Abstract
Fas-associated protein with death domain/mediator of receptor induced toxicity (FADD/MORT1) was first described as a transducer of death receptor signalling but was later recognized also to be important for proliferation of T cells. B-cell lymphoma 3 (Bcl-3) is a relatively little understood member of the nuclear factor (NF)-κB family of transcription factors. We recently found that Bcl-3 is up-regulated in T cells from mice where FADD function is blocked by a dominant negative transgene (FADD-DN). To understand the importance of this, we generated FADD-DN/bcl-3−/− mice. Here, we report that T cells from these mice show massive cell death and severely reduced proliferation in response to T-cell receptor (TCR) stimulation in vitro. Transgenic co-expression of Bcl-2 (FADD-DN/bcl-3−/−/vav-bcl-2 mice) rescued the survival but not the proliferation of T cells. FADD-DN/bcl-3−/− mice had normal thymocyte numbers but reduced numbers of peripheral T cells despite an increase in cycling T cells in vivo. However, activation of the classical NF-κB and extracellular regulated kinase (ERK) pathways and expression of interleukin (IL)-2 mRNA upon stimulation were normal in T cells from FADD-DN/bcl-3−/− mice. These data suggest that FADD and Bcl-3 regulate separate pathways that both contribute to survival and proliferation in mouse T cells.
Keywords: apoptosis, Bcl-3, FADD, proliferation, T cell
Introduction
The pathway that leads to caspase activation and apoptosis upon ligation of death receptors has been extensively studied and well characterized. Ligation of, for instance, Fas by Fas ligand (FasL) causes the clustering of Fas and the intracellular recruitment and clustering of the adapter protein Fas-associated protein with death domain (FADD) and pro-caspase-8 (in humans also pro-caspase-10). The high local concentration of pro-caspase-8 induced in this process leads to its auto-activation, whereupon active caspase-8 cleaves cell death substrates that cause apoptosis of the cell.1 The protein cellular FLICE-inhibitory protein (cFLIP) which has structural similarity to pro-caspase-8 but lacks enzymatic activity, can either enhance or reduce caspase-8 activation, probably dependent on its expression levels.2,3
In addition to this function in apoptosis, the components of death receptor signalling have now well-established roles in other cellular processes. Mice deficient in FADD, caspase-8 or cFLIP die during embryogenesis, probably as a result of defects in the formation of endothelia.4–8 Within the haemopoietic system, FADD and caspase-8 have been shown to be required for progenitor cell proliferation in response to cytokines9 and for proliferative responses in B cells.10 In T cells, loss of FADD or caspase-8 function leads to a defect in proliferation and slightly enhanced apoptosis in response to antigen or mitogen in models of transgenic mice expressing dominant negative (dn) FADD,11,12 in FADD−/− T cells (reconstituted by injection into rag-deficient blastocysts5) and in caspase-8-deficient T cells (from mice with lineage-specific gene targeting13). A small set of human patients with autoimmune lymphoproliferative syndrome further show mutations in the caspase-8 gene and defects in T-cell proliferation.14 Intriguingly, the phenotype of T cells without cFLIP is similar but even stronger, with more severe defects in proliferation and survival, leading to depletion of peripheral T cells.15,16
Although this defect in proliferation is very clear, it is unknown which T-cell receptor (TCR)-triggered pathways are disturbed in T cells in the absence of FADD/caspase-8/cFLIP. In T cells expressing dnFADD, a number of possibilities have been tested: activation of nuclear factor (NF)-κB, mitogen-activated protein (MAP)-kinase activation, calcium flux and production of interleukin (IL)-2 were all normal in the absence of functional FADD.17,18 However, gene expression analysis by DNA array showed the deregulation of a number of genes in resting and mitogen-activated T cells.19 Although there were no obvious candidates whose deregulation would have explained the proliferative defect, it was interesting to note the up-regulation of B-cell lymphoma 3 (Bcl-3) in T cells from FADD-DN mice. Bcl-3 is a relatively poorly characterized member of the NF-κB family of transcription factors. Although structurally related to the inhibitory members of this family, Bcl-3 has been shown to be able to form transcriptionally active complexes.20 Bcl-3 is over-expressed in some forms of human lymphoma21 and has been implicated in both cell cycle progression22 and survival.23,24 The reported strong proliferative defect and weak survival defect in mice without functional FADD suggested that the up-regulation of Bcl-3 might contribute to the phenotype of FADD-DN T cells.
Here, we report an analysis of mice over-expressing dnFADD in their T cells and lacking Bcl-3 (FADD-DN/bcl-3−/− mice). Unexpectedly, T cells from these mice showed even further reduced proliferation and enhanced cell death during mitogenic stimulation. Additional expression of transgenic Bcl-2 (FADD-DN/bcl-3−/−/vav-bcl-2 mice) improved survival but did not rescue proliferation of T cells. FADD and Bcl-3 therefore appear to act in complementary pathways that contribute to both survival and proliferation in mouse T cells.
Materials and methods
Mice
C57BL/6 mice were purchased from Harlan-Winckelmann (Borchen, Germany). The strains FADD-DN,11vav-bcl-225 and bcl-3−/−26 have been described previously. FADD-DN and vav-bcl-2 transgenic mice were generated on a C57BL/6 background. The bcl-3−/− mice were generated on a mixed C57BL/6x129SV background [using 129SV embryonic stem (ES) cells] and were backcrossed onto the C57BL/6 background for 12 (bcl-3−/−) generations. Mice were kept under specific pathogen-free conditions. Double and triple mutant mice were generated by interbreeding and typed by western blotting (vav-bcl-2) and polymerase chain reaction (PCR) (FADD-DN and bcl-3−/−). FADD-DN and vav-bcl-2 animals analysed were heterozygous for the transgenes.
T-cell isolation and stimulation
Single-cell suspensions were prepared from spleens. Following red blood cell lysis, B and natural killer (NK) cells were labelled with fluorescein isothiocyanate (FITC)-coupled antibodies (anto-B220 and NK1.1; BD Biosciences Pharmingen, San Diego, CA), followed by anti-FITC antibodies coupled to magnetic beads. Labelled cells were removed by passage over a magnetic antibody cell sorting (MACS) column (Miltenyi Biotech, Bergisch Gladbach, Germany). Negatively selected cells were seeded in flat-bottom 96-well plates and stimulated with plate-coated anti-CD3 antibodies, anti-CD3 plus anti-CD28 (BD Biosciences Pharmingen), phorbol 12-myristate 13-acetate (PMA) (2 ng/ml) plus ionomycin (200 ng/ml; Sigma-Aldrich, Steinheim, Germany) or concanavalin A (Con A) (2 μg/ml; GE Healthcare, Munich, Germany). After 42 hr, cultures were pulsed with 3H-thymidine for 6 hr, and thymidine incorporation was measured by direct beta-counting (Beckman-Coulter-Packard, Krefeld, Germany).
Analysis of subpopulations and of cell death
Single-cell suspensions of spleen cells were prepared and stained with Cy5-coupled antibodies to CD3 and FITC-coupled anti-B220 antibodies (BD Biosciences Pharmingen). Analysis was performed by flow cytometry. For detection of cell death, cultures were stained with FITC-coupled annexin V (Caltag Laboratories, Burlingame, CA) and propidium iodide (PI) (Sigma-Aldrich) followed by flow cytometry.
Analysis of cell cycle and proximal TCR signalling
For cell cycle analysis, cells were fixed with 70% ethanol, washed in phosphate-buffered saline (PBS) containing 2% fetal calf serum (FCS), stained with PI and analysed by flow cytometry. To analyse kinase activation, 106 cells were stimulated with PMA/ionomycin for various times. Cells were lysed in lysis buffer [50 mm HEPES, pH 7·5, 150 mm NaCl, 1 mm ethylenediaminetetraacetic acid (EDTA), 10% glycerol, 1% Triton X-100, 10 mm pyrophosphat Na4P2O7, 10 mm NaF, 1 mm Na-O-vanadat and protease inhibitors] and subjected to western blotting with antibodies specific for IκBa, phospho-IκBa, phospho-extracellular regulated kinase (ERK) (all from Cell Signalling Technology, Frankfurt, Germany) and β-actin (Sigma).
Preparation of nuclear extracts
Purified T cells (2 × 106) stimulated as indicated were washed once with PBS and re-suspended in cold buffer A [10 mm HEPES, 10 mm KCl, 0·1 mm EDTA, 0·1 mm ethyleneglycoltetraacetic acid (EGTA), 1 mm dithiothreitol (DTT), 5 mm NaF, 1 mm sodium orthovanadate and protease inhibitor cocktail (Roche, Mannheim, Germany)]. After 15 min on ice, 0·6% Igepal CA-630 was added and tubes were vigorously vortexed for 10 seconds. Nuclei were pelleted at 500 g for 3 min and re-suspended in Laemmli buffer. After boiling at 95° for 5 min, samples were subjected to western blot analysis with antibodies specific for p65 (C-20; Santa Cruz Biotechnology, Santa Cruz, CA) and poly-ADP-ribose-polymerase 1 (PARP) (Cell Signalling Technology).
Real-time PCR
106 T cells were solubilized in 0·5 ml TriFast reagent (Peqlab, Erlangen, Germany), and RNA was isolated following the manufacturer’s instructions. Two micrograms of total RNA was translated into cDNA (Superscript II system; Invitrogen, Carlsbad, CA). IL-2 PCR reactions were performed using cyclophilin as internal control and SybrGreen for detection (Applied Biosystems, Foster City, CA) as described previously.19 Within one experiment, wild-type (WT) results were set to one, and relative levels of the various genotypes were calculated.
Results
Reduced proliferation and enhanced apoptosis in FADD-DN/bcl-3−/− mouse T cells
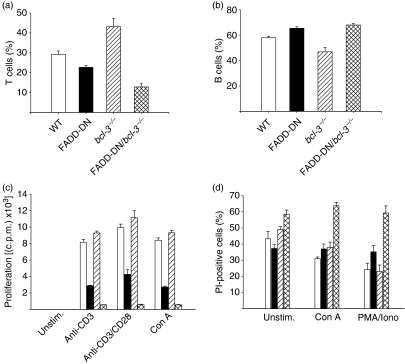
In microarray experiments, Bcl-3 mRNA was previously found to be up-regulated by a factor of 2·9 in resting FADD-DD T cells and by a factor of 5·3 upon 20 hr of stimulation with PMA/ionomycin.19 Analysis of Bcl-3 mRNA by RT-PCR in resting cells from FADD-DN mice showed an up-regulation of about a factor of two (data not shown). To investigate whether this up-regulation contributed to the phenotype of FADD-DN mice, we generated FADD-DN/bcl-3−/− mice by interbreeding of FADD-DN mice with bcl-3−/− mice. Analysis of thymic subpopulations by testing CD4 and CD8 expression showed no discernible difference compared with WT mice (not shown). However, the proportion of T cells in spleens from FADD-DN/bcl-3−/− mice was reduced by about half (Fig. 1a), accompanied by a relative increase in B cells (Fig. 1b), an effect that was largely a result of a reduction in T-cell numbers (see below) and that was seen neither in FADD-DN nor in bcl-3−/− single-mutant mice (bcl-3−/− mice develop only variably hypoplastic lymph nodes,27 restricting the analysis to the spleen). The ratio of CD4/CD8 T cells was unchanged (not shown).
Figure 1.
Loss of peripheral T cells, proliferation defect and enhanced cell death in T cells in Fas-associated protein with death domain/B-cell lymphoma 3 (FADD-DN/bcl-3−/−) mice. (a, b) Percentages of T and B cells in spleens were assessed by staining for CD3 and B220, followed by flow cytometry. Data represent mean ± standard error of the mean (SEM) for four mice of each genotype. (c) T cells were purified by magnetic sorting from spleens of wild-type (WT) mice (open bars), FADD-DN transgenic mice (filled bars), bcl-3−/− mice (hatched bars) and FADD-DN/bcl-3−/− mice (cross-hatched bars). Purified T cells (3 × 105) were stimulated with the mitogens indicated. Proliferation was measured as incorporation of 3H-thymidine at 48 hr. One representative experiment is shown (mean ± standard deviation). A similar reduction of proliferation of T cells from FADD-DN/bcl-3−/− mice for the various stimuli was seen in at least five experiments. (d) T cells were purified by magnetic sorting from spleens of WT mice (open bars), FADD-DN transgenic mice (filled bars), bcl-3−/− mice (hatched bars) and FADD-DN/bcl-3−/− mice (cross-hatched bars). Purified T cells (3 × 105) were cultured without stimulation or stimulated with the mitogens indicated for 24 hr, and cell death was measured as uptake of propidium iodide (PI). Data represent mean ± SEM for at least three mice of each genotype analysed in independent experiments. Con A, concanavalin A; c.p.m., counts per minute; Iono, ionomycin; PMA, phorbol 12-myristate 13-acetate; Unstim., unstimulated.
As the major phenotypical abnormality in FADD-DN mice is the reduced proliferative capacity, we examined proliferation of T cells from FADD-DN/bcl-3−/− mice in response to mitogenic stimulation. Surprisingly, these cells showed an almost complete lack of response when challenged with anti-CD3, anti-CD3/CD28, Con A or PMA/ionomycin (Fig. 1c and data not shown). Loss of Bcl-3 thus strongly enhanced the effect of the loss of FADD function.
A proliferation defect may be caused by both a failure of the cells to divide and the induction of apoptosis during culture. Indeed, the proliferation defect appeared to be connected to a survival defect in T cells from FADD-DN/bcl-3−/− mice. When cultured without stimulation overnight, there was considerable spontaneous cell death of WT T cells, which was not greatly different from that of cells from FADD-DN or bcl-3−/− single-mutant mice (proliferation was normal in bcl-3−/− mice). Mitogenic stimulation afforded a degree of protection in that the portion of dead cells was lower in stimulated cell cultures (Fig. 1d). In T cells from FADD-DN/bcl-3−/− mice, a higher percentage of dead cells was observed in unstimulated cultures. Strikingly, stimulation failed to reduce the number of dead cells in T-cell populations from these mice (Fig. 1d). These data suggest that FADD and Bcl-3 regulate complementary pathways that both impact on T-cell survival. Loss of Bcl-3 on its own produces no obvious phenotype while loss of FADD function produces a clear defect in proliferation and to a lesser extent in survival. However, loss of Bcl-3 in FADD-DN mice exacerbates this phenotype. This suggests that the FADD-regulated pathway plays an important role while Bcl-3 may be auxiliary. However, the function of Bcl-3 becomes critical in the absence of FADD. Transduction of T cells from bcl-3−/− mice with retrovirus driving expression of FADD-DN also caused higher levels of apoptosis than in WT cells (not shown). This survival defect may account for the measured loss of T cells in spleens of FADD-DN/bcl-3−/− mice (Fig. 1a and below).
Reduced proliferation of T cells from FADD-DN/bcl-3−/−/vav-bcl-2 mice
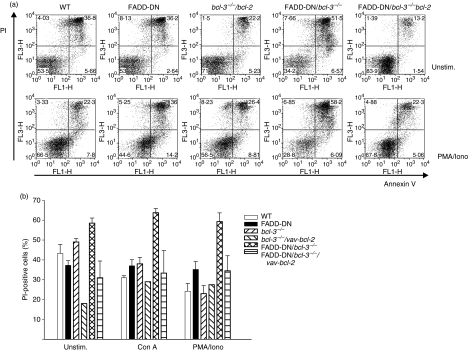
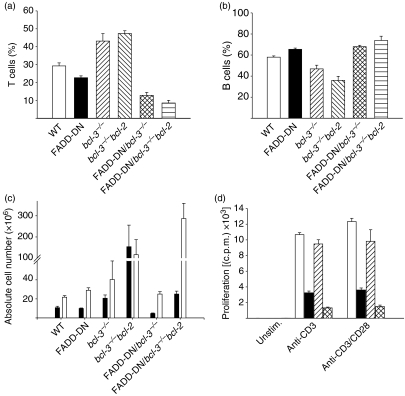
Increased apoptosis might obscure the proliferative potential of FADD-DN/bcl-3−/− T cells. Therefore, we crossed FADD-DN/bcl-3−/− mice with vav-bcl-2-transgenic mice, which express Bcl-2 throughout their haemopoietic compartment.25 Transgenic expression of Bcl-2 rescued T cells lacking the functions of FADD and Bcl-3 from cell death. As shown in Fig. 2, levels of cell death were strongly reduced in T cells from FADD-DN/bcl-3−/−/vav-bcl-2 mice as compared with FADD-DN/bcl-3−/− mice. In the spleen, there was a reduction of T cells in FADD-DN/bcl-3−/− mice compared with the respective single-mutant genotypes, in terms of both percentages and absolute numbers (Fig. 3a–c). Absolute T-cell numbers were increased in FADD-DN/bcl-3−/−/vav-bcl-2 as compared with FADD-DN/bcl-3−/− mice but severely reduced in comparison with bcl-3−/−/vav-bcl-2 mice. Remarkably, FADD-DN/bcl-3−/−/vav-bcl-2 mice showed an increase in B-cell numbers compared with bcl-3−/−/vav-bcl-2 mice, although the expression of FADD-DN would be limited to T cells in these mice. FADD-DN/bcl-3−/−/vav-bcl-2 mice died within the first year, which was probably linked to the very noticeable development of splenomegaly. A slight increase in B-cell numbers has been reported in mice lacking caspase-8 in their T-cell lineage.13 It is therefore conceivable that the increase in B-cell numbers in FADD-DN/bcl-3−/−/vav-bcl-2 mice is attributable to the same unidentified mechanism, which may be aggravated by the expression of Bcl-2 in the B cells.
Figure 2.
B-cell lymphoma 2 (Bcl-2) over-expression blocks the abnormally increased death of T cells from Fas-associated protein with death domain (FADD-DN)/bcl-3−/− mice. T cells were purified as described in the text from spleens of mice with the genotypes indicated. Purified T cells (3 × 105) were cultured without stimulation (Unstim.) or stimulated with the agents indicated overnight. Cell death was measured by staining with annexin V and propidium iodide (PI). (a) Examples of cultures of unstimulated (top) and phorbol 12-myristate 13-acetate/ionomycin (PMA/Iono)-stimulated cells (bottom). (b) Summary of the data obtained for several mice. Data represent means for two bcl-3−/−/vav-bcl-2 mice [individual values: unstimulated, 19 and 17%; concanavalin A (Con A), 33 and 25%; PMA/Iono, 29 and 26%] and mean ± standard error of the mean for three or four FADD-DN-bcl-3−/−/vav-bcl-2 mice. For comparison, data for the other genotypes are reproduced from Fig. 1(d).
Figure 3.
Cell numbers and proliferation defect of T cells from Fas-associated protein with death domain/B-cell lymphoma 3 (FADD-DN/bcl-3−/−)/vav-bcl-2 mice. (a–c) Comparison of relative numbers of T cells (a) and B cells (b) and of absolute lymphocyte numbers in spleens from mice of the various genotypes [(c) filled bars: T cells; open bars: B cells]. Data represent mean ± standard error of the mean for four mice (a, b) or five or six mice (c) with the exception of bcl-3−/−/vav-bcl-2 mice (three mice). Data from single- and double-mutant mice are reproduced from Fig. 1 for better comparison. (d) T cells were purified by magnetic sorting from spleens of wild-type (WT) mice (open bars), FADD-DN mice (filled bars), bcl-3−/−/vav-bcl-2 mice (hatched bars) and FADD-DN/bcl-3−/−/vav-bcl-2 mice (cross-hatched bars). Purified T cells (3 × 105) were stimulated with the agents indicated. Proliferation was measured as incorporation of 3H-thymidine at 48 hr. One representative experiment is shown (mean ± standard deviation). A similar reduction of proliferation of T cells from FADD-DN/bcl-3−/−/vav-bcl-2 mice was seen in five experiments. c.p.m., counts per minute.
Surprisingly, although cell death was blocked by Bcl-2 expression, this improved proliferation only marginally. T cells from FADD-DN/bcl-3−/−/vav-bcl-2 mice showed proliferation similar to that of FADD-DN/bcl-3−/− mice, i.e. a very clearly reduced proliferative response in comparison with FADD-DN mice (Fig. 3d). These data strongly suggest that two pathways exist, one regulated by FADD-DN, and the other downstream of Bcl-3. Loss of Bcl-3 on its own produces no overt phenotype but the role of Bcl-3 is unmasked in the absence of FADD function. These pathways impact not only on survival but also on proliferation of primary T cells.
A portion of T cells from FADD-DN/bcl-3−/− mice are in cell cycle in vivo
Of the group of mutant T cells lacking functional FADD, caspase-8 or cFLIP, the most striking phenotype has been found for T cells deficient in cFLIP. Although there were differences in the two models described, evidence of disturbed thymic maturation was found in both strains of mice.15,16 Furthermore, numbers of T cells lacking cFLIP were greatly diminished, and proliferation of these cells when stimulated in vitro was much more markedly reduced compared with FADD- or caspase-8-deficient T cells.15,16 Despite this reduced proliferative response in vitro, there was evidence for activation in the absence of immunization in vivo, such as a higher number of freshly isolated cells in cell cycle.15
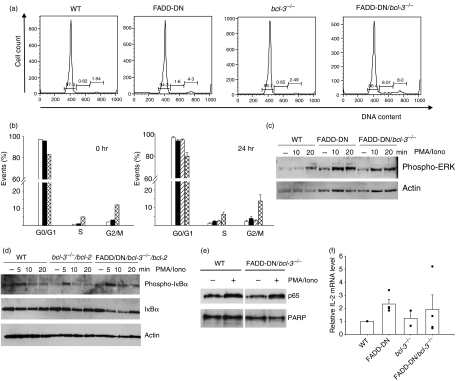
Like T cells lacking cFLIP, T cells from FADD-DN/bcl-3−/− mice are reduced in number (although not as markedly) and show a similarly near-complete lack of proliferation, accompanied by increased cell death. We therefore tested whether these T cells also show signs of activation in vivo. As shown in Fig. 4(a,b), T cells isolated from these mutant mice contained a larger proportion of cells in the S and G2/M cell cycle phases. This increase in the number of cycling cells was visible both immediately upon isolation and after 24 hr in culture without stimulation (Fig. 4b) and might be a consequence of T cell loss, which could cause increased proliferation in the attempt to preserve homeostasis. It also indicates that, in vivo, mutant T cells are principally capable of cell cycle progression.
Figure 4.
Increased numbers of cycling cells and normal signalling events in T cells from Fas-associated protein with death domain/B-cell lymphoma 3 (FADD-DN/bcl-3−/−) mice. (a) Cell cycle analysis of purified T cells from spleens of mice of the indicated genotypes. Cells were fixed in ethanol, stained with propidium iodide (PI) and analysed by flow cytometry. Cells with an apparent sub-G1 DNA content were gated out, and percentages of cells in the cell cycle phases and of sub-G1 cells are given. (b) Summary of cell cycle analyses. Cells from wild-type (WT) mice (open bars), FADD-DN mice (filled bars), bcl-3−/− mice (hatched bars) and FADD-DN/bcl-3−/− mice (cross-hatched bars) were analysed as in (a), either immediately after isolation (0 hr, left) or after 24 hr in culture without stimulation (24 hr, right). Data represent the mean for two mice (0 hr) or mean ± standard error of the mean (SEM) for four mice of each genotype (24 hr). (c) Purified T cells from mice of the genotypes indicated were stimulated with phorbol 12-myristate 13-acetate/ionomycin (PMA) plus ionomycin (Iono) for the times shown. Cells were lysed and analysed by western blotting for IκB phosphorylation and degradation. Similar results were obtained in three experiments with FADD-DN/bcl-3−/− mice and two with FADD-DN/bcl-3−/−/vav-bcl-2 mice. (d) Purified T cells from mice of the genotypes indicated were stimulated with PMA plus ionomycin for the times shown. Cells were lysed and analysed by western blotting for levels of phospho-extracellular regulated kinase (ERK). Similar results were obtained in two separate experiments. (e) Purified T cells of the genotypes indicated were either stimulated with PMA plus ionomycin for 15 min or were left untreated. Nuclear extracts were prepared and analysed by western blotting for nuclear translocation of p65. Probing with an antibody to poly-ADP-ribose-polymerase (PARP) served as a control for the loading and purity of nuclear fractionation. Similar results were obtained in two independent experiments. (f) Expression of interleukin (IL)-2 mRNA was analysed by reverse transcriptase–polymerase chain reaction (RT-PCR). T cells from mice of the genotypes indicated were stimulated for 4 hr with PMA plus ionomycin, and RNA was extracted and analysed by quantitative PCR for expression of IL-2 mRNA. In each experiment, values for WT mice were set to one, and relative values are shown. Columns represent means ± SEM for two (bcl-3−/−) or four (all other genotypes) mice from independent experiments. The individual values are shown as dots.
Normal activation of NF-κB and MAPK in T cells from FADD-DN/bcl-3−/− mice
The signalling events during TCR stimulation of mice deficient in FADD, caspase-8 or cFLIP have been investigated in the past. However, no significant difference or defect has been reported. In T cells from FADD-DN mice, activation of NF-κB, MAPK and Ca2+ flux were normal.17 NF-κB activation and ERK phosphorylation were normal in T cells lacking caspase- 8,13 and even cFLIP-deficient T cells showed normal ERK phosphorylation upon stimulation,15 while cFLIP-deficient thymocytes were shown to have normal ERK phosphorylation and IκB-α degradation upon stimulation, indicative of normal activation of the classical NF-κB pathway. We investigated these events in T cells from FADD-DN/bcl-3−/− mice and also found normal phosphorylation and degradation of NF-κB as well as normal phosphorylation of ERK (Fig. 4c,d). Similarly, nuclear translocation of NF-κB p65/RelA upon stimulation was normal in FADD-DN/bcl-3−/− cells, as assessed by western blot analysis (Fig. 4e) and immunostaining and confocal microscopy (not shown). To analyse a more complex T-cell function, we further tested for expression of IL-2 mRNA (which requires the interplay and thus normal activation of NF-κB, activator protein 1 (AP-1) and NF-AT transcription factors).28 As shown in Fig. 4(f), levels of IL-2 mRNA were similar to those of WT in stimulated T cells from FADD-DN/bcl-3−/− mice, although somewhat lower than in T cells from FADD-DN mice (which have increased levels of Bcl-2 mRNA upon stimulation).19 This suggests that none of the relevant pathways was greatly affected. Furthermore, addition of IL-2 to the cultures failed to rescue proliferation of T cells from FADD-DN/bcl-3−/− mice (data not shown). Reduced proliferation in these T cells is therefore not caused by a lack of IL-2 production. Thus, the combined loss of FADD function and Bcl-3 causes a defect in proliferation and survival despite normal proximal TCR signalling.
Discussion
This study demonstrates a link between the death receptor signalling pathway and a Bcl-3-regulated pathway. Although T cells that lack the function of either pathway show relatively small or no phenotypical changes, the combined loss causes overt, marked deficiencies in both proliferative capacity and survival. A Bcl-3-controlled pathway is therefore likely to interconnect with the death receptor pathway (at least with its non-apoptotic functions), and Bcl-3 is thus likely to play a role in both proliferation and survival in T cells. It appears likely that these capacities of Bcl-3 contribute to its oncogenic potential.
The comparison of T cells lacking functional FADD, caspase-8 or cFLIP has shown similarities and some differences. T cells of all genotypes have proliferative defects. Beyond this, numbers of T cells lacking caspase-8 declined over time.13 T cells engineered to lack cFLIP have the most striking abnormalities but basic characteristics of T cells are shared among the genotypes, most notably the reduced proliferation despite normal activation of NF-κB and MAPK. This could be interpreted as evidence that the three proteins act in concert during non-apoptotic signalling, as they do during death receptor-induced apoptosis. However, the phenotype of cFLIP loss is clearly the most severe. Two models of cFLIP deletion have been reported, which used either the reconstitution of rag−/− blastocysts with cFLIP-deficient ES cells or the deletion of cFLIP in thymocytes by crossing mice bearing a loxP-flanked cFLIP locus with lymphocyte-specific protein tyrosine kinase-cre (lck-cre) transgenic mice. While both strains of mice showed markedly reduced T-cell numbers in the periphery, only the first model had thymic abnormalities. This may be a result of the fact that lck-cre will delete the flip gene only at the late CD4−8− stage of T-cell differentiation, whereas in the former model FLIP will be absent already from the haemopoietic stem cell stage. Similarly, the first study reported normal levels of the activation marker CD69 on peripheral T cells15 while the second report showed very clear signs of T-cell activation on peripheral cFLIP-deficient T cells, as indicated by enhanced expression of CD25 and CD69 and reduced expression of CD62L.16 As cFLIP can be both an inhibitor and an activator of death receptor signalling, such an extended role may explain the stronger phenotype. The combined loss of FADD function and Bcl-3 greatly aggravated the abnormalities of FADD loss and caused a phenotype that more closely resembles that observed in T cells lacking cFLIP. One feature that is peculiar to cFLIP-deficient T cells but is not seen in FADD-DN T cells is that cFLIP-deficient T cells are to a larger extent in cycle ex vivo, and this feature was reproduced in FADD-DN/bcl-3−/− T cells. This appears paradoxical, as the same cells fail to proliferate in vitro, even when apoptosis is blocked by Bcl-2. The most likely explanation seems to be that T cells show higher levels of proliferation in vivo to make up for increased cell death. This assumption still leaves unexplained why the cells fail to proliferate in vitro, but we propose that this difference is attributable to differential signalling requirements during antigen-driven and homeostatic proliferation (the latter being the form that would replenish T-cell pools as a result of enhanced apoptosis in vivo). Although more details are yet to be worked out, it has been recently shown that such differences exist,29 and it is plausible that FADD-DN/bcl-3−/− T cells can still undergo homeostatic proliferation.
In FADD-DN mice, the expression of Bcl-3 is increased. One possibility is that this increase is an attempt to compensate for the loss of FADD function by invoking a second pathway that also regulates proliferation and survival. As T cells from Bcl-3-deficient mice have no overt proliferative defect, mitogen-induced proliferation requires FADD but not Bcl-3. However, our data indicate the possibility that the defect in T cells from FADD-DN mice would be more severe unless an increase in Bcl-3 provided additional input into pathways that regulate proliferation and survival. If this conjecture was correct, it could be argued that the phenotype of the cFLIP-deficient mouse was more severe because this loss also prevents the up-regulation of Bcl-3. It is also possible that the combined loss of FADD and Bcl-3 functions reduces the level of c-FLIP. However, PCR analyses of mRNA levels showed no obvious reduction in c-FLIP expression in the FADD-DN/bcl-3−/−mice (data not shown), making this possibility unlikely.
As a transcription factor, the role of Bcl-3 is very likely the induction or repression of target genes, probably together with NF-κB1 or NF-κB2. We can at this stage only speculate as to which genes are important for T-cell proliferation and survival. It is still unclear which pathway is defective in FADD/caspase-8/cFLIP-deficient T cells in terms of reducing proliferation. One possibility is that this unidentified pathway still has some activity in single-mutant mice but is completely inactive in T cells from FADD-DN/bcl-3−/− mice. Alternatively, there may be two independent pathways, one regulated by FADD/caspase-8 and the other by a Bcl-3-dependent mechanism. The survival defect of FADD-DN/bcl-3−/− T cells was rescued by Bcl-2 over-expression. This indicates that Bcl-3 acts upstream of Bcl-2. As the molecular function of Bcl-2 is the inhibition of pro-apoptotic Bcl-2 family members, it is likely that Bcl-3 somehow blocks the activation of this pathway.30 In activated T-cell death, Bcl-3 appears to regulate the activation of the pro-apoptotic Bcl-2 homology domain 3 (BH3)-only protein Bcl-2 interacting mediator of cell death (Bim)24 although the molecular mechanism remains unclear. Perhaps a similar mechanism operates in mitogen-activated FADD-DN/bcl-3−/− cells. There were no differences in Bim protein expression among WT, FADD-DN and FADD-DN/bcl-3−/− mice (data not shown). However, Bim expression levels do not in all situations correlate with Bim-induced apoptosis,31 and a contribution of Bim cannot therefore be excluded. The available information appears to indicate that FADD, caspase-8 and cFLIP co-operate in some way during T-cell activation, perhaps by direct complex formation, as they do for cell death induction. Bcl-3-dependent events probably contribute to the downstream pathways promoting both proliferation and survival. Although the molecular details will need to be worked out, it is clear from these results that Bcl-3 can, at least in some situations, impact on the control of both proliferation and cell survival. Appreciation of these functions will help to elucidate the physiological and oncogenic properties of Bcl-3.
Acknowledgments
We thank Dr R. M. Schmid, Munich, for the gift of bcl-3−/−-mice. This work was supported by grants and fellowships from the Deutsche Forschungsgemeinschaft through SFB 391 (to GH) and National Health and Medical Research Council (Australia; program #257502), the Leukemia and Lymphoma Society (New York; SCOR grant #7015) and the National Cancer Institute (NIH, US; CA 80188 and CA 43540).
References
- 1.Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–8. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 2.Chang DW, Xing Z, Pan Y, Algeciras-Schimnich A, Barnhart BC, Yaish-Ohad S, Peter ME, Yang X. c-FLIP(L) is a dual function regulator for caspase-8 activation and CD95-mediated apoptosis. EMBO J. 2002;21:3704–14. doi: 10.1093/emboj/cdf356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Micheau O, Thome M, Schneider P, Holler N, Tschopp J, Nicholson DW, Briand C, Grutter MG. The long form of FLIP is an activator of caspase-8 at the Fas death-inducing signaling complex. J Biol Chem. 2002;277:45162–71. doi: 10.1074/jbc.M206882200. [DOI] [PubMed] [Google Scholar]
- 4.Yeh WC, Pompa JL, McCurrach ME, et al. FADD: essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science. 1998;279:1954–8. doi: 10.1126/science.279.5358.1954. [DOI] [PubMed] [Google Scholar]
- 5.Zhang J, Cado D, Chen A, Kabra NH, Winoto A. Fas-mediated apoptosis and activation-induced T-cell proliferation are defective in mice lacking FADD/Mort1. Nature. 1998;392:296–300. doi: 10.1038/32681. [DOI] [PubMed] [Google Scholar]
- 6.Yeh WC, Itie A, Elia AJ, et al. Requirement for Casper (c-FLIP) in regulation of death receptor-induced apoptosis and embryonic development. Immunity. 2000;12:633–42. doi: 10.1016/s1074-7613(00)80214-9. [DOI] [PubMed] [Google Scholar]
- 7.Varfolomeev EE, Schuchmann M, Luria V, et al. Targeted disruption of the mouse Caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity. 1998;9:267–76. doi: 10.1016/s1074-7613(00)80609-3. [DOI] [PubMed] [Google Scholar]
- 8.Kang TB, Ben-Moshe T, Varfolomeev EE, et al. Caspase-8 serves both apoptotic and nonapoptotic roles. J Immunol. 2004;173:2976–84. doi: 10.4049/jimmunol.173.5.2976. [DOI] [PubMed] [Google Scholar]
- 9.Pellegrini M, Bath S, Marsden VS, Huang DC, Metcalf D, Harris AW, Strasser A. FADD and caspase-8 are required for cytokine-induced proliferation of hemopoietic progenitor cells. Blood. 2005;106:1581–9. doi: 10.1182/blood-2005-01-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imtiyaz HZ, Rosenberg S, Zhang Y, Rahman ZS, Hou YJ, Manser T, Zhang J. The Fas-associated death domain protein is required in apoptosis and TLR-induced proliferative responses in B cells. J Immunol. 2006;176:6852–61. doi: 10.4049/jimmunol.176.11.6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newton K, Harris AW, Bath ML, Smith KC, Strasser A. A dominant interfering mutant of FADD/MORT1 enhances deletion of autoreactive thymocytes and inhibits proliferation of mature T lymphocytes. EMBO J. 1998;17:706–18. doi: 10.1093/emboj/17.3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walsh CM, Wen BG, Chinnaiyan AM, O’Rourke K, Dixit VM, Hedrick SM. A role for FADD in T cell activation and development. Immunity. 1998;8:439–49. doi: 10.1016/s1074-7613(00)80549-x. [DOI] [PubMed] [Google Scholar]
- 13.Salmena L, Lemmers B, Hakem A, et al. Essential role for caspase 8 in T-cell homeostasis and T-cell-mediated immunity. Genes Dev. 2003;17:883–95. doi: 10.1101/gad.1063703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chun HJ, Zheng L, Ahmad M, et al. Pleiotropic defects in lymphocyte activation caused by caspase-8 mutations lead to human immunodeficiency. Nature. 2002;419:395–9. doi: 10.1038/nature01063. [DOI] [PubMed] [Google Scholar]
- 15.Chau H, Wong V, Chen NJ, et al. Cellular FLICE-inhibitory protein is required for T cell survival and cycling. J Exp Med. 2005;202:405–13. doi: 10.1084/jem.20050118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang N, He YW. An essential role for c-FLIP in the efficient development of mature T lymphocytes. J Exp Med. 2005;202:395–404. doi: 10.1084/jem.20050117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newton K, Kurts C, Harris AW, Strasser A. Effects of a dominant interfering mutant of FADD on signal transduction in activated T cells. Curr Biol. 2001;20:273–6. doi: 10.1016/s0960-9822(01)00067-7. [DOI] [PubMed] [Google Scholar]
- 18.Mack A, Hacker G. Inhibition of caspase or FADD function blocks proliferation but not MAP kinase-activation and interleukin-2-production during primary stimulation of T cells. Eur J Immunol. 2002;32:1986–92. doi: 10.1002/1521-4141(200207)32:7<1986::AID-IMMU1986>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 19.Chaneva S, Schneider G, Siegmund D, Wajant H, Mages J, Hacker G. Enhanced basal AP-1 activity and de-regulation of numerous genes in T cells transgenic for a dominant interfering mutant of FADD/MORT1. Eur J Immunol. 2004;34:3006–15. doi: 10.1002/eji.200425381. [DOI] [PubMed] [Google Scholar]
- 20.Bates PW, Miyamoto S. Expanded nuclear roles for IkappaBs. Sci STKE. 2004;2004:pe48. doi: 10.1126/stke.2542004pe48. [DOI] [PubMed] [Google Scholar]
- 21.Mathas S, Johrens K, Joos S, et al. Elevated NF-kappaB p50 complex formation and Bcl-3 expression in classical Hodgkin, anaplastic large-cell, and other peripheral T-cell lymphomas. Blood. 2005;106:4287–93. doi: 10.1182/blood-2004-09-3620. [DOI] [PubMed] [Google Scholar]
- 22.Westerheide SD, Mayo MW, Anest V, Hanson JL, Baldwin AS Jr. The putative oncoprotein Bcl-3 induces cyclin D1 to stimulate G(1) transition. Mol Cell Biol. 2001;21:8428–36. doi: 10.1128/MCB.21.24.8428-8436.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitchell TC, Hildeman D, Kedl RM, et al. Immunological adjuvants promote activated T cell survival via induction of Bcl-3. Nat Immunol. 2001;2:397–402. doi: 10.1038/87692. [DOI] [PubMed] [Google Scholar]
- 24.Bauer A, Villunger A, Labi V, Fischer SF, Strasser A, Wagner H, Schmid RM, Hacker G. The NF-kappaB regulator Bcl-3 and the BH3-only proteins Bim and Puma control the death of activated T cells. Proc Natl Acad Sci USA. 2006;103:10979–84. doi: 10.1073/pnas.0603625103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogilvy S, Metcalf D, Print CG, Bath ML, Harris AW, Adams JM. Constitutive Bcl-2 expression throughout the hematopoietic compartment affects multiple lineages and enhances progenitor cell survival. Proc Natl Acad Sci USA. 1999;96:14943–8. doi: 10.1073/pnas.96.26.14943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paxian S, Merkle H, Riemann M, et al. Abnormal organogenesis of Peyer’s patches in mice deficient for NF-kappaB1, NF-kappaB2, and Bcl-3. Gastroenterology. 2002;122:1853–68. doi: 10.1053/gast.2002.33651. [DOI] [PubMed] [Google Scholar]
- 27.Franzoso G, Carlson L, Scharton-Kersten T, et al. Critical roles for the Bcl-3 oncoprotein in T cell-mediated immunity, splenic microarchitecture, and germinal center reactions. Immunity. 1997;6:479–90. doi: 10.1016/s1074-7613(00)80291-5. [DOI] [PubMed] [Google Scholar]
- 28.Serfling E, Avots A, Neumann M. The architecture of the interleukin-2 promoter: a reflection of T lymphocyte activation. Biochim Biophys Acta. 1995;19:1263. doi: 10.1016/0167-4781(95)00112-t. [DOI] [PubMed] [Google Scholar]
- 29.Chang JT, Palanivel VR, Kinjyo I, et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 2007;315:1687–91. doi: 10.1126/science.1139393. [DOI] [PubMed] [Google Scholar]
- 30.Bouillet P, Strasser A. BH3-only proteins – evolutionarily conserved proapoptotic Bcl-2 family members essential for initiating programmed cell death. J Cell Sci. 2002;115:1567–74. doi: 10.1242/jcs.115.8.1567. [DOI] [PubMed] [Google Scholar]
- 31.Bauer A, Kirschnek S, Hacker G. Inhibition of apoptosis can be accompanied by increased Bim levels in T lymphocytes and neutrophil granulocytes. Cell Death Differ. 2007;14:1714–6. doi: 10.1038/sj.cdd.4402185. [DOI] [PubMed] [Google Scholar]