Abstract
In this study, we tested the effect of different T-cell subpopulations on antigen driven effector cell expansion in lymphopenic hosts, making use of an experimental model of graft-versus-host disease (GVHD). Fluorescence-activated cell sorted (FACS) naïve CD4 T cells from C57BL/6 mice, transferred into lymphopenic F1 (C57BL/6 × BALB/c) Rag-deficient hosts, proliferated extensively and migrated systemically causing acute GVHD within 4 weeks after transfer. Adoptive hosts of CD4 memory T cells on the other hand developed milder symptoms of GVHD with later onset. T-cell expansion and migration to peripheral sites as well as development of GVHD were prevented when naïve T cells were transferred together with CD4+ CD25+ T cells, but co-transfer of memory T cells with naïve T cells could not prevent GVHD, although its onset was delayed. OX40, a costimulatory marker that is upregulated at an early time point after T-cell activation and enhances T-cell proliferation, cytokine secretion and survival, was strongly upregulated during GVH responses. Naïve T cells deficient in OX40 expression caused markedly reduced GVH in onset and severity despite some level of expansion in the adoptive host, suggesting an important role of this molecule in the immune pathology of GVHD.
Keywords: CD4, graft-versus-host disease, regulation
Introduction
Regulation of peripheral T-cell responses is an essential component of the immune system, preventing not only the development of autoimmune diseases, but also excessive responses to foreign antigen that could lead to immune pathology. The control mechanisms that operate in a healthy immune system and help to prevent disproportionate damage are compromised in acute lymphopenia. Immune responses to cognate antigens are exaggerated in lymphopenic hosts,1,2 which may be beneficial in the case of responses against tumours, but could also be detrimental for the host. We have been studying T-cell regulation in lymphopenic models focusing on competition for limited resources as an underlying principle in the maintenance of homeostasis. While so-called natural regulatory T cells with a CD4+ CD25+ phenotype are known to be important regulators of immune pathology,3 we have shown that other T-cell populations that are not dedicated to immune regulation can be regulatory as a side effect of their own response to homeostatic or antigenic stimulation.4 For example, memory CD4 T cells fulfil an important function in the control of lymphopenia-induced proliferation of naïve CD4 T cells5 and thus contribute to homeostasis in the immune system.
In this study, we tested the effect of different T-cell subpopulations on antigen-driven expansion during lymphopenia, making use of an experimental model of graft-versus-host disease (GVHD). GVH-mediated disease is a life-threatening complication of donor lymphocyte infusion (DLI) into allogeneic hosts during bone marrow reconstitution of lymphocyte-depleted hosts. DLI accompanying stem cell transplantation for the treatment of haematological malignancies was found to increase the likelihood of beneficial immune responses against reoccurring tumour cells.6–9 Although there have been examples of separating GVHD from graft-versus-leukaemia reactions,7,10 the substantial potential for crossreactivity in T cells11 may defeat attempts to fully separate GVH and GVL reactions. The parent-into-F1 murine model of GVH disease has been widely studied, with notable differences in the disease pathogenesis depending on the genetic background of the donor strain.12–14 Either an acute or a chronic disease state can be induced depending on the parental strain used as the donor. In this study, we analysed the effect of CD4 T-cell subpopulations on development of acute GVHD in Rag-deficient F1 hosts, which allowed us to follow the fate of adoptively transferred cells. Transferred naïve cells proliferated extensively and migrated systemically causing acute GVHD within 4 weeks after transfer. Both T-cell expansion and development of GVHD were almost completely prevented when naïve T cells were transferred together with CD4+ CD25+ T cells. While transfer of memory T cells on their own caused only mild symptoms, co-transfer with naïve T cells resulted in a slight delay of onset and severity of symptoms, but could not prevent GVHD. OX40, a costimulatory marker that is upregulated at an early time point after T-cell activation and enhances T-cell proliferation, cytokine secretion and survival,15–17 was strongly upregulated during GVH responses. Interestingly, naïve T cells deficient in OX40 expression show markedly reduced GVHD responses both with respect to onset as well as severity, suggesting an important role of this molecule in the immune pathology of GVHD.
Material and methods
Mice
C57BL/6, C57BL/6 CD45.1, VA-DSRED.B-B6,18 GFP.D-B6,19 C57BL/6 Yeti,20 OX40 Cre-B6 hom,21 OX40 Cre-B6 × R26R EYFP (OX40 Rosa 26 reporter mice)22 and F1 Rag−/− (Rag2 KO B6 × Rag2 knockout (KO) BALB/c) were bred under specified pathogen-free conditions at the MRC National Institute for Medical Research in accordance with local and Home Office guidelines. VA-DSRED are transgenic mice expressing DSRED under control of the human CD2 promoter and GFP.D-B6 likewise are transgenic mice expressing GFP under control of the human CD2 promoter. C57BL/6 Yeti mice are interferon-γ (IFN-γ) gene knockin bicistronic reporter mice expressing a yellow fluorescent protein (eYFP) upon IFN-γ activation which can be directly detected by flow cytometry. OX40 Cre-B6 mice were generated by N. Killeen, have an IRES-Cre inserted in the OX40 locus and are therefore OX40 deficient. When crossed with the reporter strain R26R EYFP, cells from the resultant OX40 Cre-B6 × R26R EYFP mice that express OX40 produce EYFP; therefore OX40+ cells can be directly detected by flow cytometry. All strains used, excluding F1 Rag−/− mice, were fully backcrossed to the C57BL/6 background.
Cell purification
For CD4+ CD25+ T cells single-cell suspensions from spleens and lymph nodes were positively selected with anti-CD25-phycoerythrin (PE) microbeads (Miltenyi Biotech, Bisley, UK) on an AutoMACS according to the manufacturer’s instructions. The positive fraction was further sorted on a MoFlo cytometer (Cytomation, Fort Collins, CO) to obtain populations of CD4+ CD25+ T cells with >99% purity. For naïve and memory CD4 T cells, AutoMACS positive selection for CD4 T cells was performed, followed by MoFlo sorting of CD25, CD44 and CD4 to select populations of memory CD4+ CD44hi CD25− and naïve CD4+ CD44lo CD25− cells with >99% purity.
Antibodies and flow cytometry
Antibodies to CD4, CD44, CD62L, CD45.1, CD45.2, TCR (H57), IFN-γ, CD25, CD49b (DX5) and NKG2D were obtained conjugated with fluorescein isothiocyanate (FITC), PE, peridinin chlorophyll (PerCP), allophycocyanin (APC) from BD Bioscience UK as well as eBioScience (San Diego, CA). Anti-NK1.1 (PK136) was purified from hybridoma supernatant using standard procedures.
Induction and monitoring of GVHD
Recipient Rag 2 KO B6 × Rag 2 KO BALB/c (abbreviated as F1 Rag−/− in main text) were pretreated 2 days prior to cell transfer by 500 rad sublethal total body irradiation and injection of 150 μg anti-NK1.1 (PK136) depleting antibody intraperitoneally. To induce GVHD, sorted CD57BL/6 CD4+ CD25−CD44lo or CD4+ CD25− CD44hi cells (1 × 106) were transferred intravenously to recipient mice on day 0. For co-transfers, sorted were transferred in equal numbers (1 × 106). To measure disease incidence and severity, each of the following observed symptoms was assigned a score: 0 no external signs, 1 piloerection on back and underside, 1 hunched posture/lethargy, 1 weight loss, 0·5 alopecia or scaling (<1 cm2), 1 alopecia or scaling (>1 cm2). The severity score is the sum of these values and ranges from 0 (unaffected) to a maximum of 4. Mice were monitored daily, and the disease incidence and severity recorded every 2–4 days. Mice with severe disease, typically corresponding to a score of 3 or greater, were culled. Severity scores for an experiment are calculated as the average of the total scores for the recipients, with the score for mice culled for humane reasons kept in subsequent calculations for that experiment. Data from mice culled for analysis prior to developing extreme disease were not included in curves showing incidence and severity. Mice with no signs of disease, i.e. a severity score of 0 are defined as ‘unaffected’, with the incidence calculated as the proportion of hosts ‘unaffected’ at a particular time point. The following organs were taken for analysis at autopsy: mesenteric lymph nodes (MLN) spleen, lung and bone marrow (BM) flushed from a single femur and tibia.
Statistical analysis
Kaplan–Meyer incidence curves were graphed by prism (GraphPad Software, San Diego, CA) and compared using logrank. Comparisons between transferred cell conditions for cell counts, cytokine production and reporter expression were performed by two-way anova analysis. Two-tailed Student’s t-test was used to compare data grouped by organ. For all tests P ≤ 0·05 was considered significant and is indicated on bar graphs by *. Data are presented as mean ± standard deviation.
Stereomicroscopy
GVHD was induced as above with donor cells sorted from VA-DSRED.B-B6 or GFP.D-B6 mice. For co-transfers, to distinguish the transferred populations CD4+ CD25− CD44lo were purified from DSRED strains with CD4+ CD25+, CD4+ CD25− CD44hi or CD4+ CD25− CD44lo cells purified from GFP.D-B6 mice. Cells of each population were transferred in equal numbers (1 × 106). At autopsy, recipient tissues were imaged for DS-Red and GFP expression using a Zeiss (Jena, Germany) M2Bio stereofluorescent microscope and open lab software (Improvision, Warwick, UK).
Results
Development of GVH pathology after adoptive transfer of naïve or memory CD4 T cells
FACS sorted naïve CD4 parental C57BL/6 T cells (1 × 106) were adoptively transferred into F1 Rag−/− hosts. The hosts were pretreated 2 days before transfer with sublethal irradiation of 500 rad and injection of antibody NK1.1 to deplete host natural killer (NK) cells. As shown in supplementary Fig. S1, untreated F1 Rag−/− hosts showed substantially reduced numbers of injected donor cells because of rejection by host NK cells, which was abrogated by the combined treatment.
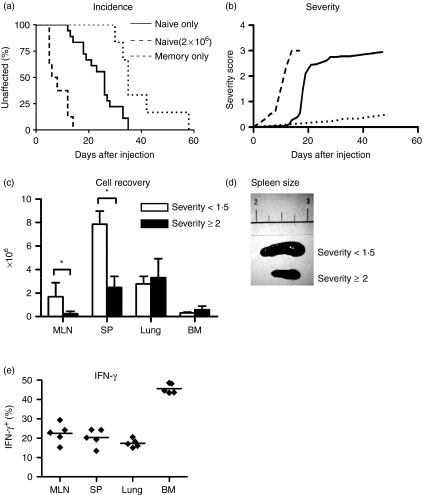
The adoptive hosts developed signs of GVHD within 10 days after transfer of naïve CD4 T cells and all mice had to be culled 47 days after injection because of severe disease (Fig. 1a). Figure 1(b) shows a gradual increase in the severity index of symptoms ranging from mild signs such as piloerection to a combination of several disease indicators. When the number of CD4 T naïve cells adoptively transferred was increased to 2 × 106 the disease onset was more rapid with all mice culled by day 19 because of severe disease (Fig. 1a,b). Following transfer of 1 × 106 naïve T cells, CD4 T-cell numbers were assessed in lymphoid organs such as MLN and spleen as well as peripheral tissues for which lung and bone marrow were taken as examples because they are easy to sample without the requirement for excessive tissue digestion steps. Cell recoveries were assessed at an early time point after transfer when the combined disease severity score was less than 1·5 and at a later time point with severe clinical signs resembling a score above 2 (Fig. 1c). At early time points, the most pronounced expansion of CD4 T cells was seen in the spleen (7·8 × 106 ± 1·1 × 106), coinciding with severe splenomegaly (Fig. 1d), but also MLN and peripheral tissues such as the lung showed greatly expanded T-cell numbers (1·7 × 106 ± 1·1 × 106, 2·7 × 106 ± 0·6 × 106, respectively).
Figure 1.
Adoptively transferred naïve CD4 T cells cause GVHD with more rapid incidence with increased cell number transferred. (a) the graph shows incidence of GVH (as % affected) in adoptive hosts of FACS sorted naïve CD4+ CD44lo CD25− cells (1 × 106 cells/host, n = 18 hosts, solid line; 2 × 106cells/host, n = 8 hosts, dashed line) or sorted memory CD4+ CD44hi CD25− cells (1 × 106 cells/host, n = 8 hosts, dotted line) from C57Bl/6 CD45.1 mice. Recipient mice with no signs of disease, i.e. a severity score of 0 are defined as ‘unaffected’. Mice with severe disease or in obvious distress, typically correlating to a score of 3 or greater, were culled. All host mice were culled within 47 days of cell transfer due to severe disease. (b) Disease severity scores for each host were assigned using a scale of 0 to a maximum of 4 as detailed in Materials and methods. Naïve cells (1 × 106), solid line; naïve cells (2 × 106), dashed line; memory cells (1 × 106), dotted line. For each observed symptom, the corresponding scores were added. (c) Recovery of CD4 T cells in early stages of disease following transfer of 1 × 106 naïve cells, i.e. from hosts with a clinical score less than 1·5 (white bars) and of CD4 T cells at a late stage of disease, i.e. from hosts with a clinical score more than 2 (black bars). Cell recovery was calculated by measuring total cell counts per organ and using flow cytometry to determine the proportion of CD4+ TCR+ cells within each organ. The data shown is representative of three independent experiments, with between three and five mice/group. (d) Ex vivo spleens taken from recipients following transfer of 1 × 106 naïve cells, one at severity <1·5 and one at severity >2. Photo representative of three independent experiments, with three to five mice in each group.
At later time points during full-blown GVHD splenomegaly subsided and the mice showed wasting disease with severe weight loss. While T-cell numbers in the spleen had significantly declined (7·8 × 106 ± 1·1 × 106–2·4 × 106 ± 0·9 × 106, P < 0·001), spleen and lung still contained significantly more T cells than originally injected (2·4 × 106 ± 0·9 × 106 and 3·3 × 106 ± 1·6 × 106; P = 0·02, P = 0·03, respectively).
The evidence for the ability of memory T cells to induce GVHD is controversial with several reports suggesting that memory CD4 T cells themselves do not induce GVHD23–26 whereas others show that memory T cells cause a chronic form of GVHD.27,28 However, these data were obtained in an experimental GVH model which involved differences in minor histocompatibility antigens only, whereas in our case much stronger major histocompatibility complex differences are involved. We determined the role of CD4 memory T cells in GVHD in our experimental model. F1 Rag−/− hosts of FACS-sorted CD4+ CD25− CD44hi memory CD4 T cells showed a delay in onset of GVHD symptoms compared to transfer of naïve CD4 T cells alone (Fig. 1a, median incidence 35 days versus 26 days, P < 0·001), which were much milder (Fig. 1b, maximum severity 2·9 versus 0·5, respectively) and did not cause the severe wasting disease observed after transfer of naïve CD4 T cells, nor result in any deaths.
Acute CD4 T-cell-mediated GVHD is classically thought to be caused by the action of T helper 1 (Th1) cells,10,29 characterized by their secretion of IFN-γ. To visualize T cells that make IFN-γ, we made use of IFN-γ gene knock-in bicistronic reporter mice (Yeti) expressing a yellow fluorescent protein (eYFP) upon IFN-γ activation.20 This allows visualizing IFN-γ-producing T cells without the requirement for in vitro reactivation and intracellular staining and thus gives a more accurate picture of the in vivo cytokine status of Th1 T cells. Following adoptive transfer of sorted YFP negative naïve CD4 T cells from Yeti mice, we observed strong upregulation of YFP on a large proportion of T cells in all peripheral organs, notably the bone marrow (Fig. 1e, P < 0·001). Nevertheless, not all CD4 T cells isolated from organs of the adoptive host produced IFN-γ, but we did not detect significant amounts of other cytokines such as tumour necrosis factor-α (TNF-α), interleukin (IL)-17, IL-4 or IL-5 (data not shown).
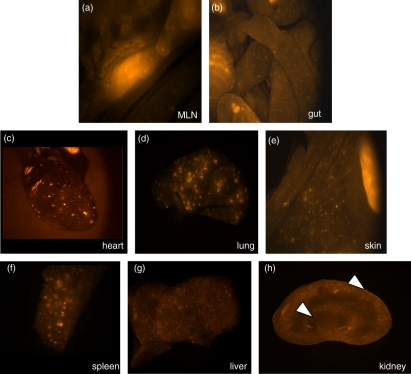
Notably, tissue infiltration was widespread all over the body and not restricted to the organs sampled as assessed by stereomicroscopy of organs from recipients of naïve CD4 T cells expressing fluorescent DS-Red on all T cells (Fig. 2). Stereomicroscopy images from control F1 Rag−/− recipients were negative for DS-Red (data not shown).
Figure 2.
Widespread organ infiltration following naïve CD4 T cell transfer in GVHD. The photos show stereomicroscopy images of postmortem organs from F1 Rag−/− hosts 4 weeks after transfer of FACS sorted naïve CD4+ CD44lo CD25− cells from VA-DSRED.B-B6 mice. Fluorescent DSRED expression, indicating the location of transferred cells, was detected in the organs indicated and is visible as bright areas in the photo. (a) Mesenteric lymph node; (b) gut; (c) heart; (d) lung; (e) skin flank; (f) spleen; (g) liver; (h) kidney section. Infiltration was visible in the kidney cortex; two areas of infiltration are highlighted with white arrows. Data are representative of five hosts.
CD4+ CD25+ T cells prevent expansion of naïve CD4 T cells and development of GVHD
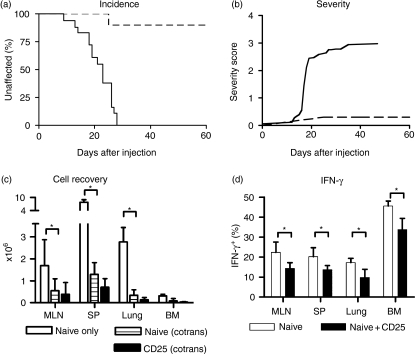
CD4+ CD25+ T cells have been shown to prevent GVHD and bone marrow rejection.30 In order to test the effect of CD4+ CD25+ T cells in our model, we co-transferred equal numbers (1 × 106) of naïve CD4 T cells and CD4+ CD25+ T cells, distinguished by their CD45 allotype into conditioned F1 Rag−/− hosts as above. Adoptive hosts that received co-transfer of CD4+ CD25+ T cells were protected from GVHD as shown in Fig. 3(a,b). These cells had a major impact on the expansion of the naïve T cell input, as recovery from lymphoid organs as well as peripheral tissues was greatly reduced (Fig. 3c, P = 0·006) compared with hosts that received only naïve T cells. For example, in MLN mean recovery was 0·5 × 106 ± 0·4 × 106 after co-transfer compared to 1·7 × 106 ± 1·1 × 106 when naïve T cells were transferred alone. CD4+ CD25+ T cells did not expand much themselves and were in fact only detectable in appreciable numbers in lymphoid organs and not in peripheral tissues (Fig. 3c). In adoptive hosts of co-transferred naïve T cells and CD4+ CD25+ T cells, the presence of CD4+ CD25+ T cells significantly reduced the proportion of YFP positive (IFN-γ positive) Th1 effector cells from the naïve input compared with hosts where naïve cells were transferred alone (Fig. 3d, P < 0·001).
Figure 3.
Co-transfer CD4+ CD25+ T cells protects against disease. (a, b) Adoptive hosts of co-transferred FACS sorted naïve and CD4+ CD25+ T cells were monitored for signs of disease incidence (a) and severity (b). Hosts of naïve T cells alone (n = 18) are shown as solid line, hosts of co-transfers (n = 10) are shown in dashed lines, open circles. (c, d) Cell recovery from adoptive hosts of co-transferred naïve CD45.2 T cells from B6 mice and CD4+ CD25+ T cells from C57BL/6 CD45.1 mice is shown. The naïve input (striped bar) and CD4+ CD25+ input (black bar) were distinguished by their CD45 allotype. The data shown is representative of three independent experiments, with between two and four mice per group. The white bar represents naïve alone transfer as described in Fig. 1c.
Memory CD4 T cells delay, but do not prevent T-cell expansion and GVHD
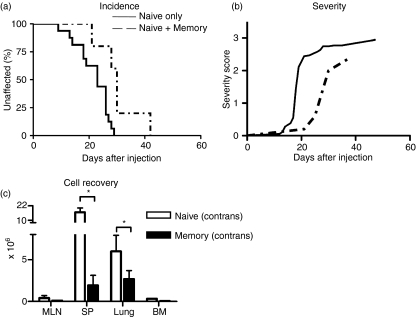
CD4 memory T cells are very effective at preventing lymphopenia-induced expansion and phenotypic conversion of naïve T cells.5 While transfer of small numbers of naïve CD4 T cells into T cell deficient Rag−/− hosts might indeed cause lymphopenia-induced expansion, our previous results indicated that in the face of antigenic stimulation, lymphopenia-induced expansion is negligible and delayed compared with the rapid expansion of antigen-activated effector T cells.5 Thus it is not clear whether memory T cells influence the expansion of antigen-driven T cells. Indeed, co-transfer of naïve and memory CD4 T cells only showed a small effect of memory CD4 T cells on the naïve T-cell input with GVHD developing in a slightly delayed fashion, with a median incidence of 26 days in the naïve only transfer compared to 30 days for the co-transfer with memory T cells (Fig. 4a, not significantly different, P = 0·17). In the co-transfer, severity of symptoms reached those of mice that received only naïve CD4 T cells (Fig. 4b, maximum severity 2·4 versus 2·9, respectively). Also in terms of cell expansion, memory CD4 T cells had little influence on the expansion of the naïve T-cell input (Fig. 4c). Memory cell themselves did not expand strongly, but were recovered from all peripheral tissues sampled, in line with their capacity to access lymphoid as well as non-lymphoid sites (Fig. 4c).
Figure 4.
Effect of memory T cells on GVH. (a, b) disease incidence and severity in adoptive hosts of FACS-sorted CD4+ CD44hi CD25− naïve cells from C57BL/6 CD45.1 mice on their own (n = 8, solid line) or co-transferred with CD4+ CD44hi CD25− memory T cells (n = 5, dashed line). In both cases 1 × 106cells of each cell type were transferred. (c) The cell recovery following naïve and memory cell co-transfer is shown for the naïve input (white bar) and for the memory input (black bar) for three mice using antibodies to CD45.1/CD45.2 to distinguish the two populations.
OX40 is upregulated during GVHD development and OX40−/− T cells do not induce GVHD
Molecules thought to be important in GVHD induction include the costimulatory molecule OX40. OX40 (CD134) belongs to the TNF receptor family and is expressed transiently on naïve T cells post-stimulation via the T-cell receptor with the peak of expression on days 2–3.31 Memory T cells upregulate OX40 rapidly following antigen re-challenge. The interaction between OX40 and its ligand OX40L, expressed on antigen-presenting cells, including dendritic cells and macrophages, has a role in enhancing T-cell proliferation, survival and cytokine secretion.17,32–34 Indeed blockade of OX40/OX40L interactions with antibodies to OX40L proved to be protective against GVHD.35,36 In order to visualize the transient upregulation of OX40 on transferred CD4 T cells in vivo, we made use of an OX40 reporter system developed by N. Killeen.21 Mice with a Cre insertion in the OX40 locus were mated with Rosa26 reporter mice which contain a YFP reporter transgene inserted into the Rosa26 locus followed by a stop codon that is flanked by loxP sites. In these reporter F1 mice, YFP will be expressed following activation of the OX40 locus, which upregulates Cre expression, resulting in excision of the stop codon and permanent expression of YFP. Thus, these mice permit the detection of any cell that has ever switched on OX40 regardless of whether it is currently expressed or not. Furthermore, mice homozygous for the Cre insertion into the OX40 locus, which disables the gene can be used as knockout for OX40.
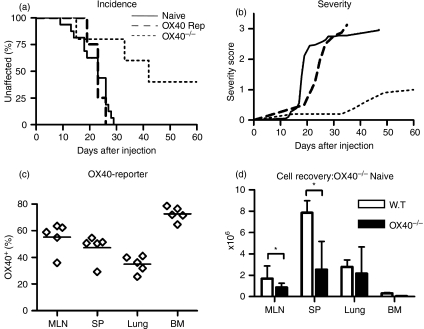
Naïve YFP negative T cells FACS purified from OX40 Rosa26 reporter mice were transferred into F1 Rag−/− hosts. As shown in Fig. 5(a) these hosts developed GVHD with similar severity as hosts of wild type naïve T cells (Fig. 5b) and a large proportion of the T cells recovered from these mice were YFP positive (Fig. 5c), indicating that they had at some point upregulated OX40. In contrast, when naïve T cells lacking OX40 (from OX40 Cre homozygous mice) were transferred, GVHD was strongly reduced both with respect to incidence (Fig. 4a, 100% versus 60%, P = 0·01) and severity (Fig. 4b, maximum severity 2·9 versus 1·1, respectively), indicating that OX40 interactions play an important role in the development of pathology. Recovery of cell numbers in recipients of OX40−/− T cells was reduced compared to recipients of wild type T cells (Fig. 4d), in spleen 2·6 × 106 ± 2·5 × 106 versus 7·9 × 106 ± 1·1 × 106, but they were not as strongly suppressed as in recipients of co-transfers of naïve and CD4 + CD25 + T cells where spleen recovery was 1·3 × 106 ± 0·5 × 106.
Figure 5.
OX40 is involved in development of GVHD. (a, b) disease incidence and severity in adoptive hosts of naïve T cells FACS purified from OX40-deficient mice (n = 5, dotted lines), OX40 Cre B6 R26R EYFP reporter mice (n = 4, dashed lines), compared with wild type naïve T cells (n = 8, solid lines). (c) Percentage of YFP-positive CD4 T cells in recipients of sorted YFP negative naïve T cells from OX40 Cre B6 R26R EYFP reporter mice assessed 2 weeks after transfer. The data is representative of two independent experiments with between two and five recipients per group. (d) Cell recovery from recipients of wildtype (white bar) or OX40−/− naïve T cells (black bar) 8 weeks after transfer. The data shown is representative of two independent experiments with three to four mice per group.
Discussion
In this study we made use of a murine GVH model to study whether immune pathology initiated by transfer of naïve CD4 T cells into allogeneic lymphopenic hosts could be influenced by other CD4 T-cell subpopulations. We show here that memory CD4 T cells, which have a strong inhibitory effect on the lymphopenia-induced expansion of naïve CD4 T cells,5 however, have little protective effect in the development of GVHD by adoptively transferred naïve CD4 T cells. Unlike the protective effect on lymphopenia-induced expansion of naïve CD4 T cells4 increasing the number of adoptively transferred naïve CD4 T cells to 2 × 106 to mimic the total number of cells given in the co-transfer experiments, resulted in more rapid onset of severe GVHD. Notably, recent reports indicate that memory and memory-like T cells, i.e. donor cells that have undergone lymphopenia-induced expansion and have a CD44hi phenotype, caused a milder GVHD37 or indeed a beneficial graft-versus-leukaemia response without GVHD.38
Interestingly, adoptive transfer of pure CD25− CD44hi memory T cells on their own did not cause lethal GVHD, although the mice still developed signs of GVHD, mostly affecting the skin. A previous study on the effect of memory CD4 T cells in a GVH model employing minor histocompatibility antigenic differences reported that these cells did not induce any GVHD.23 Our results indicate that the apparent ‘innocence’ of memory CD4 T cells does not extend to more profound antigenic differences and one might also expect extensive cross-reactive activation in memory cells resulting from previous exposure to pathogens, a situation that more likely resembles the conditions of humans rather than mice that are kept in pathogen-free facilities. Upon co-transfer of naïve and memory CD4 T cells, GVHD was slightly delayed both in onset and severity, but all mice succumbed to lethal disease eventually. It is not clear what causes the delay and we cannot exclude the possibility that the memory population contained some regulatory T cells that had downregulated CD25, but were nevertheless active suppressors. Studies using FoxP3 reporter mice noted that while most lymph-node-derived FoxP3-positive CD4 T cells expressed CD25, the spleen as well as peripheral organs contain a substantial proportion of CD25 negative FoxP3-positive cells that appear equally potent in in vitro suppressor assays.39
In accordance with the reported potency of CD4+ CD25+ T cells in preventing immune pathology in allo-transplantation,40 we found that co-transfer with CD4+ CD25+ T cells was very effective in preventing GVHD in our model. Development of GVHD is closely linked with the uncontrolled expansion of transferred T cells and their widespread dissemination throughout the body. CD4+ CD25+ T cells prevented effector T-cell expansion in lymphoid organs, thus curbing their access to peripheral tissues. Because CD4+ CD25+ T cells themselves were almost exclusively recovered from lymphoid organs and were difficult to find in peripheral tissues, we assume that the control they exerted over the naïve CD4 T-cell input took place in lymphoid organs. This is in agreement with previous reports that suggested that only CD62Lhi lymph node homing CD4+ CD25+ T cells are effective in controlling GVHD41,42 and a recent report that demonstrates that blocking entry to lymphoid organs prevents acute GVHD.43 The mechanism of action is not clear although the protective effect in GVHD was partly dependent on IL-10;44 also transforming growth factor-β is linked with the regulatory function of CD4+ CD25+ T cells.45 Furthermore, CD4+ CD25+ T cells are capable of modulating the cytokine profile of dendritic cells at an early stage of interaction promoting Toll-like receptor-independent IL-10 secretion by dendritic cells,46 which may contribute to reducing the expansion of the transferred naïve CD4 T cells during activation in lymphoid organs. Another possibility is that CD4+ CD25+ T cells block costimulatory interactions that are crucial for the expansion of effector T cells. A candidate interaction is between OX40 expressed transiently on activated T cells and OX40 ligand which is expressed by APC.47 Activation of the OX40/OX40L pathway has been implicated in a number of clinical models including allergic asthma,48 heart transplantation,49 virus-induced lung inflammation,50 corneal allograft51 and GVHD, where disruption of OX40/OX40L interactions via blockade or deletion of OX40 or OX40L has been shown to have a protective effect;35,36 OX40 is constitutively expressed on 50% of CD4+ CD25+ T cells52 and is involved in their suppressive function. These cells would therefore be better equipped to compete for interaction with OX40L expressing APC compared with conventional T cells that upregulate this receptor only transiently days after initial antigen contact. Recent reports have demonstrated that costimulation of effector T cells with OX40 also prevents the induction of new inducible CD4+ CD25+ regulatory T cells.53–55 Interference with the OX40/OX40L pathway would affect the expansion, cytokine production and survival of activated CD4 T cells and has been shown to reduce T-cell-mediated immune pathology during lung viral infection without affecting recall responses.50 In our study, recipients of OX40 deficient naïve T cells developed only milder symptoms of GVHD with delayed onset. T-cell expansion varied considerably between individual mice, but even in mice that showed an expanded T-cell population in lymphoid or peripheral organs severe GVHD was absent. OX40 promotes effector cell generation while its absence facilitates the conversion of naïve T cells to Foxp3-expressing regulatory T cells in vitro,54 which is in line with our results. Given the essential function of donor lymphocytes in mounting immune responses that can prevent the recurrence of malignant cells, any interference strategy that will limit GVH pathology without eliminating the antitumour response potential of transferred cells will be advantageous. There is a concern, however, that CD4+ CD25+ T cells will prove too strong in their inhibition as it is known that they interfere with effective antitumour responses56,57 and would thus defeat the objective of combating tumour reoccurrence in patients receiving donor lymphocyte infusion. It remains to be seen whether OX40/OX40L blockade, on the other hand, will prove sufficient to avoid excessive effector differentiation and survival of aggressive T cells without abrogating effective responses against malignant cells.
Acknowledgments
We would like to thank Christopher J. Atkins for FACS sorting. This study was supported by the Medical Research Council, UK.
Supplementary material
The following supplementary material is available for this article online:
The density plots show NK cell (a, b) and T cell (c, d) recovery from mesenteric lymph nodes of F1 Rag−/− hosts pretreated with 500 rad irradiation and additionally anti-NK1.1 to deplete NK cells (right panels) or without anti-NK1.1 (left panels). MLN were harvested two days after transfer of donor C57Bl/6 T cells. Data is representative of two hosts.
The photos show stereomicroscopy images of organs from F1 Rag−/−hosts 4 weeks after co-transfer of naïve cells FACS sorted from VA-DSRED.B-B6 mice and CD4+CD25+ from GFP.D-B6 mice. DS-Red and GFP –expressing cells were found in spleen, MLN (not shown) and gut. Very few cells expressing DS-Red expressing cells but no GFP positive cells were found in skin and kidney. Data is representative of 5 hosts. Magnification of spleen x32, gut and skin x16.
Please note: Blackwell Publishing is not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
This material is available as part of the online article from http://www.blackwell-synergy.com/doi/abs/10.1111/j.1365-2567.2008.02861.x.
(This link will take you to the article abstract.)
Please note: Blackwell Publishing is not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Gattinoni L, Finkelstein SE, Klebanoff CA, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005;202:907–12. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mackall CL, Bare CV, Granger LA, Sharrow SO, Titus JA, Gress RE. Thymic-independent T cell regeneration occurs via antigen-driven expansion of peripheral T cells resulting in a repertoire that is limited in diversity and prone to skewing. J Immunol. 1996;156:4609–16. [PubMed] [Google Scholar]
- 3.Maloy KJ, Powrie F. Regulatory T cells in the control of immune pathology. Nat Immunol. 2001;2:816–22. doi: 10.1038/ni0901-816. [DOI] [PubMed] [Google Scholar]
- 4.Barthlott T, Kassiotis G, Stockinger B. T cell regulation as a side effect of homeostasis and competition. J Exp Med. 2003;197:451–60. doi: 10.1084/jem.20021387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bourgeois C, Kassiotis G, Stockinger B. A major role for memory CD4 T cells in the control of lymphopenia-induced proliferation of naive CD4 T cells. J Immunol. 2005;174:5316–23. doi: 10.4049/jimmunol.174.9.5316. [DOI] [PubMed] [Google Scholar]
- 6.Kolb HJ, Schmid C, Barrett AJ, Schendel DJ. Graft-versus-leukemia reactions in allogeneic chimeras. Blood. 2004;103:767–76. doi: 10.1182/blood-2003-02-0342. [DOI] [PubMed] [Google Scholar]
- 7.Dazzi F, Goldman J. Donor lymphocyte infusions. Curr Opin Hematol. 1999;6:394–9. doi: 10.1097/00062752-199911000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Troeger A, Meisel R, Moritz T, Dilloo D. Immunotherapy in allogeneic hematopoietic stem cell transplantation – not just a case for effector cells. Bone Marrow Transplant. 2005;35(Suppl. 1):S59–64. doi: 10.1038/sj.bmt.1704849. [DOI] [PubMed] [Google Scholar]
- 9.Simpson E, Scott D, James E, et al. Minor H antigens: genes and peptides. Transpl Immunol. 2002;10:115–23. doi: 10.1016/s0966-3274(02)00057-6. [DOI] [PubMed] [Google Scholar]
- 10.Blazar BR, Korngold R, Vallera DA. Recent advances in graft-versus-host disease (GVHD) prevention. Immunol Rev. 1997;157:79–109. doi: 10.1111/j.1600-065x.1997.tb00976.x. [DOI] [PubMed] [Google Scholar]
- 11.Mason D. A very high level of crossreactivity is an essential feature of the T-cell receptor. Immunol Today. 1998;19:395–404. doi: 10.1016/s0167-5699(98)01299-7. [DOI] [PubMed] [Google Scholar]
- 12.Rolink AG, Radaszkiewicz T, Pals ST, van der Meer WG, Gleichmann E. Allosuppressor and allohelper T cells in acute and chronic graft-vs-host disease. I. Alloreactive suppressor cells rather than killer T cells appear to be the decisive effector cells in lethal graft-vs.-host disease. J Exp Med. 1982;155:1501–22. doi: 10.1084/jem.155.5.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rus V, Svetic A, Nguyen P, Gause WC, Via CS. Kinetics of Th1 and Th2 cytokine production during the early course of acute and chronic murine graft-versus-host disease. Regulatory role of donor CD8+ T cells. J Immunol. 1995;155:2396–406. [PubMed] [Google Scholar]
- 14.Tschetter JR, Mozes E, Shearer GM. Progression from acute to chronic disease in a murine parent-into-F1 model of graft-versus-host disease. J Immunol. 2000;165:5987–94. doi: 10.4049/jimmunol.165.10.5987. [DOI] [PubMed] [Google Scholar]
- 15.Flynn S, Toellner KM, Raykundalia C, Goodall M, Lane P. CD4 T cell cytokine differentiation: the B cell activation molecule, OX40 ligand, instructs CD4 T cells to express interleukin 4 and upregulates expression of the chemokine receptor, Blr-1. J Exp Med. 1998;188:297–304. doi: 10.1084/jem.188.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maxwell JR, Weinberg A, Prell RA, Vella AT. Danger and OX40 receptor signaling synergize to enhance memory T cell survival by inhibiting peripheral deletion. J Immunol. 2000;164:107–12. doi: 10.4049/jimmunol.164.1.107. [DOI] [PubMed] [Google Scholar]
- 17.Pippig SD, Pena-Rossi C, Long J, et al. Robust B cell immunity but impaired T cell proliferation in the absence of CD134 (OX40) J Immunol. 1999;163:6520–9. [PubMed] [Google Scholar]
- 18.Veiga-Fernandes H, Coles MC, Foster KE, et al. Tyrosine kinase receptor RET is a key regulator of Peyer’s patch organogenesis. Nature. 2007;446:547–51. doi: 10.1038/nature05597. [DOI] [PubMed] [Google Scholar]
- 19.de Boer J, Williams A, Skavdis G, et al. Transgenic mice with hematopoietic and lymphoid specific expression of Cre. Eur J Immunol. 2003;33:314–25. doi: 10.1002/immu.200310005. [DOI] [PubMed] [Google Scholar]
- 20.Stetson DB, Mohrs M, Reinhardt RL, et al. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J Exp Med. 2003;198:1069–76. doi: 10.1084/jem.20030630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu J, Min B, Hu-Li J, et al. Conditional deletion of Gata3 shows its essential function in T(H)1-T(H)2 responses. Nat Immunol. 2004;5:1157–65. doi: 10.1038/ni1128. [DOI] [PubMed] [Google Scholar]
- 22.Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson BE, McNiff J, Yan J, Doyle H, Mamula M, Shlomchik MJ, Shlomchik WD. Memory CD4+ T cells do not induce graft-versus-host disease. J Clin Invest. 2003;112:101–8. doi: 10.1172/JCI17601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen BJ, Deoliveira D, Cui X, Le NT, Son J, Whitesides JF, Chao NJ. Inability of memory T cells to induce graft-versus-host disease is a result of an abortive alloresponse. Blood. 2007;109:3115–23. doi: 10.1182/blood-2006-04-016410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen BJ, Cui X, Sempowski GD, Liu C, Chao NJ. Transfer of allogeneic CD62L– memory T cells without graft-versus-host disease. Blood. 2004;103:1534–41. doi: 10.1182/blood-2003-08-2987. [DOI] [PubMed] [Google Scholar]
- 26.Beilhack A, Schulz S, Baker J, et al. In vivo analyses of early events in acute graft-versus-host disease reveal sequential infiltration of T-cell subsets. Blood. 2005;106:1113–22. doi: 10.1182/blood-2005-02-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Joe G, Hexner E, Zhu J, Emerson SG. Alloreactive memory T cells are responsible for the persistence of graft-versus-host disease. J Immunol. 2005;174:3051–8. doi: 10.4049/jimmunol.174.5.3051. [DOI] [PubMed] [Google Scholar]
- 28.Dutt S, Tseng D, Ermann J, et al. Naive and memory T cells induce different types of graft-versus-host disease. J Immunol. 2007;179:6547–54. doi: 10.4049/jimmunol.179.10.6547. [DOI] [PubMed] [Google Scholar]
- 29.Antin JH, Ferrara JL. Cytokine dysregulation and acute graft-versus-host disease. Blood. 1992;80:2964–8. [PubMed] [Google Scholar]
- 30.Joffre O, van Meerwijk JP. CD4(+)CD25(+) regulatory T lymphocytes in bone marrow transplantation. Semin Immunol. 2006;18:128–35. doi: 10.1016/j.smim.2006.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weinberg AD, Vella AT, Croft M. OX-40: life beyond the effector T cell stage. Semin Immunol. 1998;10:471–80. doi: 10.1006/smim.1998.0146. [DOI] [PubMed] [Google Scholar]
- 32.Gramaglia I, Jember A, Pippig SD, Weinberg AD, Killeen N, Croft M. The OX40 costimulatory receptor determines the development of CD4 memory by regulating primary clonal expansion. J Immunol. 2000;165:3043–50. doi: 10.4049/jimmunol.165.6.3043. [DOI] [PubMed] [Google Scholar]
- 33.Rogers PR, Song J, Gramaglia I, Killeen N, Croft M. OX40 promotes Bcl-xL and Bcl-2 expression and is essential for long-term survival of CD4 T cells. Immunity. 2001;15:445–55. doi: 10.1016/s1074-7613(01)00191-1. [DOI] [PubMed] [Google Scholar]
- 34.Lane P. Role of OX40 signals in coordinating CD4 T cell selection, migration, and cytokine differentiation in T helper (Th)1 and Th2 cells. J Exp Med. 2000;191:201–6. doi: 10.1084/jem.191.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsukada N, Akiba H, Kobata T, Aizawa Y, Yagita H, Okumura K. Blockade of CD134 (OX40)–CD134L interaction ameliorates lethal acute graft-versus-host disease in a murine model of allogeneic bone marrow transplantation. Blood. 2000;95:2434–9. [PubMed] [Google Scholar]
- 36.Blazar BR, Sharpe AH, Chen AI, et al. Ligation of OX40 (CD134) regulates graft-versus-host disease (GVHD) and graft rejection in allogeneic bone marrow transplant recipients. Blood. 2003;101:3741–8. doi: 10.1182/blood-2002-10-3048. [DOI] [PubMed] [Google Scholar]
- 37.Maeda Y, Tawara I, Teshima T, et al. Lymphopenia-induced proliferation of donor T cells reduces their capacity for causing acute graft-versus-host disease. Exp Hematol. 2007;35:274–86. doi: 10.1016/j.exphem.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 38.Zheng H, Matte-Martone C, Li H, et al. Effector memory CD4+ T cells mediate graft-versus-leukemia without inducing graft-versus-host disease. Blood. 2008;111:2476–84. doi: 10.1182/blood-2007-08-109678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–41. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 40.Hoffmann P, Ermann J, Edinger M. CD4+ CD25+ regulatory T cells in hematopoietic stem cell transplantation. Curr Top Microbiol Immunol. 2005;293:265–85. doi: 10.1007/3-540-27702-1_12. [DOI] [PubMed] [Google Scholar]
- 41.Taylor PA, Panoskaltsis-Mortari A, Swedin JM, et al. L-Selectin(hi) but not the L-selectin(lo) CD4+ 25+ T-regulatory cells are potent inhibitors of GVHD and BM graft rejection. Blood. 2004;104:3804–12. doi: 10.1182/blood-2004-05-1850. [DOI] [PubMed] [Google Scholar]
- 42.Ermann J, Hoffmann P, Edinger M, et al. Only the CD62L+ subpopulation of CD4+ CD25+ regulatory T cells protects from lethal acute GVHD. Blood. 2005;105:2220–6. doi: 10.1182/blood-2004-05-2044. [DOI] [PubMed] [Google Scholar]
- 43.Beilhack A, Schulz S, Baker J, et al. Prevention of acute graft-versus-host disease by blocking T-cell entry to secondary lymphoid organs. Blood. 2008;111:2919–28. doi: 10.1182/blood-2007-09-112789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S. Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J Exp Med. 2002;196:389–99. doi: 10.1084/jem.20020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Powrie F, Carlino J, Leach MW, Mauze S, Coffman RL. A critical role for transforming growth factor-beta but not interleukin 4 in the suppression of T helper type 1-mediated colitis by CD45RB(low) CD4+ T cells. J Exp Med. 1996;183:2669–74. doi: 10.1084/jem.183.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Veldhoen M, Moncrieffe H, Hocking RJ, Atkins CJ, Stockinger B. Modulation of dendritic cell function by naive and regulatory CD4+ T cells. J Immunol. 2006;176:6202–10. doi: 10.4049/jimmunol.176.10.6202. [DOI] [PubMed] [Google Scholar]
- 47.Gramaglia I, Weinberg AD, Lemon M, Croft M. Ox-40 ligand: a potent costimulatory molecule for sustaining primary CD4 T cell responses. J Immunol. 1998;161:6510–7. [PubMed] [Google Scholar]
- 48.Burgess JK, Blake AE, Boustany S, et al. CD40 and OX40 ligand are increased on stimulated asthmatic airway smooth muscle. J Allergy Clin Immunol. 2005;115:302–8. doi: 10.1016/j.jaci.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 49.Curry AJ, Chikwe J, Smith XG, Cai M, Schwarz H, Bradley JA, Bolton EM. OX40 (CD134) blockade inhibits the co-stimulatory cascade and promotes heart allograft survival. Transplantation. 2004;78:807–14. doi: 10.1097/01.tp.0000131670.99000.54. [DOI] [PubMed] [Google Scholar]
- 50.Humphreys IR, Walzl G, Edwards L, Rae A, Hill S, Hussell T. A critical role for OX40 in T cell-mediated immunopathology during lung viral infection. J Exp Med. 2003;198:1237–42. doi: 10.1084/jem.20030351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hattori T, Usui Y, Okunuki Y, et al. Blockade of the OX40 ligand prolongs corneal allograft survival. Eur J Immunol. 2007;37:3597–604. doi: 10.1002/eji.200636975. [DOI] [PubMed] [Google Scholar]
- 52.Valzasina B, Guiducci C, Dislich H, Killeen N, Weinberg AD, Colombo MP. Triggering of OX40 (CD134) on CD4(+)CD25+ T cells blocks their inhibitory activity: a novel regulatory role for OX40 and its comparison with GITR. Blood. 2005;105:2845–51. doi: 10.1182/blood-2004-07-2959. [DOI] [PubMed] [Google Scholar]
- 53.Vu MD, Xiao X, Gao W, et al. OX40 costimulation turns off Foxp3+ Tregs. Blood. 2007;110:2501–10. doi: 10.1182/blood-2007-01-070748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.So T, Croft M. Cutting edge: OX40 inhibits TGF-beta- and antigen-driven conversion of naive CD4 T cells into CD25+ Foxp3+ T cells. J Immunol. 2007;179:1427–30. doi: 10.4049/jimmunol.179.3.1427. [DOI] [PubMed] [Google Scholar]
- 55.Niedbala W, Cai B, Liu H, Pitman N, Chang L, Liew FY. Nitric oxide induces CD4+ CD25+ Foxp3 regulatory T cells from CD4+ CD25 T cells via p53, IL-2, and OX40. Proc Natl Acad Sci USA. 2007;104:15478–83. doi: 10.1073/pnas.0703725104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Golgher D, Jones E, Powrie F, Elliott T, Gallimore A. Depletion of CD25+ regulatory cells uncovers immune responses to shared murine tumor rejection antigens. Eur J Immunol. 2002;32:3267–75. doi: 10.1002/1521-4141(200211)32:11<3267::AID-IMMU3267>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 57.Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+ CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol. 1999;163:5211–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The density plots show NK cell (a, b) and T cell (c, d) recovery from mesenteric lymph nodes of F1 Rag−/− hosts pretreated with 500 rad irradiation and additionally anti-NK1.1 to deplete NK cells (right panels) or without anti-NK1.1 (left panels). MLN were harvested two days after transfer of donor C57Bl/6 T cells. Data is representative of two hosts.
The photos show stereomicroscopy images of organs from F1 Rag−/−hosts 4 weeks after co-transfer of naïve cells FACS sorted from VA-DSRED.B-B6 mice and CD4+CD25+ from GFP.D-B6 mice. DS-Red and GFP –expressing cells were found in spleen, MLN (not shown) and gut. Very few cells expressing DS-Red expressing cells but no GFP positive cells were found in skin and kidney. Data is representative of 5 hosts. Magnification of spleen x32, gut and skin x16.
Please note: Blackwell Publishing is not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.