Abstract
In light of an increasing awareness of the presence of bone marrow (BM)-derived macrophages in the normal cornea and their uncertain role in corneal diseases, it is important that the turnover rate of these resident immune cells be established. The baseline density and distribution of macrophages in the corneal stroma was investigated in Cx3cr1gfp transgenic mice in which all monocyte-derived cells express enhanced green fluorescent protein (eGFP). To quantify turnover, BM-derived cells from transgenic eGFP mice were transplanted into whole-body irradiated wild-type recipients. Additionally, wild-type BM-derived cells were injected into irradiated Cx3cr1+/gfp recipients, creating reverse chimeras. At 2, 4 and 8 weeks post-reconstitution, the number of eGFP+ cells in each corneal whole mount was calculated using epifluorescence microscopy, immunofluorescence staining and confocal microscopy. The total density of myeloid-derived cells in the normal Cx3cr1+/gfp cornea was 366 cells/mm2. In BM chimeras 2 weeks post-reconstitution, 24% of the myeloid-derived cells had been replenished and were predominantly located in the anterior stroma. By 8 weeks post-reconstitution 75% of the myeloid-derived cells had been replaced and these cells were distributed uniformly throughout the stroma. All donor eGFP+ cells expressed low to moderate levels of CD45 and CD11b, with approximately 25% coexpressing major histocompatibility complex class II, a phenotype characteristic of previous descriptions of corneal stromal macrophages. In conclusion, 75% of the myeloid-derived cells in the mouse corneal stroma are replenished after 8 weeks. These data provide a strong basis for functional investigations of the role of resident stromal macrophages versus non-haematopoietic cells using BM chimeric mice in models of corneal inflammation.
Keywords: chimera, cornea, eGFP, macrophage, turnover
Introduction
Disturbances of the avascular and highly organized structure of the cornea secondary to injury or infection may lead to impaired visual acuity. Indeed, five million people globally are blind as a result of corneal disease.1 It had been thought that the central and paracentral regions of the normal cornea were devoid of bone marrow (BM)-derived cells, including dendritic cells (DCs) and macrophages.2,3 However, several recent studies have demonstrated the presence of populations of BM-derived cells in the corneal stroma.4–6 Whereas some laboratories found major histocompatibility complex (MHC) class II− CD11c+ DCs in the corneal epithelium and anterior stroma and MHC class II− CD11b+ macrophages in the posterior stroma,5,7,8 other investigators proposed that the majority of BM-derived cells throughout the corneal stroma are CD11b+ CD11c− macrophages4 and have now concluded that CD11c+ DCs are rare in the normal mouse cornea.6,9 Although the specific lineage of BM-derived cells in various layers and regions of the cornea is still unclear, there is universal agreement that a more extensive population of these cells is present in the murine cornea than had been recognized when the presence of macrophages or DCs in the cornea was considered a hallmark of active immunopathological changes.
As a result of the increased interest in the role of BM-derived cells in innate and adaptive immune responses in the cornea, we sought a better understanding of the turnover of these cell populations in this unique tissue microenvironment. Obtaining data on the recruitment rate and turnover of macrophages and DCs following whole body irradiation was an essential prerequisite to the design of chimera experiments investigating the functional role of BM-derived cells in models of bacterial keratitis currently underway in our laboratory. The creation of BM chimeras has allowed the visualization of donor cells, which are readily identifiable post-reconstitution because of their expression of enhanced green fluorescent protein (eGFP) using in vivo epifluorescent microscopy and ex vivo confocal microscopy of the transparent cornea.10,11
In the present study, we sought to provide accurate quantitative information on the rate of recruitment and phenotype of donor BM-derived cells in the murine cornea by analysing corneal whole mounts, a method which provides a more precise means of assessing topographical distribution. Additionally, the current investigation took advantage of Cx3cr1+/gfp transgenic mice, as well as the more traditional eGFP mice, as BM donors. Cx3cr1 is the sole receptor for the chemokine Cx3cl1 (fractalkine)12,13 and is expressed predominantly by cells of myeloid origin: namely monocytes, natural killer cells, DCs and macrophages, including microglia.13–15 The development of transgenic mice in which the gene encoding eGFP was inserted into one or both copies of the Cx3cr1 locus16 has been a powerful tool in providing novel insights into both the phenotype and behaviour of blood monocytes in vivo17,18 and the characterization of resident tissue macrophages and DCs in situ in a range of tissues.19–22 The present investigation used heterozygous Cx3cr1+/gfp transgenic mice as a tool to investigate the specific turnover of myeloid-derived cells in the naïve cornea. Our findings revealed that approximately 25% of BM-derived cells had repopulated the corneal stroma by 2 weeks post-reconstitution, increasing to 75% by the 8th week post-reconstitution. The initial migration of eGFP+ cells was mostly confined to the anterior portion of the stroma.
Materials and methods
Animals
Transgenic heterozygote Cx3cr1+/gfp mice16 which retain a wild-type copy of the Cx3cr1 receptor were used in the present study. These mice, on a C57BL/6 background, were housed at the Animal Resources Centre, Murdoch, Western Australia and were used to generate baseline densities of BM-derived stromal macrophages (n = 6). For BM chimeric experiments, 6- to 12-week-old eGFP C57BL/6TgN (ACTbEGFP)10sb and Cx3cr1+/gfp mice were used as donors, with C57BL/6 and Toll-like receptor-4 double-negative (TLR4−/−) mice as recipients. At least four animals were used for each time-point (Jackson Laboratories; Bar Harbor, ME). The TLR4−/− mice, kindly provided by Shizao Akira (Research Institute for Microbial Diseases, Osaka University, Osaka, Japan), were fully backcrossed to C57BL/6 mice, and age-matched littermates were used as controls. All animals were treated in accordance with the guidelines provided in the ARVO Statement for the use of Animals in Ophthalmic and Vision Research.
Generation of eGFP chimeric mice
Either C57BL/6TgN (ACTbEGFP)10sb or heterozygote Cx3cr1+/gfp mice were used as donor mice to generate eGFP BM chimeras. Following euthanasia by CO2 asphyxiation, the femurs and tibias were harvested, the distal and proximal ends of the bones were removed and the shafts were centrifuged at 9300 g for 30 seconds at 4°. The pellet was resuspended in 1 ml of sterile red blood cell lysis buffer for 2–3 min. Cells were centrifuged at 290 g for 5 min at room temperature and washed once using sterile Dulbecco’s modified Eagles’ medium (DMEM). Recipient mice received 2 × 600 Gy doses of whole-body irradiation 3 hr apart. Immediately following the second dose, mice were injected via the tail vein with 200 μl DMEM containing approximately 5 × 106 bone marrow cells.
Epifluorescence microscopy
Mice were killed by CO2 asphyxiation and positioned in a three-point stereotactic mouse restrainer. Corneas were visualized using a high-resolution stereo fluorescence MZFLIII microscope (Leica Microsystems Inc., Bannockburn, IL) and images were captured using a digital camera (SpotCam RT Slider KE; Diagnostics Instruments, Sterling Heights, MI). The eGFP+ cells were visualized using a 465 nm laser and low- and high-magnification images of all corneas were captured using identical exposure times.
Tissue collection and immunostaining
Animals were killed at 2, 4 and 8 weeks post-reconstitution. Eyes were enucleated and fixed in 4% paraformaldehyde and stored at 4° until further processing. Corneas were dissected free from the eye and radial incisions were made to produce eight pie-shaped wedges consisting of limbus/conjunctiva peripherally and central cornea apically as previously documented.23 Tissue pieces were rehydrated in phosphate-buffered saline (PBS), incubated in 20 mm prewarmed ethylenediaminetetraacetic acid tetrasodium for 30 min at 37°, followed by incubation with a 0·2% solution of Triton-X in PBS plus 1% bovine serum albumin for 5 min at room temperature. For Cx3cr1+/gfp mice, corneas were double stained with anti-GFP and either anti-MHC class II (M5/114; 1/200; Pharmingen, San Diego, CA), anti-CD45 (1/100; Serotec, Oxford, UK), anti-CD11b (1/100; Serotec) or anti-CD11c (1/100; Pharmingen). Following overnight incubation with rabbit anti-GFP (1/200; Chemicon, Temecula, CA) at 4°, tissues were washed then incubated with Alexa Fluor 488-conjugated goat anti-rabbit (1/200; Molecular Probes, Eugene, OR) plus 10% mouse serum for 60 min at room temperature. Following further washes with PBS, tissues were incubated overnight at 4° with monoclonal antibodies (mAbs). The following day, tissues were incubated with biotinylated goat anti-rat immunoglobulin G (IgG; 1/100; Amersham Biosciences, Piscataway, NJ), for 60 min at room temperature, washed three times then incubated in Streptavidin Alexa Fluor 568 (1/100; Mol. Probes) for 60 min at room temperature. Tissues were incubated in DAPI (1/200 in PBS; Roche Molecular Biochemicals, Mannheim, Germany) for 10 min at room temperature. Stained tissue whole mounts were placed in aqueous mounting medium (Thermo Shandon, Pittsburgh, PA) onto glass slides and coverslips were added. As negative controls, isotype rat IgG2b was substituted for primary antibodies.
Examination of corneal mounts and quantitative analysis of stromal myeloid-derived cells
Corneal whole mounts were examined using both conventional epifluorescence microscopy (Olympus, Tokyo, Japan; DMRBE, Leica, Hawthorn East, Vic., Australia) and confocal microscopy (Leica TCS SP2). To perform quantitative evaluation of BM-derived cells each wedge-shaped portion of the corneal whole mount was divided into three topographical regions, namely the central, paracentral and peripheral regions.8 Confocal microscopy was used to generate series of Z-stacks through the entire thickness of the corneal stroma from the posterior aspect of the basement membrane to the anterior aspect of the endothelium in 0·5-μm increments. From these videos, the density of eGFP+ cells was averaged from two samples from each region of the cornea. To view the anterior–posterior profile of the corneal stromas, the Z-series were merged then rotated at a 90° angle using LeicaConfocal software. Final image processing was performed using AdobePhotoshop (version 7·0). To determine the turnover rate of stromal myeloid-derived cells, the density of donor eGFP+ cells was divided by the baseline density of myeloid-derived cells in the normal corneal stroma, established using conventional Cx3cr1+/gfp mice. Since there were no differences in the turnover rate observed depending on whether recipients were TLR4−/− mice or wild-type mice, all data were pooled.
Statistics
Statistical significance was determined with an unpaired t-test (Prism; Graph Pad Software, San Diego, CA). P<0·05 was considered to be significant.
Results
Quantification of the baseline density of myeloid-derived cells in the corneal stroma
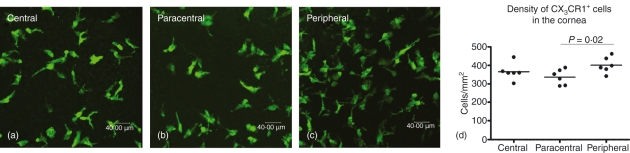
The eGFP+ cells were evident in the stroma of naïve corneas from Cx3cr1+/gfp mice (Fig. 1a–c). Extensive quantification of eGFP+ cells in Cx3cr1+/gfp mice indicated that the average density of myeloid-derived cells in the naïve cornea was 366 cells/mm2 (Fig. 1). Representative images of Z-stacked corneal samples from each region reveal a dense network of pleomorphic cells in each region of the cornea (Fig. 1a–c). There was no significant difference in the density of eGFP+ cells in the central and peripheral corneal regions but there was a significant reduction in the density of cells in the paracentral cornea compared with the peripheral cornea (P< 0·02; Fig. 1d).
Figure 1.
eGFP+ cells in the naïve corneal stroma of Cx3cr1+/gfp mice. Confocal Z-series of corneal whole mounts from the central (a), paracentral (b) and peripheral (c) corneal stroma were used to quantify the density of eGFP+ cells (d). The density of eGFP+ cells in the peripheral corneal stroma was greater than in the paracentral corneal stroma, but not than in the central corneal stroma. n = 6 animals.
Quantitative analysis of donor BM-derived eGFP+ cells in the mouse cornea
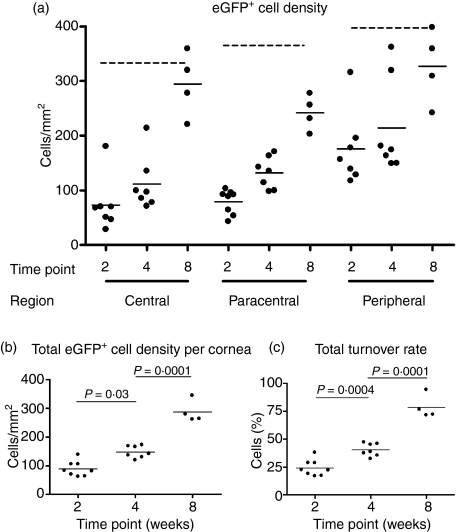
Ex vivo epifluorescence microscopy reveal numerous eGFP+ cells throughout the entire host cornea at both 2 weeks (Fig. 2a,b) and 4 weeks post-reconstitution (Fig. 2c,d) with the latter time-point demonstrating a slightly increased number of eGFP+ cells. In the central and paracentral regions of the corneal stroma, quantitative analysis confirmed a significant increase in the mean density of eGFP+ cells at 4 weeks compared to 2 weeks post-reconstitution (Fig. 3a). There was no significant increase in the density of donor derived eGFP+ cells in the peripheral cornea between the 2 and 4 weeks post-reconstitution. The mean total density (± SD) of donor eGFP+ cells in the corneas was 89 (± 26) cells/mm2 at 2 weeks, 147 (± 20) cells/mm2 at 4 weeks and 287 (± 39) cells/mm2 at 8 weeks post-reconstitution (Fig. 3b). Turnover rate (calculated by dividing the density of donor eGFP+ cells by the density of Cx3cr1+/gfp cells present in a normal cornea) increased significantly from 24% at 2 weeks through 40% at 4 weeks to 78% at 8 weeks post-reconstitution (Fig. 3c). No eGFP+ cells were observed at 1 week post-reconstitution (data not shown).
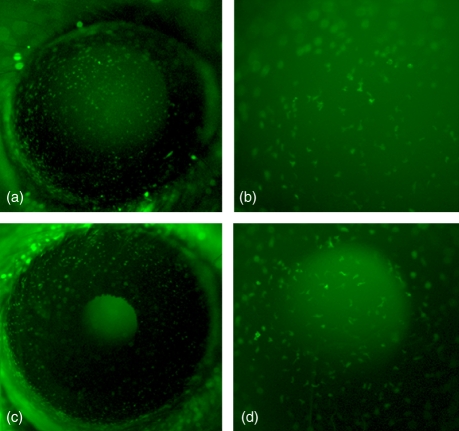
Figure 2.
Representative in vivo epifluorescent images of eGFP+ cells in the naïve corneas of eGFP chimeric mice 2 weeks (a, b) and 4 weeks (c, d) post-reconstitution. Low-power images at both time-points (a, c) reveal numerous eGFP+ cells distributed throughout the cornea, with intense staining around the limbal border. Higher magnification images of the central cornea at 2 weeks (b) and 4 weeks (d) post-reconstitution demonstrate a similar density of eGFP+ cells between the two time-points. Magnification × 32 (a, b) and × 80 (c, d).
Figure 3.
Turnover of eGFP+ cells in the corneal stroma of at 2, 4 and 8 weeks post-reconstitution. In all three anatomical regions of the cornea, there was a gradual increase in the density of donor eGFP+ cells in the corneal stroma (a), with the greatest difference in turnover between 2 and 8 weeks occurring in the central cornea. Dotted lines depict the normal mean density of eGFP+ cells in the specific regions of the corneal stroma. The total density of eGFP+ cells throughout the cornea, calculated by pooling all the regions, clearly illustrates a gradual increase from 2 to 8 weeks (b). The turnover rate increased from approximately 25% at 2 weeks to 75% at 8 weeks (c). Each data point represents one animal.
‘Reverse’ chimera demonstrating host eGFP+ myeloid-derived cells persist in the cornea 12 weeks post-reconstitution
To confirm the validity of the chimeras in measuring the turnover of BM-derived cells in the cornea, we created ‘reverse’ chimeras whereby normal, bone marrow isolated from C57BL/6 mice was transplanted into irradiated Cx3cr1+/gfp mice. This allows us to visualize the diminution of the resident eGFP+ myeloid lineage cell populations. At 12 weeks following reconstitution (Fig. 4) there were 80% fewer eGFP+ cells in the cornea than in naïve Cx3cr1+/gfp mice, suggesting that only 20% of the original Cx3cr1+ cells had not been replenished in the cornea since irradiation. This corresponds closely to the 75% replenishment rate of eGFP+ cells seen in the opposite version of the BM chimeras. As for the fate of the remaining 80% of resident myeloid-derived cells, it is not known whether these cells migrate out of the cornea or undergo apoptosis in situ during normal homeostatic conditions.
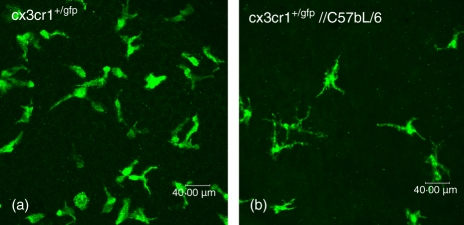
Figure 4.
Confocal Z-stack of the central corneal stroma of a ‘reverse’ chimeric, namely a Cx3cr1+/gfp, mouse which received non-eGFP bone marrow from wild-type C57BL/6 mice. Normal density of eGFP+ resident corneal macrophages in the naïve central corneal stroma (a) compared to 12 weeks post-reconstitution (b) demonstrating a diminution in the eGFP+ cell population.
Immunophenotypic characterization of donor eGFP+ myeloid-derived cells
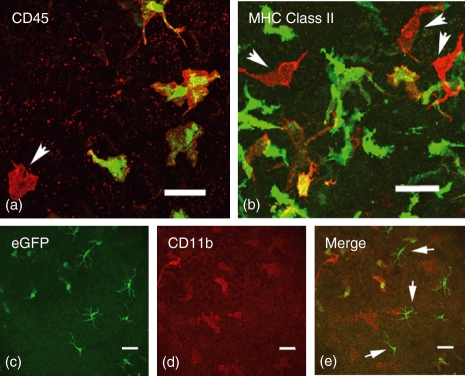
To confirm the validity of our observations, we sought to confirm that in both forms of chimeras the cells that repopulated the cornea were indeed of myeloid lineage. Immunostaining of corneal whole mounts confirmed that all eGFP+ cells expressed low to moderate levels of CD45 (Fig. 5a) and approximately 25% of donor eGFP+ cells expressed MHC class II (Fig. 5b). Cells that were MHC class II+ or CD45+ but were eGFP−, and so host-derived, were commonly located in the posterior stroma of the peripheral cornea (Fig. 5b, arrows). Further immunophenotypic studies confirmed our previous immunophenotypic analysis of Cx3cr1 eGFP+ cells in the naïve corneal stroma, which showed that the majority of these cells are CD45+, CD11b+, CD169+ and CD68+ and approximately 30% are MHC class II+, which is consistent with a macrophage phenotype (Fig. 5c–e).19 CD11c staining, although performed, provided unsatisfactory data (not shown). A population of eGFP+ CD11b− cells was observed in the epithelium of the peripheral cornea, which represent the well-recognized corneal Langerhans cells (LCs) (Fig. 5c,e).
Figure 5.
Confocal microscopy images of immunostained corneal whole mounts. All eGFP+ donor cells (green) expressed varying levels of CD45 (red; colocalized results in yellow). Red only cells are of host origin (arrow). In major histocompatibility complex (MHC) class II stained whole mounts (b) approximately 25% of donor eGFP+ cells were MHC class II+ (yellow), with host MHC class II+ cells (red only) tending to be located in the posterior stroma of the cornea (b; arrows). The eGFP+ cells in the corneal stroma were CD11b+ (c–e) whereas the eGFP+ cells observed in the epithelium were CD11b− and had the characteristic morphology of intraepithelial dendritic cells or Langerhans cells (e; arrows). Scale bars = 40 μm.
BM-derived cells repopulating the corneal stroma migrate through the anterior stroma
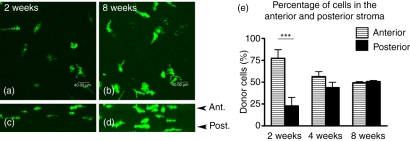
The precise mode of entry of BM-derived cells replenishing the resident population in the stroma is unknown. Therefore, in chimeric tissues, we analysed the Z-profiles of a large number of Z-series (Fig. 6a–d) and revealed that at early time-points (i.e. 2 weeks) the majority of eGFP+ cells were located in the anterior stroma (Fig. 6a,c), suggesting that the initial wave of migration of BM-derived cells occurs through the anterior rather than the posterior stroma. By 8 weeks post-reconstitution, donor eGFP+ cells became more uniformly distributed throughout the entire corneal stroma (Fig. 6b,d,e).
Figure 6.
Immigration of eGFP+ cells occurs through the anterior stroma of the cornea. Rotation of the Z-series (a) reveals eGFP+ donor cells confined mostly to the anterior stroma (c; Z-profile of a) at 2 weeks post-reconstitution. This contrasts with the uniform distribution of eGFP+ cells 8 weeks post-reconstitution (d; Z-profile of b). Quantification of the percentage of cells in the anterior stroma at 2, 4 and 8 weeks post-reconstitution showed significantly more eGFP+ donor cells in the anterior stroma at 2 weeks (P< 0·0001). n = 10 at 2 weeks, n = 7 at 4 weeks and n = 4 at 8 weeks. Ant, anterior stroma; Post, posterior stroma.
Discussion
An increased appreciation that dense networks of macrophages and DCs populate the normal mouse cornea has led to renewed interest in their role in local innate and adaptive immune responses in this unique tissue microenvironment that shares characteristics of both mucosa and skin. To investigate the function of these resident immune cells in the cornea, a detailed understanding of their distribution, regional density and turnover rate is required. In the present study, heterozygous Cx3cr1+/gfp transgenic mice, in which monocyte-derived cells that express this chemokine receptor are eGFP+, provide a powerful tool to investigate macrophage and DC populations by allowing a combination of intravital microscopy and ex vivo phenotypic analysis of corneal whole mounts. Robust quantitative data on the numbers and distribution of eGFP-expressing cells in situ can be readily obtained in comparison to the limited sampling provided by conventional histological or frozen sections. The approaches chosen in the present study have demonstrated a relatively evenly distributed network of monocyte-derived cells in the resting mouse corneal stroma, which has until now not been clearly appreciated.24
It is well accepted that circulating monocytes derived from BM enter peripheral tissues and replenish the resident tissue macrophage populations.25 The present study revealed that by 2 weeks post-reconstitution a significant number of BM-derived cells had commenced repopulation of the central cornea. In the only previous study of this nature, Nakamura and colleagues reported that the earliest migration of donor cells into the cornea occurs at around 4 weeks post-reconstitution.11 A number of possible explanations exist for the difference between the two studies. First, different methods were used to detect eGFP fluorescence. Second, lead-shielding in the previous study may have protected the corneas from the potentially harmful local effects of X-irradiation; however, it should be noted that X-irradiation at the dose chosen targets cells which are actively dividing, and there is no evidence that macrophages in the cornea actively divide in normal resting conditions. There is a single report of a small population of slow-cycling BrdU+ Langerhans cells in the rat basal limbal epithelium around the corneal margin but not in the corneal epithelium, but it is likely that these represent the only actively proliferating BM-derived cells in the cornea.26 In the absence of evidence that corneal macrophages divide in situ, we suggest that these resident cells, similar to the radioresistant microglia of the retina, remain unaffected by X-irradiation in the absence of lead shielding. Previous investigations of the turnover of resident macrophages/microglia in the retina and uveal tract did not lead-shield the eyes or heads of recipient mice.27,28 The last point to consider in comparing our data with that of Nakamura et al.11 is that to quantify eGFP+ cells, these investigators analysed in vivo images of the eye. This analysis has inherent problems because of the limited focal depth, which makes resolution of individual eGFP+ cells throughout the entire thickness and width of the convex cornea difficult. By contrast, the present study allowed regional comparison by confocal optical sectioning of the entire corneal thickness in ex vivo flattened corneal whole mounts, a far more reliable method of accurately quantifying donor cell numbers throughout the various anatomical zones of the host cornea.
The turnover rate of BM-derived cells in the cornea reported here is relatively fast compared to other non-lymphoid tissues such as lung, liver and brain, which exhibit a turnover of less than 5% by 4 weeks.29 Not surprisingly, the turnover rate in the cornea is slower than that in lymphoid tissues, for example the spleen, in which macrophages exhibit 90% turnover by 4 weeks.29,30 The faster turnover rate of eGFP+ cells in the peripheral cornea compared to the central zone of the cornea is most likely a reflection of the proximity to the vascular limbus, where all haematogenously derived cells extravasate from limbal vessels to enter the avascular cornea and where egressing cells enter the lymphatic system.31
In support of previous studies our data confirm that donor eGFP+ cells entering the host cornea were of haematopoietic origin (CD45+).11,32 Furthermore, the use of Cx3cr1 transgenic mice confirmed the myeloid origin of these cells. Approximately 25% of donor eGFP+ cells expressed MHC class II, a pattern that closely matches that described in the naïve cornea,6,19 suggesting not only that there is no specific activation of the cornea as the result of irradiation but also that the donor BM-derived cells adopt a phenotype similar to the resident host corneal macrophage populations. The eGFP+ cells in the stroma expressed CD11b, which is consistent with a macrophage phenotype, whereas the few eGFP+ cells in the peripheral corneal epithelium were MHC class II+ CD11b−, which is a DC phenotype.8,19 There was no evidence of CD11c staining in the corneal epithelium or stroma in the present study, a technical limitation experienced by other laboratories that has prevented the definitive characterization of DCs in corneal whole mounts.4,6,19,33
The observation that the initial recruitment of eGFP+ cells in the naïve cornea occurred through the anterior corneal stroma was surprising and novel. This pattern may reflect the well-described different arrangements of collagen lamellae in the anterior and posterior stromas (the lamellae in the mid and posterior stroma being arranged orthogonally compared to an oblique orientation with undulating pattern in the anterior stroma)34–39 or the embryological development of the corneal stroma from two distinct evolutionary components.40 In birds, fish, reptiles and amphibians, collagen bundles gradually turn clockwise in the anterior stroma but remain unrotated in the posterior stroma.41–43 A study of the ultrastructural organization of human corneal keratocytes suggested that a gradual clockwise shift is also seen in the anterior stroma.36 The physical environment, and possibly the extracellular matrix, may therefore favour the centripetal migration of newly recruited donor eGFP+ cells into these anterior layers. The superficial location of limbal vessels, where haematogenous cells originate, is also likely to strongly influence this pattern.
The establishment of the dynamics of myeloid-derived cell turnover in the corneas of whole-body irradiated mice will allow us to carefully select appropriate time-points in BM chimeras to compare the function of macrophages with non-haematopoietic cells in experimental models of keratitis.
Acknowledgments
We thank Dr Eric Carlson (Case Western Reserve University, Cleveland, Ohio), Dr Valentina Voigt (Lions Eye Institute, Perth, Western Australia) and Jelena Kezic (University of Western Australia) for their help in generating the bone marrow chimeras. All confocal microscopy was carried out using facilities at the Centre for Microscopy, Characterization and Analysis, The University of Western Australia, which are supported by University, State and Federal Government funding. We also acknowledge support from National Institutes of Health grants RO1EY14362 and P30EY11373 (E.P.) and the Research to Prevent Blindness Foundation and the Ohio Lions Eye Research Foundation.
References
- 1.Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: a global perspective. Bull World Health Organ. 2001;79:214–21. [PMC free article] [PubMed] [Google Scholar]
- 2.Niederkorn JY. The immune privilege of corneal allografts. Transplantation. 1999;67:1503–8. doi: 10.1097/00007890-199906270-00001. [DOI] [PubMed] [Google Scholar]
- 3.Streilein JW, Toews GB, Bergstresser PR. Corneal allografts fail to express Ia antigens. Nature. 1979;282:326–7. doi: 10.1038/282326a0. [DOI] [PubMed] [Google Scholar]
- 4.Brissette-Storkus CS, Reynolds SM, Lepisto AJ, Hendricks RL. Identification of a novel macrophage population in the normal mouse corneal stroma. Invest Ophthalmol Vis Sci. 2002;43:2264–71. [PMC free article] [PubMed] [Google Scholar]
- 5.Hamrah P, Huq SO, Liu Y, Zhang Q, Dana MR. Corneal immunity is mediated by heterogeneous population of antigen-presenting cells. J Leukoc Biol. 2003;74:172–8. doi: 10.1189/jlb.1102544. [DOI] [PubMed] [Google Scholar]
- 6.Sosnova M, Bradl M, Forrester JV. CD34+ corneal stromal cells are bone marrow-derived and express hemopoietic stem cell markers. Stem Cells. 2005;23:507–15. doi: 10.1634/stemcells.2004-0291. [DOI] [PubMed] [Google Scholar]
- 7.Dana MR. Corneal antigen-presenting cells: diversity, plasticity, and disguise: the Cogan lecture. Invest Ophthalmol Vis Sci. 2004;45:722–7. 1. doi: 10.1167/iovs.03-0803. [DOI] [PubMed] [Google Scholar]
- 8.Hamrah P, Zhang Q, Liu Y, Dana MR. Novel characterization of MHC class II-negative population of resident corneal Langerhans cell-type dendritic cells. Invest Ophthalmol Vis Sci. 2002;43:639–46. [PubMed] [Google Scholar]
- 9.Kuffova L, Netukova M, Duncan L, Porter A, Stockinger B, Forrester JV. Cross presentation of antigen on MHC class II via the draining lymph node after corneal transplantation in mice. J Immunol. 2008;180:1353–61. doi: 10.4049/jimmunol.180.3.1353. [DOI] [PubMed] [Google Scholar]
- 10.Carlson EC, Drazba J, Yang X, Perez VL. Visualization and characterization of inflammatory cell recruitment and migration through the corneal stroma in endotoxin-induced keratitis. Invest Ophthalmol Vis Sci. 2006;47:241–8. doi: 10.1167/iovs.04-0741. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura T, Ishikawa F, Sonoda KH, et al. Characterization and distribution of bone marrow-derived cells in mouse cornea. Invest Ophthalmol Vis Sci. 2005;46:497–503. doi: 10.1167/iovs.04-1154. [DOI] [PubMed] [Google Scholar]
- 12.Bazan JF, Bacon KB, Hardiman G, et al. A new class of membrane-bound chemokine with a CX3C motif. Nature. 1997;385:640–4. doi: 10.1038/385640a0. [DOI] [PubMed] [Google Scholar]
- 13.Imai T, Hieshima K, Haskell C, et al. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell. 1997;91:521–30. doi: 10.1016/s0092-8674(00)80438-9. [DOI] [PubMed] [Google Scholar]
- 14.Nishiyori A, Minami M, Ohtani Y, et al. Localization of fractalkine and CX3CR1 mRNAs in rat brain: does fractalkine play a role in signaling from neuron to microglia? FEBS Lett. 1998;429:167–72. doi: 10.1016/s0014-5793(98)00583-3. [DOI] [PubMed] [Google Scholar]
- 15.Papadopoulos EJ, Sassetti C, Saeki H, et al. Fractalkine, a CX3C chemokine, is expressed by dendritic cells and is up-regulated upon dendritic cell maturation. Eur J Immunol. 1999;29:2551–9. doi: 10.1002/(SICI)1521-4141(199908)29:08<2551::AID-IMMU2551>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 16.Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol. 2000;20:4106–14. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Auffray C, Fogg D, Garfa M, et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–70. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- 18.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 19.Chinnery HR, Ruitenberg MJ, Plant GW, Pearlman E, Jung S, McMenamin PG. The chemokine receptor CX3CR1 mediates homing of MHC class II-positive cells to the normal mouse corneal epithelium. Invest Ophthalmol Vis Sci. 2007;48:1568–74. doi: 10.1167/iovs.06-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kezic J, Xu H, Chinnery HR, Murphy CC, McMenamin PG. Retinal microglia and uveal tract dendritic cells and macrophages are not CX3CR1 dependent in their recruitment and distribution in the young mouse eye. Invest Ophthalmol Vis Sci. 2008;49:1599–608. doi: 10.1167/iovs.07-0953. [DOI] [PubMed] [Google Scholar]
- 21.Niess JH, Brand S, Gu X, et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–8. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 22.Soos TJ, Sims TN, Barisoni L, Lin K, Littman DR, Dustin ML, Nelson PJ. CX3CR1+ interstitial dendritic cells form a contiguous network throughout the entire kidney. Kidney Int. 2006;70:591–6. doi: 10.1038/sj.ki.5001567. [DOI] [PubMed] [Google Scholar]
- 23.McMenamin PG. Optimal methods for preparation and immunostaining of iris, ciliary body, and choroidal wholemounts. Invest Ophthalmol Vis Sci. 2000;41:3043–8. [PubMed] [Google Scholar]
- 24.Hamrah P, Liu Y, Zhang Q, Dana MR. The corneal stroma is endowed with a significant number of resident dendritic cells. Invest Ophthalmol Vis Sci. 2003;44:581–9. doi: 10.1167/iovs.02-0838. [DOI] [PubMed] [Google Scholar]
- 25.Volkman A, Gowans JL. The origin of macrophages from bone marrow in the rat. Br J Exp Pathol. 1965;46:62–70. [PMC free article] [PubMed] [Google Scholar]
- 26.Chen W, Hara K, Tian Q, Zhao K, Yoshitomi T. Existence of small slow-cycling Langerhans cells in the limbal basal epithelium that express ABCG2. Exp Eye Res. 2007;84:626–34. doi: 10.1016/j.exer.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 27.Xu H, Chen M, Mayer EJ, Forrester JV, Dick AD. Turnover of resident retinal microglia in the normal adult mouse. Glia. 2007;55:1189–98. doi: 10.1002/glia.20535. [DOI] [PubMed] [Google Scholar]
- 28.Tomita M, Yamada H, Adachi Y, et al. Choroidal neovascularization is provided by bone marrow cells. Stem Cells. 2004;22:21–6. doi: 10.1634/stemcells.22-1-21. [DOI] [PubMed] [Google Scholar]
- 29.Kennedy DW, Abkowitz JL. Kinetics of central nervous system microglial and macrophage engraftment: analysis using a transgenic bone marrow transplantation model. Blood. 1997;90:986–93. [PubMed] [Google Scholar]
- 30.Krall WJ, Challita PM, Perlmutter LS, Skelton DC, Kohn DB. Cells expressing human glucocerebrosidase from a retroviral vector repopulate macrophages and central nervous system microglia after murine bone marrow transplantation. Blood. 1994;83:2737–48. [PubMed] [Google Scholar]
- 31.Liu Y, Hamrah P, Zhang Q, Taylor AW, Dana MR. Draining lymph nodes of corneal transplant hosts exhibit evidence for donor major histocompatibility complex (MHC) class II-positive dendritic cells derived from MHC class II-negative grafts. J Exp Med. 2002;195:259–68. doi: 10.1084/jem.20010838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hisatomi T, Sonoda KH, Ishikawa F, et al. Identification of resident and inflammatory bone marrow derived cells in the sclera by bone marrow and haematopoietic stem cell transplantation. Br J Ophthalmol. 2007;91:520–6. doi: 10.1136/bjo.2006.102046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang M, Kitahara Y, Yoshida A, Hori J. Latanoprost does not affect immune privilege of corneal allografts. Exp Eye Res. 2008;86:394–402. doi: 10.1016/j.exer.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 34.Daxer A, Fratzl P. Collagen fibril orientation in the human corneal stroma and its implication in keratoconus. Invest Ophthalmol Vis Sci. 1997;38:121–9. [PubMed] [Google Scholar]
- 35.Komai Y, Ushiki T. The three-dimensional organization of collagen fibrils in the human cornea and sclera. Invest Ophthalmol Vis Sci. 1991;32:2244–58. [PubMed] [Google Scholar]
- 36.Muller LJ, Pels L, Vrensen GF. Novel aspects of the ultrastructural organization of human corneal keratocytes. Invest Ophthalmol Vis Sci. 1995;36:2557–67. [PubMed] [Google Scholar]
- 37.Pouliquen YJ. 1984 Castroviejo lecture. Fine structure of the corneal stroma. Cornea. 1984;3:168–77. [PubMed] [Google Scholar]
- 38.Radner W, Zehetmayer M, Aufreiter R, Mallinger R. Interlacing and cross-angle distribution of collagen lamellae in the human cornea. Cornea. 1998;17:537–43. doi: 10.1097/00003226-199809000-00012. [DOI] [PubMed] [Google Scholar]
- 39.Smolek MK. Interlamellar cohesive strength in the vertical meridian of human eye bank corneas. Invest Ophthalmol Vis Sci. 1993;34:2962–9. [PubMed] [Google Scholar]
- 40.Walls GL. The Vertebrate Eye and its Adaptive Radiation. Bloomfield Hills: Cranbrook Institute of Science; 1942. [Google Scholar]
- 41.Coulombre J, Coulombre A. Corneal development. V. Treatment of five-day-old embryos of domestic fowl with 6-diazo-5-oxo-l-norleucine (DON) Dev Biol. 1975;45:291–303. doi: 10.1016/0012-1606(75)90067-6. [DOI] [PubMed] [Google Scholar]
- 42.Hay ED. Development of the vertebrate cornea. Int Rev Cytol. 1979;63:263–322. doi: 10.1016/s0074-7696(08)61760-x. [DOI] [PubMed] [Google Scholar]
- 43.Trelstad RL, Coulombre AJ. Morphogenesis of the collagenous stroma in the chick cornea. J Cell Biol. 1971;50:840–58. doi: 10.1083/jcb.50.3.840. [DOI] [PMC free article] [PubMed] [Google Scholar]