Abstract
CD66a (CEACAM1), an adhesion molecule that has regulatory function on T lymphocytes, was found to be expressed on a minority of mouse natural killer (NK) cells, especially in the liver. CD66a expression on NK cells depended on their differentiation stage, with highest levels on immature CD49b−NK cells. Expression of CD66a on NK cells was strongly enhanced by in vitro activation with interleukin-12 (IL-12) and IL-18. However, in vivo NK cell stimulation by infection with lactate dehydrogenase-elevating virus did not lead to strong CD66a expression, even on activated interferon--γ-producing NK cells. These results indicate that CD66a expression is differently regulated, depending on the NK cell activation pathway, which may lead to distinct regulatory mechanisms of the functional subpopulations of these cells.
Keywords: carcinoembryonic antigen-related cell adhesion molecule 1, cell marker, lactate dehydrogenase-elevating virus, mouse, natural killer cells
Introduction
CD66a or CEACAM1 (carcinoembryonic antigen-related cell adhesion molecule 1) is an adhesion molecule that is expressed, in addition to other cell types, on a large set of immune cells, and that can regulate some immune functions (reviewed in ref. 1). In the mouse, CD66a is expressed on macrophages, dendritic cells, thymic epithelial cells, B lymphocytes and activated T lymphocytes.1–5 On activated T cells, ligation of CD66a leads to inhibition of effector functions including allogeneic mixed lymphocyte reaction and delayed-type hypersensitivity.6 CD66a is also expressed on human natural killer (NK) cells, causing inhibition of their cytolytic activity by homophilic or heterophilic binding with molecules expressed on potential target cells.7–9
Little is known about the expression of CD66a on mouse NK cells. However, the ability of some mouse hepatitis virus (MHV) strains to infect NK cells10 suggests that CD66a, which serves as the major MHV receptor,11 is expressed on NK cells. The purpose of this study was to analyse CD66a expression on mouse NK cells.
Materials and methods
Animals
Specific pathogen-free female BALB/c, 129/Sv and C57/BL6 mice were bred at the Ludwig Institute for Cancer Research (Brussels) by G. Warnier and used when they were 8–20 weeks old. The project was approved by the local commission for animal care.
Virus
Mice were infected by intraperitoneal injection of approximately 2 × 107 50% infectious doses (ID50) of lactate dehydrogenase-elevating virus (LDV, Riley strain; American Type Culture Collection, Rockville, MD) in 500 μl saline.
Flow cytometry analysis
Liver immune cells were isolated by two successive centrifugations on 40% Percoll (Amersham Biosciences, Uppsala, Sweden) containing 100 U/ml heparin (Leo Pharma, Zaventem, Belgium). After lysis of erythrocytes for 5 min in ACK lysis buffer (0·15 m NH4Cl, 10 mm KHCO3, 0·1 mm Na2EDTA, pH 7·3) liver, spleen or peritoneal cells were incubated in 100 μl HAFA buffer (137 mm NaCl, 5 mm KCl, 0·4 mm MgSO4, 0·3 mm MgCl2, 5 mm glucose, 4 mm NaHCO3, 1 mm EDTA, 1 mm phosphate, 20 mm NaN3, 100 U/ml penicillin, 100 μg/ml streptomycin, pH 7·4, supplemented with 3% decomplemented fetal calf serum) with biotinylated anti-CD66a monoclonal antibody (mAb) (CC1,12 kind gift of Kathryn V. Holmes) followed by streptavidin–phycoerythrin (streptavidin-PE, ref. 349023; BD Biosciences, Erembodegem, Belgium), streptavidin–peridinin chlorophyll-a protein (streptavidin-PerCP, ref. 554064; BD Biosciences), and with antibodies recognizing different cell markers: fluorescein isothiocyanate (FITC) -labelled anti-mouse CD49b mAb (DX5 mAb, ref. 553857; BD Biosciences); PE-labelled anti-mouse CD49b mAb (ref. 553858, BD Biosciences); FITC-labelled anti-mouse NK1.1 mAb (ref. 553164, BD Biosciences); FITC-labelled anti-mouse Ly-49D mAb (ref. 555313; BD Biosciences); FITC-labelled anti-mouse Ly-49C/Ly-49I mAb (ref. 553276, BD Biosciences); FITC-labelled anti-mouse Ly-49G2 mAb (ref. 555315; BD Biosciences); FITC-labelled anti-mouse NKG2A/C/E mAb (ref. 550520; BD Biosciences); PE-labelled anti-mouse CD3 mAb (ref. 555275; BD Biosciences). After fixation in 0·62% paraformaldehyde, fluorescence was analysed with a FACScan flow cytometer (Becton Dickinson, Erembodegem, Belgium), as described previously.13 Interferon-γ (IFN-γ) producing cells were detected with a mouse IFN-γ secretion assay detection kit following the manufacturer’s instructions (Miltenyi Biotec, Bergisch Gladbach, Germany).
In vitro NK cell stimulation
BALB/c spleen cells (3 × 106/ml) were incubated for 3 days in H16 medium containing 10% fetal calf serum and supplemented with 0·24 mm l-asparagine, 0·55 mm l-arginine, 1·5 mm l-glutamine and 0·05 mm 2-mercaptoethanol in the presence of 1 ng/ml murine interleukin-12 (IL-12; R&D Systems, Abingdon, UK) and 100 ng/ml murine IL-18 (Peprotech EC, London, UK).14
IFN-γ assay
Interferon-γ was measured by enzyme-linked immunosorbent assay using a commercial kit (R&D Systems), following the manufacturer’s recommendations.
Results
CD66a expression on resting mouse NK cells
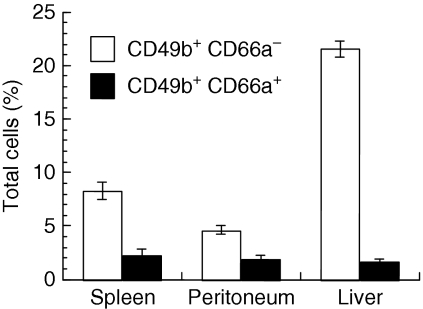
CD66a expression was analysed by flow cytometry on resting BALB/c NK cells, which were identified by CD49b expression. The NK cells represented less than 12% of cells in the spleen and peritoneum, but 23% of immune cells in the liver (Fig. 1). In all three organs, the majority of these resting NK cells did not express CD66a. However, the proportion of CD66a+ cells differed from one organ to another, from 7% of NK cells in the liver to 21% of NK cells in the spleen and 29% in the peritoneal cavity.
Figure 1.
CD66a expression by mouse natural killer (NK) cells. Peritoneal, spleen and liver cells were analysed by flow cytometry for CD49b and CD66a expression. The proportion of CD49b+ CD66a− (open columns) and CD49b+ CD66a+ cells (closed columns) is shown as mean ± SEM for three groups of two BALB/c animals.
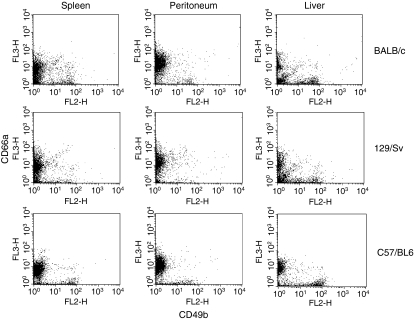
In other mouse strains, a similar high proportion of CD49b+ NK cells (more than 20%) was found in immune liver cells when compared with spleen and peritoneal cells. As in BALB/c animals, low levels of CD66a expression were observed on liver NK cells (3–13%, Fig. 2). In the spleen and the peritoneal cavity, the proportion of CD49b+ cells that expressed CD66a was higher in all three mouse strains, although the majority of the resting NK cells did not express CD66a (Fig. 2). Similar results were obtained in three, two and four independent experiments for BALB/c, 129/Sv and C57/BL6 mice, respectively.
Figure 2.
CD66a expression by natural killer (NK) cells from different mouse strains. Pooled peritoneal, spleen and liver cells from groups of two to five mice were analysed by flow cytometry for CD49b and CD66a expression. BALB/c and 129/Sv cells were analysed in the same experiment, C57/BL6 cells in an independent experiment.
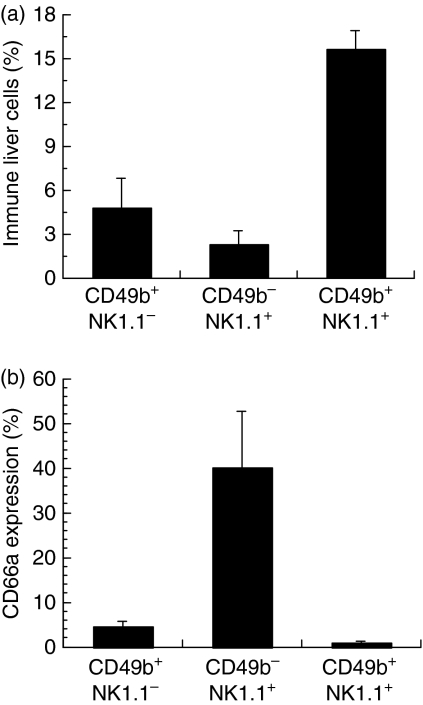
Expression of NK1.1 and of CD49b antigens defines distinct NK cell subpopulations so CD66a expression was analysed on NK cells identified in the liver of C57/BL6 mice with mAbs directed against these markers. As shown in Fig. 3(a), double-positive CD49b+ NK1.1+ cells represented a large majority of NK cells in the liver. Very few of these double-positive NK cells expressed CD66a (Fig. 3b). In sharp contrast, CD66a was expressed on more of the CD49b− NK1.1+ cells, which were a minority of NK cells in the liver. This pattern of NK cell subpopulations and CD66a expression was also observed in NK cells from the spleen and from the peritoneum (data not shown).
Figure 3.
CD66a expression by liver natural killer (NK) subpopulations. Pooled liver cells from groups of five or six C57/BL6 mice were analysed by flow cytometry for CD49b, NK1.1 and CD66a expression. (a) Proportion of CD49b+ NK1.1−, CD49b− NK1.1+ and CD49b+ NK1.1+ cells. (b) Expression of CD66a by each NK subpopulation shown in (a). Results are shown as mean ± SEM of three independent experiments.
CD66a expression was also analysed on CD49b+ cells subpopulations defined by the expression of distinct markers. As shown in Table 1, there was no difference in the percentage of these NK cell subpopulations that expressed CD66a. Similar results were observed in BALB/c and C57/BL6 mice, in three independent experiments.
Table 1.
CD66a expression on natural killer cell subpopulations
| DX5+ cell subtype1 | Proportion in DX5+ cells (%)2 | CD66a expression (% of subtype)3 |
|---|---|---|
| LY-49G2 | 48·6 | 7·1 |
| Ly-49C | 35·7 | 11·8 |
| Ly-49D | 33·7 | 8·4 |
| NK2A/C/E | 39·9 | 8·7 |
Analysis by flow cytometry of pooled liver immune cells from a group of 9 C57/BL6 mice.
Percentage of DX5+ cells that express the specified marker.
Percentage of cells positive for DX5 and for the specified marker that express CD66a.
CD66a expression on activated mouse NK cells
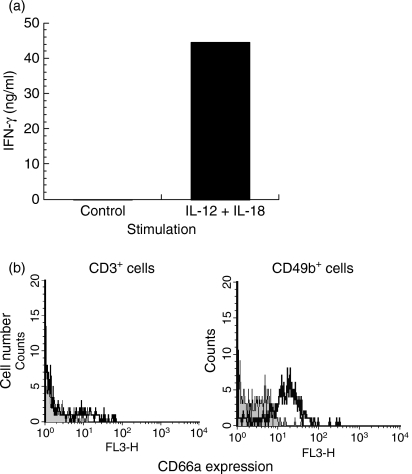
To determine whether NK cell activation would lead to enhanced CD66a expression, spleen cells from BALB/c mice were incubated with IL-12 and IL-18, following a classical protocol for NK cell stimulation.14 This treatment resulted in strong IFN-γ production, indicating that the NK cells were indeed activated (Fig. 4a). Almost all of these activated NK cells expressed CD66a (Fig. 4b), whereas no CD66a expression was detected on CD3+ cells in the same culture. Induction of CD66a expression in activated CD49b+ NK cells was observed in three independent experiments. No binding of a control antibody to these NK cells was detected (not shown).
Figure 4.
CD66a expression by in vitro activated natural killer (NK) cells. (a) Interferon-γ (IFN-γ) was measured by enzyme-linked immunosorbent assay in the supernatant of 3 × 106 BALB/c spleen cells incubated in the absence or in the presence of interleukin-12 (IL-12) and IL-18. (b) CD66a expression, analysed by flow cytometry, by CD3+ and CD49b+ BALB/c spleen cells cultured for 3 days in the presence of IL-12 and IL-18. For each cell population identified with specific marker, the grey zone represents background fluorescence, the bold line indicates labelling with CC1 anti-CD66a antibody.
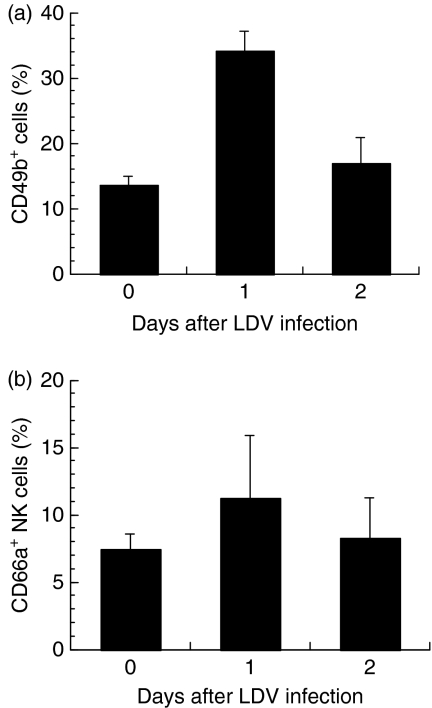
Mouse LDV infection activated NK cells, leading to enhanced cytotoxicity and IFN-γ production.13,15 CD66a expression on NK cells was therefore analysed after LDV infection. One day after infection, a moderate increase in the proportion of CD49b+ cells was observed in the liver (Fig. 5). Little or no change in the percentage of NK cells expressing CD66a was observed at 1 or 2 days after LDV inoculation (Fig. 5) in four independent experiments with BALB/c mice, and three independent experiments with C57/BL6 mice, using anti-CD49b and anti-NK1.1 antibodies as NK cell markers (data not shown). Moreover, no significant enhancement of expression of CD66a in the NK cells was found after inoculation with LDV, either in splenic or peritoneal NK cells (data not shown).
Figure 5.
CD66a expression by mouse liver natural killer (NK) cells after LDV infection. Liver immune cells were analysed by flow cytometry for CD49b and CD66a expression in control BALB/c mice and in animals infected for 1 and 2 days with LDV. (a) Proportion of CD49b+ cells. (b) Proportion od CD66a+ cells among these CD49b+ cells. Results are shown as mean ± SEM of three independent experiments, each with pooled cells from groups of three mice.
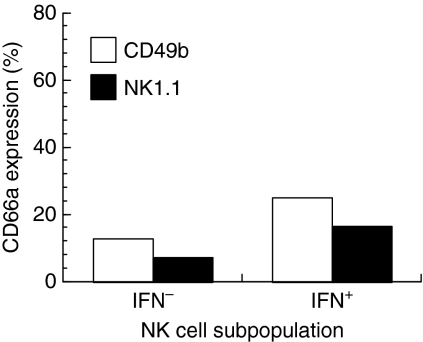
To determine which NK cells were activated after LDV infection, cells were identified by flow cytometry by their IFN-γ production and their expression of either CD49b or NK1.1. Although a slight increase in the proportion of IFN-γ-producing NK cells that expressed CD66a was observed, the majority of these activated NK cells still did not express CD66a (Fig. 6). This observation was made in two independent experiments.
Figure 6.
CD66a expression by activated mouse liver NK cells after lactate dehydrogenase-elevating virus (LDV) infection. Pooled liver immune cells from a group of five C57/BL6 mice were analysed by flow cytometry for CD49b or NK1.1, and CD66a expression, and interferon-γ (IFN-γ) production 1 day after LDV infection. The proportion of CD66a+ cells is shown for IFN-γ+ and IFN-γ– NK cells identified by expression of CD49b (open columns) or NK1.1 (closed columns).
Discussion
CD66a expression has been reported to be enhanced by activation of murine T lymphocytes,6 whereas this adhesion molecule is only weakly expressed by resting T cells.2 Similar results were reported here with murine NK cells. Few resting NK cells in the liver expressed CD66a. The specific pathogen-free status of our animals makes it likely that NK stimuli, such as viruses, were not present in the liver. In the spleen and in the peritoneal cavity, where the proportion of NK cells expressing CD66a was larger, it is quite possible that stimuli, including some originating from the commensal microflora, provided a higher level of activation to this cell population.
A previous report has shown that in vitro addition of IL-12 and IL-18 to spleen cells triggers the activation of CD3– CD49b+ NK cells, but not of NK/T cells, and induces IFN-γ production by these activated NK cells.14 We confirmed here that NK cells were activated in these culture conditions, and we showed that this activation led to CD66a expression by these cells. In contrast, CD3+ T lymphocytes from the same in vitro experiments did not increase their CD66a expression. Although it cannot be excluded that indirect mechanisms involving stimulation of other cell types by IL-12 and IL-18 play a role in this NK cell activation, this result suggests that the NK cell activation pathway that is induced by the combination of these cytokines leads to enhanced CD66a expression. Similarly, CD66a expression on human NK cells was enhanced after IL-2 stimulation.16 However, in vivo, LDV infection, which has been reported to activate NK cells to produce both cytokines and cytotoxicity,13 did not lead to a significant increase of CD66a expression. This difference may be related to a lesser stimulation of NK cells by this in vivo stimulus rather than by in vitro culture with IL-12 and IL-18, or to different activation pathways. Indeed, we have reported that LDV-induced IFN-γ production by NK cells is an IL-12-independent phenomenon in normal mice.15 Therefore, it will be interesting to analyse whether IL-12 stimulation is required for CD66a expression by murine NK cells or whether other activation pathways, such as those induced by tumour growth factor, type I IFNs, IL-2, or IL-15,17 may similarly upregulate the expression of CD66a.
On both murine and human activated T lymphocytes CD66a acts as an immunoregulatory molecule, and its ligation triggers inhibition of T-cell effector functions.6,18–20 A similar inhibitory function of CD66a has been reported on human NK cells.7–9,21 It is therefore likely that a similar immunomodulatory role corresponds to increased expression of CD66a after activation of murine NK cells. However, we were not able to inhibit murine NK cell function by CD66a ligation with the specific CC1 antibody (not shown). Whether this was the result of expression by murine NK cells of CD66a isoforms lacking immunomodulatory function,1 in our experimental model, or to the inability of CD66a to regulate some mouse NK cell functions, like IFN-γ production, remains to be determined.
Interestingly, although classical CD49b+ NK1.1+ NK cells from C57/BL6 liver expressed CD66a at very low levels, the CD49b− NK1.1+ subpopulation expressed high levels of CD66a. Because these cells have been shown to correspond to immature NK cells,22,23 this observation indicates that CD66a expression is lost with NK cell maturation, and then re-expressed following activation of mature cells through some pathways. Whether CD66a plays any regulatory role on these immature NK cells is to be determined.
CD66a serves as the major receptor for MHV.11 Therefore, its expression on immature and on some activated mouse NK cells suggests that MHV infection might selectively suppress these cells. This hypothesis is supported by the observation that fulminant MHV-3-induced hepatitis correlates with NK cell depletion in mice.10 Whether such depletion preferentially affects NK subpopulations, and triggers specific loss of immune functions has yet to be analysed.
Acknowledgments
The authors are indebted to Kathryn V. Holmes and J. Van Snick for critical reading of this manuscript, to N. Ouled Haddou and A. Thonon for expert technical assistance and to Kathryn V. Holmes and B. Lauwerys for the gift of reagents. This work was supported by the Fonds National de la Recherche Scientifique (FNRS), Fonds de la Recherche Scientifique Médicale (FRSM), Loterie Nationale, Fonds de Développement Scientifique (UCL), the State-Prime Minister’s Office – S.S.T.C. (interuniversity attraction poles, grant no. 44) and the ‘Actions de recherche concertées’ from the Communauté française de Belgique – Direction de la Recherche scientifique (concerted actions, grant no. 04/09-318), Belgium. G.T. is a FRIA fellow, A.A.F. was a fellow with the ARGO Project (managed by General Foundation of University of Salamanca) and J-P.C. is a research director with the FNRS.
Abbreviations
- CEACAM1
carcinoembryonic antigen-related cell adhesion molecule 1
- IFN-γ
interferon-γ
- IL
interleukin
- LDV
lactate dehydrogenase-elevating virus
- mAb
monoclonal antibody
- MHV
mouse hepatitis virus
- NK
natural killer
References
- 1.Gray-Owen SD, Blumberg RS. CEACAM1: contact-dependent control of immunity. Nat Rev Immunol. 2006;6:433–46. doi: 10.1038/nri1864. [DOI] [PubMed] [Google Scholar]
- 2.Coutelier J-P, Godfraind C, Dveksler GS, Wysocka M, Cardellichio CB, Noël H, Holmes KV. B lymphocyte and macrophage expression of carcinoembryonic antigen-related adhesion molecules that serve as receptors for murine coronavirus. Eur J Immunol. 1994;24:1383–90. doi: 10.1002/eji.1830240622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Godfraind C, Holmes KV, Coutelier J-P. Thymus involution induced by mouse hepatitis virus A59 in BALB/c mice. J Virol. 1995;69:6541–7. doi: 10.1128/jvi.69.10.6541-6547.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Godfraind C, Coutelier J-P. Morphological analysis of mouse hepatitis virus A59-induced pathology with regard to viral receptor expression. Histol Histopathol. 1998;13:181–99. doi: 10.14670/HH-13.181. [DOI] [PubMed] [Google Scholar]
- 5.Kammerer R, Stober D, Singer BB, Öbrink B, Reimann J. Carcinoembryonic antigen-related cell adhesion molecule 1 on murine dendritic cells is a potent regulator of T cell stimulation. J Immunol. 2001;166:6537–44. doi: 10.4049/jimmunol.166.11.6537. [DOI] [PubMed] [Google Scholar]
- 6.Nakajima A, Iijima H, Neurath MF, et al. Activation-induced expression of carcinoembryonic antigen-cell adhesion molecule 1 regulates mouse T lymphocyte function. J Immunol. 2002;168:1028–35. doi: 10.4049/jimmunol.168.3.1028. [DOI] [PubMed] [Google Scholar]
- 7.Markel G, Lieberman N, Katz G, et al. CD66a interactions between human melanoma and NK cells: a novel class I MHC-independent inhibitory mechanism of cytotoxicity. J Immunol. 2002;168:2803–10. doi: 10.4049/jimmunol.168.6.2803. [DOI] [PubMed] [Google Scholar]
- 8.Markel G, Wolf D, Hanna J, Gazit R, Goldman-Wohl D, Lavy Y, Yagel S, Mandelboim O. Pivotal role of CEACAM1 protein in the inhibition of activated decidual lymphocyte functions. J Clin Invest. 2002;110:943–53. doi: 10.1172/JCI15643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stern N, Markel G, Arnon TI, Gruda R, Wong H, Gray-Owen SD, Mandelboim O. Carcinoembryonic antigen (CEA) inhibits NK killing via interaction with CEA-related cell adhesion molecule 1. J Immunol. 2005;174:6692–701. doi: 10.4049/jimmunol.174.11.6692. [DOI] [PubMed] [Google Scholar]
- 10.Lehoux M, Jacques A, Lusignan S, Lamontagne L. Murine viral hepatitis involves NK cell depletion associated with virus-induced apoptosis. Clin Exp Immunol. 2004;137:41–51. doi: 10.1111/j.1365-2249.2004.02501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams RK, Jiang G-S, Holmes KV. Receptor for mouse hepatitis virus is a member of the carcinoembryonic antigen family of glycoproteins. Proc Natl Acad Sci USA. 1991;88:5533–6. doi: 10.1073/pnas.88.13.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams RK, Jiang G-S, Snyder SW, Frana MF, Holmes KV. Purification of the 110-kilodalton glycoprotein receptor for mouse hepatitis virus (MHV)-A59 from mouse liver and identification of a nonfunctional, homologous protein in MHV resistant SJL/J mice. J Virol. 1990;64:3817–23. doi: 10.1128/jvi.64.8.3817-3823.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Markine-Goriaynoff D, Hulhoven X, Cambiaso CL, Monteyne P, Briet T, Gonzalez M-D, Coulie P, Coutelier J-P. Natural killer cell activation after infection with lactate dehydrogenase-elevating virus. J Gen Virol. 2002;83:2709–16. doi: 10.1099/0022-1317-83-11-2709. [DOI] [PubMed] [Google Scholar]
- 14.Lauwerys BR, Garot N, Renauld J-C, Houssiau FA. Cytokine production and killer activity of NK/T-NK cells derived with IL-2, IL-15, or the combination of IL-12 and IL-18. J Immunol. 2000;165:1847–53. doi: 10.4049/jimmunol.165.4.1847. [DOI] [PubMed] [Google Scholar]
- 15.Le-Thi-Phuong T, Thirion G, Coutelier J-P. Distinct gamma interferon-production pathways in mice infected with lactate dehydrogenase-elevating virus. J Gen Virol. 2007;88:3063–6. doi: 10.1099/vir.0.83242-0. [DOI] [PubMed] [Google Scholar]
- 16.Möller MJ, Kammerer R, Grunert F, von Kleist S. Biliary glycoprotein (BGP) expression on T cells and on a natural-killer-cell sub-population. Int J Cancer. 1996;65:740–5. doi: 10.1002/(SICI)1097-0215(19960315)65:6<740::AID-IJC5>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen KB, Salazar-Mather TP, Dalod MY, et al. Coordinated and distinct roles for IFN-αβ, IL-12, and IL-15 regulation of NK cell responses to viral infection. J Immunol. 2002;169:4279–87. doi: 10.4049/jimmunol.169.8.4279. [DOI] [PubMed] [Google Scholar]
- 18.Chen C-J, Shively JE. The cell–cell adhesion molecule carcinoembryonic antigen-related cellular adhesion molecule 1 inhibits IL-2 production and proliferation in human T cells by association with Src homology protein-1 and down-regulates IL-2 receptor. J Immunol. 2004;172:3544–52. doi: 10.4049/jimmunol.172.6.3544. [DOI] [PubMed] [Google Scholar]
- 19.Markel G, Seidman R, Stern N, et al. Inhibition of human tumor-infiltrating lymphocyte effector functions by the homophilic carcinoembryonic cell adhesion molecule 1 interactions. J Immunol. 2006;177:6062–71. doi: 10.4049/jimmunol.177.9.6062. [DOI] [PubMed] [Google Scholar]
- 20.Nagaishi T, Pao L, Lin S-H, et al. SHP1 phosphatase-dependent T cell inhibition by CEACAM1 adhesion molecule isoforms. Immunity. 2006;25:769–81. doi: 10.1016/j.immuni.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 21.Markel G, Mussaffi H, Ling K-L, et al. The mechanisms controlling NK cell autoreactivity in TAP2-deficient patients. Blood. 2004;103:1770–8. doi: 10.1182/blood-2003-06-2114. [DOI] [PubMed] [Google Scholar]
- 22.Vosshenrich CAJ, Samson-Villéger SI, Di Santo JP. Distinguishing features of developing natural killer cells. Curr Opin Immunol. 2005;17:151–8. doi: 10.1016/j.coi.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 23.Walzer T, Bléry M, Chaix J, et al. Identification, activation, and selective in vivo ablation of mouse NK cells via NKp46. Proc Natl Acad Sci USA. 2007;104:3384–9. doi: 10.1073/pnas.0609692104. [DOI] [PMC free article] [PubMed] [Google Scholar]