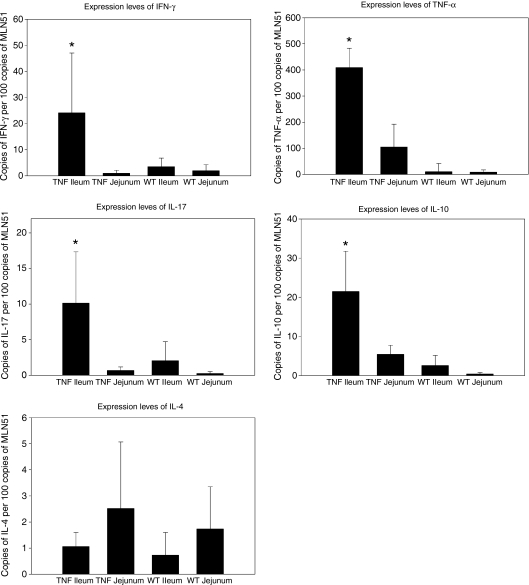
Cytokine profiles were assessed for two reasons. First, to confirm CD-like inflammatory conditions within the ileum of TNFΔARE/WT mice and second, to search for differences in the expression levels of cytokines that have been shown to influence the endocytic trafficking of exogenous antigens in vitro, such as IFN-γ, interleukin-4 (IL-4) and IL-10. Interferon-γ, TNF-α and IL-17 are key mediators of the inflammatory processes in CD. Messenger RNAs for all these proinflammatory cytokines were significantly increased in the inflamed tissue of the TNFΔARE/WT ileum compared to the other groups examined (Fig. 2). The regulatory cytokine IL-10 was also significantly up-regulated in the course of ileitis. Significant differences in the mRNA expression levels for TNF-α and IL-10 were also detected in the TNFΔARE/WT jejunum when compared to the jejunum in WT controls. In contrast, the expression levels of IL-4 mRNA did not change significantly whether the specimen was derived from the jejunum or ileum, or whether the tissue was healthy or inflamed.
Figure 2.
Cytokine expression levels in the healthy and inflamed small bowel. Tissue expression levels of interferon-γ (IFN-γ), tumour necrosis factor-α (TNF-α), interleukin-17 (IL-17), IL-10 and IL-4 were separately assessed in jejunal and ileal specimens of wild-type (WT) and TNFΔARE/WT (TNF) mice. Specimens from at least six mice were analysed by real-time quantitative polymerase chain reaction for each strain and locality. Results are shown as mean ± SD. The inflamed ileum of TNFΔARE/WT mice revealed a significant increase in the expression levels of all cytokines examined (indicated by asterisks), except for IL-4. A significant difference was assumed when P ≤ 0·05.
Multivesicular bodies of IECs accumulate MHC II and invariant chain molecules
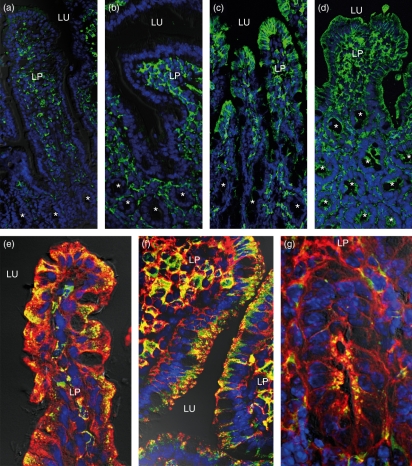
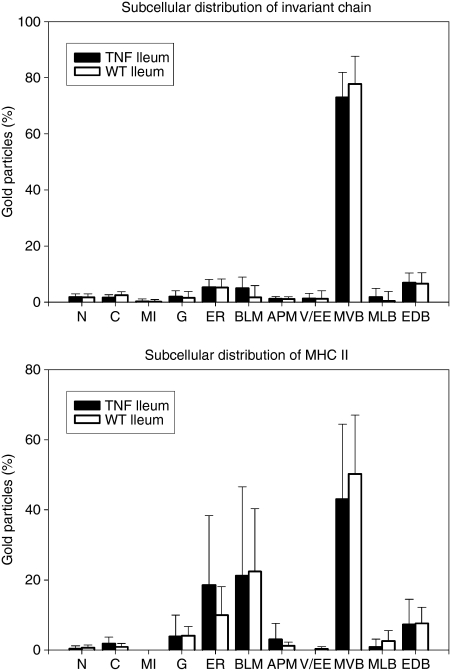
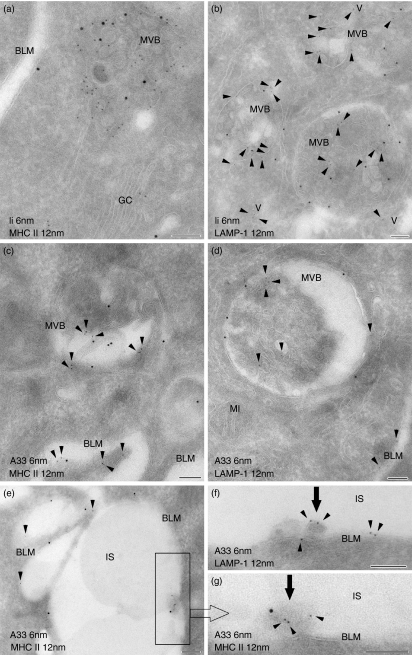
Immunofluorescence staining was used to assess regional differences in the epithelial expression of MHC II (Fig. 3) and Ii (not shown). Staining for both molecules was almost undetectable within the jejunal epithelium of control WT (Fig. 3a) and TNFΔARE/WT (Fig. 3b) mice. Faint expression was only rarely seen and was restricted to the top of the jejunal villi. In contrast, constitutive expression of MHC II and Ii molecules was found in villus IECs of the ileum. While staining in crypt IECs was absent or faint in WT mice (Fig. 3c), it was markedly up-regulated in ileitis (Fig. 3d). Double-labelling for MHC II and Ii revealed a granular pattern of colocalization in the supranuclear part of villus IECs in the healthy (Fig. 3e) and inflamed (Fig. 3f) mucosa. This granular staining was analogous to that seen in IECs of crypts in the course of ileitis (Fig. 3g). Immunoelectron microscopy was performed to further characterize and quantify the subcellular expression of MHC II and Ii within ileal IECs (Fig. 4). Additional labelling for LAMP-1 was used as a marker for late endocytic compartments. On systematically sampled images of labelled cells, the relative numbers of gold particles associated with each organelle of interest was quantified. Gold particles corresponding to the localization of MHC II and Ii were found at different intensities throughout the endocytic tract. Faint labelling for both proteins was associated with small LAMP-1-negative endosomes situated near the apical membrane, which are likely to correspond to early endosomes. In contrast, abundant levels of MHC II molecules were located in supranuclear MVB (Figs 4 and 5a). Small amounts were further detected in EDB and MLB. In line with MHC II, the majority of Ii molecules was localized in MVB (Figs 4 and 5a,b), while only small amounts were found in MLB and EDB. Double-labelling for MHC II and Ii confirmed strong colocalization for both molecules in MVB of IECs (Fig. 5a). Both molecules were expressed on the limiting membranes and internal vesicles of these structures. Whereas MLB and EDB consistently showed labelling for LAMP-1, MVB were in part found to be LAMP-1-negative (Fig. 5b). Cell surface expression of MHC II was highly polarized and predominated at the basolateral membrane. Ii molecules were only occasionally seen on cell surface membranes. Remarkably this pattern of subcellular labelling for MHC II and Ii molecules in ileal IECs was similar in TNFΔARE/WT and control mice, even in the villus and crypt IECs in inflamed mucosa.
Figure 3.
Expression of major histocompatibility complex class II (MHC II) and invariant chain (Ii) in the healthy and inflamed small bowel. Cryosections were labelled for MHC II (green) (a–d) or double-labelled for MHC II (red) and Ii (green) (e–g). The blue fluorescence stained nuclei. WT mice (a) and TNFΔARE/WT mice (b) lacked epithelial staining for MHC II in the jejunum. In contrast, both strains showed MHC II expression in intestinal epithelial cells (IECs) of ileal villi: (c) WT, (d) TNFΔARE/WT mice. The inflamed ileum of TNFΔARE/WT mice showed blunted villi and the expression of MHC II was extended to the epithelium of crypts (asterisks). The epithelial staining for MHC II was predominantly localized at the basolateral membranes and within supranuclear granules. Cells of the lamina propria (LP) revealed MHC II staining in the jejunum and ileum, independent of mucosal inflammation. The merged images (e–g) show colocalization for MHC II and Ii staining (yellow) within the supranuclear granules of IECs. Colocalization was seen in the villi of healthy (e) and inflamed (f) mucosa and was also identified in crypts during ileitis (g). LU, Lumen.
Figure 4.
The subcellular expression of major histocompatibility complex type II (MHC II) and invariant chain (Ii) in intestinal epithelial cells. The subcellular distribution of MHC II and Ii in ileal IECs was assessed on immunogold-labelled ultrathin sections as described in detail in the Materials and methods. The percentages of total gold particles corresponding to MHC II and Ii molecules within the different subcellular compartments were determined and are presented as mean ± SD of three wild-type (WT) and three TNFΔARE/WT (TNF) mice. Both proteins were found within the biosynthetic pathway and throughout the endocytic tract. In the healthy and inflamed ileum the majority was consistently seen in multivesicular bodies (MVB). Cell surface labelling for MHC II was largely associated with basolateral membranes (BLM). In contrast, labelling for Ii was only faintly detected at cell surface membranes. Labelling in mitochondria (MI), nuclei (N) and the cytosol (C) represents background and was negligible. G, Golgi complex; ER, endoplasmic reticulum; APM, apical membrane; V/EE, vesicle/early endosomes; MLB, multilamellar bodies; EDB, electron-dense bodies.
Figure 5.
The A33 antigen is localized in multivesicular bodies, enriched in major histocompatibility complex class II (MHC II) and invariant (Ii) chain molecules. MHC II, invariant chain (Ii), lysosome-associated membrane protein-1 (LAMP-1) and the A33 antigen were immunogold-labelled on ultrathin sections using 6-nm and 12-nm gold particles. Sections of ileal (a–c,e,g) and jejunal (d,f) mucosa obtained from wild-type (WT) (b) and TNFΔARE/WT (a,c–f) mice are shown. Double-labellings for Ii/MHC II (a) and Ii/LAMP-1 (b) showed strong colocalization of these molecules in the multivesicular bodies (MVB) of intestinal epithelial cells (IECs; Ii depicted by arrowheads, b). Labelling for MHC II and Ii was identified on the limiting membranes and on internal vesicles of the MVB. Ii was additionally detected in vesicles (V), small MVB lacking LAMP-1 and within the Golgi complex (GC). Labelling for the A33 antigen (arrowheads) was regularly identified on vesicles of MVB which further displayed labelling for MHC II (c) and LAMP-1 molecules (d). Cell surface expression of the A33 antigen (c–e) and MHC II molecules (a,c,e) was seen at the basolateral membrane (BLM). A33 was additionally detected on exosomes (arrows) in the intercellular spaces (IS) of the epithelium. These exosomes predominantly lacked LAMP-1 (f) but carried MHC II proteins (g, inset of e). MI, mitochondrium. Bars = 100 nm.
Lumenally applied OVA is efficiently sorted into multivesicular bodies
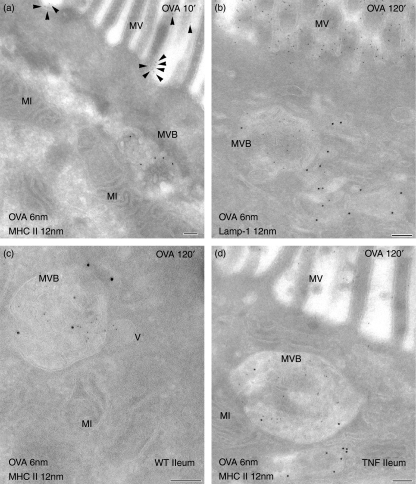
The epithelial uptake of the model protein antigen OVA was studied in vivo over different periods up to 2 hr. Using double-labellings with either LAMP-1 or MHC II its subcellular trafficking was analysed by immunoelectron microscopy in jejunal and ileal mucosa of TNFΔARE/WT and control mice. Within 10 min of exposure, OVA had passed through the epithelial barrier and was seen on microvilli and within the intercellular spaces of the epithelium and the lamina propria. Internalized OVA was found associated with LAMP-1-negative early endosomes of IECs. These endosomes were seen adjacent to the apical membranes and occasionally showed MHC II labelling. While OVA was not detectable in late endocytic compartments at 10 min (Fig. 6a), it inconsistently accessed these structures to a small extent after 30 min. At the later periods (60 and 120 min), it accumulated in class-II containing MVB (Fig. 6b–d). In contrast, only faint labelling for OVA was seen in MLB and EDB at these periods. Specimens taken from saline-injected loops did not show any labelling for OVA. The traffic routes of OVA in IECs, in particular its accumulation within MVB, were similar in jejunal and ileal tissues and were not altered in ileitis. In addition, no differences were found regarding the kinetics of the epithelial OVA transport. Table 1 summarizes the endocytic pathway of OVA in IECs, representative for all mice examined, analysing at least 100 IECs per mouse and per intestinal segment.
Figure 6.
Lumenally administered ovalbumin accumulates in major histocompatibility complex class II (MHC II) -positive multivesicular bodies. Ultrathin cryosections were immunogold-labelled for ovalbumin (OVA), lysosome-associated membrane protein-1 (LAMP-1) and MHC II and binding sites were visualized by 6-nm or 12-nm gold particles. Sections were prepared of ileal (a,c,d) and jejunal (b) tissue taken from wild-type (WT) (a,c) and TNFΔARE/WT (TNF) (b,d) mice. (a) Ten minutes after lumenal injection, OVA (arrowheads) was localized on microvilli (MV) but was not detected in MHC II-positive multivesicular bodies (MVB). After 2 hr, abundant levels of OVA were found within MVB, here underlying the apical membranes and additionally labelled for LAMP-1 (b) and MHC II molecules (c,d). The accumulation of OVA within MVB was independent of mucosal inflammation. MI, mitochondrium; V, vesicle. Bars = 100 nm.
Table 1.
The endocytic trafficking of lumenally-given ovalbumin in intestinal epithelial cells
| 10 min | 30 min | 60 min | 120 min | Control | |
|---|---|---|---|---|---|
| APM | ++ | ++ | ++ | ++ | − |
| EE | + | + | + | + | − |
| MVB | − | −/+ | ++ | ++ | − |
| MLB | − | −/+ | −/+ | −/+ | − |
| EDB | − | −/+ | −/+ | −/+ | − |
| IS | ++ | ++ | ++ | ++ | − |
APM, apical membrane; EE, early endosome; MVB, multivesicular body; MLB, multilamellar body; EDB, electron-dense body; IS, intercellular space; IECs, intestinal epithelial cells; OVA, ovalbumin.
Time-dependent subcellular distribution of OVA within the endocytic pathway of IECs after lumenal injection. Control mice were exposed to saline alone. The results presented are representative for all mice examined, independent of inflammation or the specific region within the small intestine; −, no labelling; −/+, inconsistent and faint labelling; +, consistent labelling; ++, consistent strong labelling.
Exosomes released by IECs originate from multivesicular bodies
Exosomes derived from IECs were shown to bear the A33 antigen, a definitive marker for IECs.13,15,21 Using immunoelectron microscopy, we examined the subcellular expression of A33 in IECs, to identify subcellular compartments that might function as sources of epithelial-derived exosomes. As expected, abundant levels of A33 antigen were uniformly distributed along the entire basolateral membrane and rarely detected on microvilli (Fig. 5c–g). Interestingly, A33 antigen labelling was observed within the compartments of the endocytic tract. Among these, labelling for A33 was consistently found on vesicles within MVB (Fig. 5c,d) and lower levels were seen in MLB and EDB. In these MIICs, A33 antigen colocalized with MHC II and LAMP-1. Again, part of the MVB appeared LAMP-1-negative. As observed for MHC II and Ii, the subcellular distribution of the A33 antigen in IECs was identical whether the specimen was taken from the jejunum or the ileum and was not influenced by ileal inflammation in TNFΔARE/WT mice. In addition to its labelling within IECs, the A33 antigen was also found on vesicles identified within the intercellular spaces of the epithelium (Fig. 5e–g). Double-labelling revealed colocalization for the A33 antigen and MHC II molecules on these vesicles within the ileum (Fig. 5e,g), while labelling for LAMP-1 was usually absent (Fig. 5f). The presence of A33 antigen-positive vesicles within the widened intercellular spaces of the epithelium was comparable within the jejunum and ileum and no obvious differences arose in response to gut inflammation.
Discussion
Inappropriately activated T helper type 1 (Th1) and Th–IL-17 CD4+ T cells are thought to be essential components of the loss of tolerance towards lumenal antigens (e.g. dietary or microbial); these are key elements in the development of CD.5,25,26 There is evidence that IECs are involved in the aberrant stimulation of CD4+ T cells during CD inflammation.3,27 Changes in the intracellular sorting of internalized antigens, in antigen processing and peptide loading, might be responsible for the distinctive functions of IECs as APCs (tolerogenic versus proinflammatory). Here, we focused on intracellular antigen trafficking and the involvement of MIICs. To unravel alterations in these processes by inflammatory mediators in CD, we used the TNFΔARE mice, a model for a CD-resembling ileitis. Histopathological examinations revealed a transmural ileitis in all sick TNFΔARE/WT mice studied and healthy intestinal tissue in all the other specimens examined. Both Th1 and Th–IL-17-related cytokines (among these IFN-γ, TNF-α and IL-17) play a critical role in the pathophysiology of intestinal inflammation in CD.5 This characteristic proinflammatory cytokine milieu was found in the ileal biopsies of TNFΔARE/WT mice studied, confirming that a CD-like inflammatory process was underway. Remarkably, the significantly increased levels of TNF-α mRNA, seen in the jejunum of TNFΔARE/WT mice, were not accompanied by an up-regulation of IFN-γ or IL-17 mRNAs. This might rely on a regional distinct bacterial colonization and partly explains the absence of destructive tissue inflammation in the upper small intestine.
In contrast to our previous work with conventionally housed BALB/c mice,11,12 we found a general lack of MHC II staining in all the jejunal biopsies taken from specific pathogen-free-housed mice, except for occasional faint and irregular labelling at the villus tips. Similar results for Ii staining support the validity of this finding. However, these results do not completely exclude the expression of both proteins in jejunal IECs in our mice. MHC II and Ii might be expressed at low, but potentially functionally relevant, levels that are undetectable by immunohistochemistry. Such low expression levels could be the result of insufficient inflammatory stimuli (trend towards higher levels of IFN-γ and IL-17 in the WT ileum) by jejunal colonization as a result of their maintenance in a specific pathogen-free environment. In contrast to the jejunum, villi of the ileum consistently showed staining for MHC II and Ii in IECs. This constitutive expression and its up-regulation and extension to crypt IECs during ileitis is in line with data obtained from humans.9
In professional APCs, late endosomes and lysosomes harbour most of the intracellular MHC II and related molecules responsible for antigen processing, peptide loading and editing, e.g. proteases, Ii and HLA-DM.6,7 Differences in the morphology of MIICs are thought to represent distinct stages along the endocytic pathway; MVB and MLB correspond to late endosomes and EDB to lysosome-like structures.7 Although MVB are suggested to be the main type of MIICs in which antigen loading occurs, antigen loading onto MHC molecules has been observed in all these MIICs. However, a distinct biological role in antigen presentation is speculated for the morphologically different MIICs.6 Work by Mayrhofer and Spargo yielded the first insights into the subcellular localization of MHC II in IECs.28 They found labelling for MHC II within multivesicular and morphologically heterogeneous endosomes in the supranuclear cytoplasm of jejunal IECs and this analysis has subsequently been confirmed by ourselves and others.11,12,29 We recently demonstrated MHC II molecules in late endosomes of human colonic and ileal epithelial cells during CD inflammation.30,31 The present study provides additional data differentiating the distinct subsets of MIICs and reveals the associated expression of Ii molecules. Of note, all the types of MIICs described in professional APCs could also be identified in IECs of the murine ileum. MHC II and Ii molecules were predominantly located within MVB, but could also be detected in lower amounts in the MLB and EDB. Likewise in professional APCs, the predominant location of Ii in MVB might be influenced by cleavage of Ii in MLB and especially in EDB. As also described in APCs, both MHC II and Ii were localized on the limiting and internal membranes of the MIICs. The MHC II- and Ii-enriched MVB that lack labelling for LAMP-1 might represent early MIICs as proposed by Geuze.7
The influence of extracellular mediators on the composition of MIICs remains almost unknown. Data on fibroblasts and monocytes demonstrated the induction of MVB formation by epidermal growth factor and granulocyte–macrophage colony-stimulating factor.6 The different mRNA expression levels of cytokines assessed in the intestinal segments of our mice, in particular the increase in proinflammatory mediators in TNFΔARE/WT mice, did not alter the pattern of MIICs in IECs. Jejunal IECs of both strains revealed late endocytic compartments of similar ultrastructural morphology to those of ileal IECs. Although most of the jejunal IECs in our study lacked staining for MHC II and Ii in immunohistochemistry, we assume that in the colonized gut, IECs harbour uniform subsets of MIICs, which seem not to be changed in the course of mucosal inflammation (at least in CD).
According to previous work on antigen uptake, the kinetics of antigen trafficking into MIICs varied from 10 to 60 min depending on the cell type analysed, the tracer used and the design of the experimental setting. In contrast to our previous work, the experimental procedure used in this project offered the opportunity to study the epithelial uptake of lumenal antigens for up to 2 hr. In recent studies in mice, we saw orally administered OVA in late endosomes of jejunal IECs already after 10 min of exposure.11,12 Gonnella and Wilmore showed trafficking of bovine serum albumin into class II-containing multivesicular endosomes of jejunal IECs 40 min after lumenal injection.29 This is in line with our current results, which demonstrated the delivery of lumenally applied OVA into MIICs by 30 min. Of note, OVA accumulated within MVB at the later periods (60 and 120 min), in contrast to the low levels of labelling found in MLB and EDB. This might reflect preferential targeting and retention in MVB. It might also be the result of less degradation (e.g. protection by MHC II binding) of OVA in MVB, whereas OVA transported into MLB and EBD would be subjected to more hydrolytic conditions.
In vitro studies have previously shown functionally distinct antigen presentation via MHC II by IECs dependent on late endocytic targeting from either a basolateral (induced by inflammatory stimuli) or apical pathway.4,32 However, the precise endocytic routes taken and in particular the nature of the MIICs involved remained unresolved. Little is known about the influence of inflammatory mediators on endocytic trafficking of antigens. Using macrophages, Montaner et al. demonstrated that IL-10 and IFN-γ decrease antigen uptake in vitro and IFN-γ further promoted antigen delivery into late endocytic compartments.33 By contrast, treatment with IL-4 or IL-10 reduced late endocytic transport. In experiments using specimens of human ileum and T84 cells, TNF-α increased the overall endocytic uptake of endocytic tracers in IECs.34 Using germ-free SCID (severe combined immunodeficiency) mice, we previously showed that the influence of systemically administered IFN-γ was effective in restoring antigen sorting into MIICs of jejunal IECs.12 Here, we were able to compare inflamed and non-inflamed tissues from jejunal and ileal specimens and found no differences in either the kinetics or the routeing of OVA trafficking in IECs. Antigen targeting into MVB seems to be a uniform process stimulated by constitutive cytokine expression in the colonized proximal and distal small bowel, and is not altered by additional proinflammatory stimuli.
Recent in vitro and in vivo studies have interpreted the presence of the A33 antigen to specify the IECs origin of exosomes.13–15 The A33 antigen is an immunoglobulin-like molecule that is recognized as a highly specific marker for IECs. While histological analysis has revealed a basolateral cell surface localization for this molecule,21 a precise subcellular analysis of its expression has not been undertaken. Notably exosomes derived from professional APCs have been shown to lack cell surface proteins.17 Applying immunoelectron microscopy, we found that expression of the A33 antigen in IECs is not limited to the basolateral cell surface. In all mice, and in jejunal as well as ileal IECs, the A33 antigen was also found associated with compartments of the endocytic tract, in particular on vesicles within MVB (class II-enriched in ileal IECs). Moreover, A33 antigen-positive exosomes were also identified in the intercellular spaces of the epithelium. While we cannot rule out that the extrusion of vesicles from basolateral membranes might contribute to the release of exosomes from IECs, our data strongly indicate that MVB are the source of MHC II/peptide-loaded exosomes in IECs, independent of the inflammatory state of the mucosa. Exosomes might be secreted by fusion of the MVB or endosomal intermediates with the basolateral membrane. These results further underscore the immunogenic relevance of constitutively active MVB in the class II-restricted antigen presentation by IECs.
Our data do not indicate that modulations of the intracellular antigen sorting are involved in the alternative functions of IECs as APCs. The distinctive capacities of IECs in MHC II-restricted antigen presentation in the healthy and inflamed state might rely on a differential editing and processing of internalized antigens within the MVB identified here. Future studies will concentrate on MVB and be directed towards elucidating the functional impact of modulating antigen processing and MHC II/peptide loading by inflammatory stimuli.
Acknowledgments
The authors thank Doris Stöckmann, Doreen Unmack, Heidi Schlichting, Harry Manfeldt and Christo Örün for excellent technical assistance; Dr Dominique Kaiserlian and Dr Nikolas Barois for the antibodies against MHC II and Ii. There is no financial or other conflict of interest that might have biased the work. Supported by grants from the Medical Faculty, University of Lübeck, Germany (J04-2004 and SPP Autoimmunity B6, to J.B.).
Abbreviations
- EDB
electron-dense bodies
- IECs
intestinal epithelial cells
- Ii
invariant chain
- LAMP-1
lysosome-associated membrane protein-1
- MHC II
major histocompatibility complex class II
- MIICs
MHC II-enriched compartments
- MLB
multilamellar bodies
- MVB
multivesicular bodies
- OVA
ovalbumin
References
- 1.Hershberg RM, Mayer LF. Antigen processing and presentation by intestinal epithelial cells – polarity and complexity. Immunol Today. 2000;21:123–8. doi: 10.1016/s0167-5699(99)01575-3. [DOI] [PubMed] [Google Scholar]
- 2.Westendorf AM, Bruder D, Hansen W, Buer J. Intestinal epithelial antigen induces CD4+ T cells with regulatory phenotype in a transgenic autoimmune mouse model. Ann NY Acad Sci. 2006;1072:401–6. doi: 10.1196/annals.1326.035. [DOI] [PubMed] [Google Scholar]
- 3.Dotan I, Allez M, Nakazawa A, Brimnes J, Schulder-Katz M, Mayer LF. Intestinal epithelial cells from inflammatory bowel disease patients preferentially stimulate CD4+ T cells to proliferate and secrete interferon-gamma. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1630–40. doi: 10.1152/ajpgi.00294.2006. [DOI] [PubMed] [Google Scholar]
- 4.Hershberg RM, Escola JM, Framson PE, et al. Intestinal epithelial cells use two distinct pathways for HLA class II antigen processing. J Clin Invest. 1997;100:204–15. doi: 10.1172/JCI119514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sartor RB. Mechanisms of disease: pathogenesis of Crohn’s disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol. 2006;7:390–407. doi: 10.1038/ncpgasthep0528. [DOI] [PubMed] [Google Scholar]
- 6.Stern LJ, Potolicchio I, Santambrogio L. MHC class II compartment subtypes: structure and function. Curr Opin Immunol. 2006;18:64–9. doi: 10.1016/j.coi.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Geuze HJ. The role of endosomes and lysosomes in MHC class II functioning. Immunol Today. 1998;19:283–7. doi: 10.1016/s0167-5699(98)01269-9. [DOI] [PubMed] [Google Scholar]
- 8.Watts C. Capture and processing of exogenous antigens for presentation on MHC molecules. Annu Rev Immunol. 1997;15:821–50. doi: 10.1146/annurev.immunol.15.1.821. [DOI] [PubMed] [Google Scholar]
- 9.Mayer L, Eisenhardt D, Salomon P, Bauer W, Plous R, Piccinini L. Expression of class II molecules on intestinal epithelial cells in humans. Differences between normal and inflammatory bowel disease. Gastroenterology. 1991;100:3–12. doi: 10.1016/0016-5085(91)90575-6. [DOI] [PubMed] [Google Scholar]
- 10.Lin XP, Almqvist N, Telemo E. Human small intestinal epithelial cells express the key elements for antigen processing and the production of exosomes. Blood Cells Mol Dis. 2005;35:122–8. doi: 10.1016/j.bcmd.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 11.Zimmer KP, Buning J, Weber P, Kaiserlian D, Strobel S. Modulation of antigen trafficking to MHC class II-positive late endosomes of enterocytes. Gastroenterology. 2000;118:128–37. doi: 10.1016/s0016-5085(00)70421-5. [DOI] [PubMed] [Google Scholar]
- 12.Buning J, Schmitz M, Repenning B, Ludwig D, Schmidt MA, Strobel S, Zimmer KP. Interferon-gamma mediates antigen trafficking to MHC class II-positive late endosomes of enterocytes. Eur J Immunol. 2005;35:831–42. doi: 10.1002/eji.200425286. [DOI] [PubMed] [Google Scholar]
- 13.Karlsson M, Lundin S, Dahlgren U, Kahu H, Petterson I, Telemo E. Tolerosomes are produced by intestinal epithelial cells. Eur J Immunol. 2001;31:2892–900. doi: 10.1002/1521-4141(2001010)31:10<2892::aid-immu2892>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 14.Ostmann S, Taube M, Telemo E. Tolerosome-induced oral tolerance is MHC dependent. Immunology. 2005;16:464–76. doi: 10.1111/j.1365-2567.2005.02245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Niel G, Mallegol J, Bevilacqua C, et al. Intestinal epithelial exosomes carry MHC class II/peptides able to inform the immune system in mice. Gut. 2003;52:1690–7. doi: 10.1136/gut.52.12.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mallegol J, van Niel G, Lebreton C, et al. T-84-Intestinal epithelial exosomes bear MHC class II/peptide complexes potentiating antigen presentation by dendritic cells. Gastroenterology. 2007;132:1866–76. doi: 10.1053/j.gastro.2007.02.043. [DOI] [PubMed] [Google Scholar]
- 17.Denzer K, Kleijmeer MJ, Heijnen HF, Stoorvogel W, Geuze HJ. Exosome: from internal vesicle of the multivesicular body to intercellular signalling device. J Cell Sci. 2000;113:3365–74. doi: 10.1242/jcs.113.19.3365. [DOI] [PubMed] [Google Scholar]
- 18.Raposo G, Nijman HW, Stoorvogel W, Liejemdekker R, Harding CV, Melief CJ, Geuze HJ. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183:1161–72. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kontoyiannis D, Pasparakis M, Pizarro TT, Cominelli F, Kollias G. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity. 1999;10:387–98. doi: 10.1016/s1074-7613(00)80038-2. [DOI] [PubMed] [Google Scholar]
- 20.Barois N, Forquet F, Davoust J. Selective modulation of the major histocompatibility complex class II antigen presentation pathway following B cells receptor ligation and protein kinase C activation. J Biol Chem. 1997;272:3641–7. doi: 10.1074/jbc.272.6.3641. [DOI] [PubMed] [Google Scholar]
- 21.Johnstone CN, Tebbutt NC, Abud HE, et al. Characterization of mouse A33 antigen, a definitive marker for basolateral surfaces of intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2000;279:G500–10. doi: 10.1152/ajpgi.2000.279.3.G500. [DOI] [PubMed] [Google Scholar]
- 22.Vidal K, Samarut C, Magaud JP, Revillard JP, Kaiserlian D. Unexpected lack of reactivity of allogeneic anti-Ia monoclonal antibodies with MHC class II molecules expressed by mouse intestinal epithelial cells. J Immunol. 1993;151:4642–50. [PubMed] [Google Scholar]
- 23.Griffiths G. Fine Structure Immunocytochemistry. Heidelberg, Germany: Springer; 1993. [Google Scholar]
- 24.Lucocq JM, Habermann A, Watt S, Backer JM, Mayhew TM, Griffiths G. A rapid method for assessing the distribution of gold labeling on thin sections. J Histochem Cytochem. 2004;52:991–1000. doi: 10.1369/jhc.3A6178.2004. [DOI] [PubMed] [Google Scholar]
- 25.Duchmann R, Kaiser I, Hermann E, Mayet W, Ewe K, Meyer zum Buschenfelde KH. Tolerance exists towards resident intestinal flora but is broken in active inflammatory bowel disease (IBD) Clin Exp Immunol. 1995;102:448–55. doi: 10.1111/j.1365-2249.1995.tb03836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kraus TA, Toy L, Chan L, Childs J, Mayer L. Failure to induce oral tolerance to a soluble protein in patients with inflammatory bowel disease. Gastroenterology. 2004;126:1771–8. doi: 10.1053/j.gastro.2004.03.076. [DOI] [PubMed] [Google Scholar]
- 27.Toy LS, Yio XY, Lin A, Honig S, Mayer L. Defective expression of gp180, a novel CD8 ligand on intestinal epithelial cells, in inflammatory bowel disease. J Clin Invest. 1997;100:2062–71. doi: 10.1172/JCI119739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mayrhofer G, Spargo LDJ. Distribution of class II major histocompatibility antigens in enterocytes of the rat jejunum and their association with organelles of the endocytic pathway. Immunology. 1989;70:11–9. [PMC free article] [PubMed] [Google Scholar]
- 29.Gonnella PA, Wilmore DW. Co-localisation of class II antigen and exogenous antigen in the rat enterocyte. J Cell Sci. 1993;106:937–40. doi: 10.1242/jcs.106.3.937. [DOI] [PubMed] [Google Scholar]
- 30.Buning J, Hundorfean G, Schmitz M, Zimmer KP, Strobel S, Gebert A, Ludwig D. Antigen targeting to MHC class II-enriched late endosomes in colonic epithelial cells: trafficking of luminal antigens studied in vivo in Crohn’s colitis patients. FASEB J. 2006;20:359–61. doi: 10.1096/fj.05-4807fje. [DOI] [PubMed] [Google Scholar]
- 31.Hundorfean G, Zimmer KP, Strobel S, Gebert A, Ludwig D, Buning J. Luminal antigens access late endosomes of intestinal epithelial cells enriched in MHC I and MHC II molecules –in vivo study in Crohn’s ileitis. Am J Physiol Gastrointest Liver Physiol. 2007;293:G798–808. doi: 10.1152/ajpgi.00135.2007. [DOI] [PubMed] [Google Scholar]
- 32.Hershberg RM, Cho DH, Youakim A, Bradley MB, Lee JS, Framson PE, Nepom GT. Highly polarized HLA class II antigen processing and presentation by human intestinal epithelial cells. J Clin Invest. 1998;102:792–803. doi: 10.1172/JCI3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montaner LJ, da Silva RP, Sun J, Sutterwala S, Hollinshead M, Vaux D, Gordon S. Type 1 and type 2 cytokine regulation of macrophage endocytosis: differential activation by IL-4/IL-13 as opposed to IFN-gamma or IL-10. J Immunol. 1999;162:4606–13. [PubMed] [Google Scholar]
- 34.Söderholm JD, Streutker C, Yang PC, et al. Increased epithelial uptake of protein antigens in the ileum of Crohn’s disease mediated by tumor necrosis factor α. Gut. 2004;53:1817–24. doi: 10.1136/gut.2004.041426. [DOI] [PMC free article] [PubMed] [Google Scholar]