Abstract
Neutrophil granulocytes play an important role in innate host defence against microbial invasions and they are also the key effector cells in mediating host tissue damage. These functions often rely on the production of reactive oxygen species (ROS) from the membrane-bound NADPH-oxidase system. The magnitude of ROS production varies depending on the state of the cells, i.e. resting or primed. Many priming agents as well as potent NADPH-oxidase activators have been identified and characterized for human neutrophils. The cytokine tumour necrosis factor (TNF)-α is one prominent example of a priming agent and the synthetic hexapeptide WKYMVm is an agonist that triggers an activation of the NADPH-oxidase of human neutrophils through two members of the formyl peptide family of receptors, formyl peptide receptor (FPR) and FPR-like 1 (FPRL1). This peptide also activates murine neutrophils but the precise receptor involved has not been previously characterized. We show in this study that WKYMVm activates stably transfected HL60 cells expressing murine formyl peptide receptor-related sequence 2 (Fpr-rs2) and that activation of murine neutrophils with WKYMVm is blocked by an FPRL1-specific antagonist. WKYMVm is thus an agonist for Fpr-rs2 and we suggest that this receptor is in fact the mouse orthologue of FPRL1. In addition, we show that the WKYMVm response in murine neutrophils can be primed by TNF-α and this priming process involves mobilization of subcellular granules. The results obtained using neutrophils derived from TNF receptor type I (TNFRI)-deficient animals suggest that TNF-α exerts its priming effect via the TNFRI.
Keywords: formyl peptide receptor, NADPH-oxidase, neutrophils, subcellular granules, tumour necrosis factor-α
Introduction
Neutrophil granulocytes are key players in immune reactions and, as such, not only are they responsible for the direct elimination of microbial intruders by innate immune mechanisms, but they are also important effector cells in tissue destruction during inflammation as well as in the regulation of the adaptive immune response.1,2 Many of these functions largely rely on the action of reactive oxygen species (ROS) generated by NADPH-oxidase. Upon proper activation, this electron-transporting system is assembled and can reduce molecular oxygen to a superoxide anion that further generates a number of different ROS.3–6 Many endogenous and exogenous inflammatory mediators including the most intensively studied ones, bacterial-derived formylated peptides, can trigger neutrophil production of ROS via activation of a specific cell surface receptor.7
Formylated peptides are recognized by human neutrophils primarily through a pattern recognition receptor named the formyl peptide receptor (FPR) after its ligands.8,9 Human neutrophils express an additional FPR-like receptor (FPRL1) that recognizes the prototypical fMet-Leu-Phe (fMLF) but with much lower affinity than FPR.10 To identify the biological roles of these receptors, their counterparts in mice have been cloned and investigated. In addition to the gene that encodes the mouse orthologue of FPR, Fpr, the mouse genome contains seven other closely related sequences and these are termed formyl peptide receptor-related sequence 1 (Fpr-rs1) to Fpr-rs7. At least five distinct murine members of this receptor family are expressed; among these, Fpr, Fpr-rs1 and Fpr-rs2 are expressed in leucocytes.11 The differential lineage-specific expansion of the FPR gene cluster in mammals leads to a difficulty in defining the direct relationship between the mouse and human receptors, particularly in defining the mouse orthologue of human FPRL1 as both Fpr-rs1 and Fpr-rs2 share 75% amino acid identity to FPRL1 and both murine receptors are expressed in phagocytes.11 The murine Fpr is clearly the orthologue of human FPR. However, it is important to note that the very potent activator of human cells, fMLF, is a poor activator of cells expressing murine Fpr.12 Another peptide (F2L) derived from a haem-binding protein has been suggested to bind and activate FPRL1 and FPRL2 (the latter being expressed only in monocytes) in human cells.13 This peptide was recently demonstrated also to bind Fpr-rs2 in mice.14 Fpr-rs1 is still an orphan receptor in terms of peptide/protein agonists, but it has been suggested to bind the anti-inflammatory eicosanoid lipoxin A415, a finding leading to the assumption that Fpr-rs1 is the murine orthologue of FPRL1. FPRL1 has during the last couple of years been shown to be a promiscuous receptor that binds a large number of both endogenous and exogenous peptide/protein ligands.8 One of the very potent FPRL1 agonists that also binds and activates FPR is the synthetic hexapeptide WKYMVm, and this peptide has previously been shown to be a potent stimulus also for mouse neutrophils.16 The precise receptor engaged by WKYMVm in murine neutrophils has, however, not yet been determined.
Neutrophils exert their functions in vivo mainly after leaving the blood vessels and entering inflammatory sites. During this extravasation process, the cells become primed (i.e. hyper-responsive), as illustrated by the fact that both human and murine neutrophils obtained after in vivo exudation are high ROS producers upon stimulation.17 The priming phenomenon has also been described in many in vitro experimental settings, using potent priming agents such as tumour necrosis factor (TNF)-α.18 TNF-α has been shown to exert its biological functions through either both or one of two specific receptors, TNF receptor type I (TNFRI, also called CD120a and p55/60) and TNF receptor type II (TNFRII, also called CD120b and p75/80).19,20 The precise receptor type engaged in mediating neutrophil priming has not been previously addressed. Priming can be achieved in both human and animal model systems, but the precise molecular mechanism underlying the phenomenon is still poorly understood despite extensive research using in vitro experimental settings for priming. Using human cells and model systems, we and others have proposed a plausible mechanism whereby priming is associated with mobilization of intracellular storage granules, a process that endows the plasma membrane with new receptors.21 Nevertheless, the details of the priming process and the association of priming to granule mobilization have not been investigated in murine neutrophils.
The aim of this study was to characterize the murine receptor for WKYMVm through the use of primary murine neutrophils and a cell line over-expressing Fpr-rs2. Additionally, we attempt to understand the molecular mechanism of priming in murine neutrophils using WKYMVm-mediated ROS production as our read-out system. We show that WKYMVm induces a potent calcium influx in transfected HL60 cells expressing Fpr-rs2 and that the peptide also elicits the release of ROS from primary murine neutrophils. These responses were inhibited by WRW4, an antagonist shown in earlier studies to be specific for FPRL1 in human neutrophils.22 These findings imply that Fpr-rs2 is the murine orthologue of FPRL1. In addition, we demonstrate a link between subcellular granule mobilization, receptor up-regulation and priming also in murine neutrophils. We also show that the priming effect of TNF-α was diminished in TNFRI−/− cells, suggesting a role for this receptor in priming and up-regulation of cell surface receptors.
Materials and methods
Mice
Female C57BL/6 mice were purchased from B&K Universal AB (Stockholm, Sweden) and maintained under pathogen-free conditions in the animal facility of the Department of Rheumatology and Inflammation Research, Gothenburg University. Mice 10–15 weeks old were used throughout the study. The animal study protocol was approved by the Ethical Committee for Animal Experimentation, Gothenburg, Sweden. The TNF receptor I-deficient (TNFRI−/−) mice on a pure C57BL/6 background were kindly provided by Dr Mary-Jo Wick, and age- and sex-matched healthy C57BL/6 mice were used as controls.
Isolation of mouse neutrophils from bone marrow
Mouse neutrophils were isolated as described previously.17 Briefly, a bone marrow cell suspension was collected by flushing the femurs and tibias with 10 ml of cold Krebs–Ringer phosphate buffer (KRG) containing 10 mm glucose and 1·5 mm Mg2+, pH 7·3. A three-layer Percoll density gradient [2 ml each in Hanks’ balanced salt solution (HBSS)] composed of 1·095, 1·085 and 1·070 g/ml was used to enrich neutrophils from the total leucocyte population. Neutrophils were carefully collected from the 1·085/1·095 g/ml interface after centrifugation at 500 g for 30 min at 4°. This isolation procedure routinely gives 95% purity of neutrophils as determined by May–Grünwald–Giemsa staining. The contaminating erythrocytes were lysed with ACK lysis buffer [0·15 m NH4Cl, 10 mm KHCO3 and 0·1 mm disodium ethylenediaminetetraacetic acid (Na2EDTA)] and the remaining neutrophils were washed and re-suspended in KRG containing 10 mm glucose, 1·5 mm Mg2+and 1 mm Ca2+, pH 7·3, and stored on ice until use.
Determination of neutrophil superoxide production
The release of superoxide anions from activated neutrophils was determined using an isoluminol amplified chemiluminescence (CL) technique and a six-channel Biolumat LB9505 apparatus (Bertold Co., Wildbad, Germany).7 A reaction mixture of 900 μl containing 5 × 104 neutrophils, horseradish peroxidase (HRP) and isoluminol was equilibrated in the luminometer for 5 min at 37°, after which 100 μl of the stimulus was added to activate the NADPH-oxidase. The light emission [counts per minute (c.p.m.)] was recorded continuously. By direct comparison of the superoxide dismutase (SOD)-inhibitable reduction of cytochrome c and SOD-inhibitable chemiluminescence, 7·2 × 107 c.p.m. was found to correspond to production of 1 nmol of superoxide (a millimolar extinction coefficient for cytochrome c of 21·1 was used). In vitro priming was achieved by incubation of cells with the priming agent for indicated time periods at 37° prior to the addition of a stimulus.
Determination of neutrophil granule mobilization
The degree of granule mobilization was determined by measuring the surface exposure of the marker molecule complement receptor 3 (CR3). Cells primed with TNF-α at 37° for 30 min were immediately fixed in 4% ice-cold paraformaldehyde. After fixation at room temperature for 5 min, the cells were washed once and re-suspended in fluorescence-activated cell sorter (FACS) buffer [phosphate-buffered saline (PBS) supplemented with 0·1 mm EDTA and 0·02% NaN3]. The fixed cells were stained with a fluorescein isothiocyanate (FITC)-conjugated rat anti-mouse CR3 (CD11b) for 30 min on ice and the unbound CR3 was washed away. FITC-labelled rat immunoglobulin G2b (IgG2b) antibody was used as the isotype-matched control. The cell surface expression of CR3 was examined using a FACSort (Becton Dickinson, Mountain View, CA).
Expression of murine Fpr-rs2 in undifferentiated HL60 cells
The stable expression of Fpr-rs2 in undifferentiated HL60 cells was carried out as previously described.23 In brief, the cDNA encoding Fpr-rs2 was cloned by polymerase chain reaction from genomic mouse DNA using the forward primer 5′-ACCCAAGCTTGAGCAGACAAGATGGAATCCAACTACTCCATCCATCTG-3′ and the reverse primer 5′-GCTCTAGATCACATTGCCTGTAACTCAGTCTCTGCAGGA GGTGAAGCAGGATTGGC-3′. After cleavage by HindIII and XbaI the polymerase chain reaction product was cloned into pcDNA3·1 and completely sequenced. The Fpr-rs2 cDNA was further excised and ligated into the pEF-neo plasmid cleaved by XbaI and dephosphorylated.24 Transfection of HL60 cells was performed by electroporation with a Bio-Rad Gene Pulser apparatus (Bio-Rad Laboratories, Marnes La Coquette, France), according to a slightly modified version25 of the technique described by Tonetti et al.26 After electroporation, cells were expanded for 48 hr in 20 ml of complete RPMI 1640-glutaMax I culture medium. They were further diluted to 100 ml in the presence of G418 (1 mg/ml) and distributed into four 24-well plates to select G418-resistant clones. Cells from the few wells that turned yellow were further amplified and tested for their ability to mobilize intracellular calcium upon addition of Trp-Lys-Tyr-Met-Val-D-Met-NH2 (WKYMVmNH2) at a 100 nm final concentration. One clone that exhibited a robust intracellular calcium mobilization (∼1 mm) was expanded and used for further studies. To prevent possible autodifferentiation as a result of the accumulation of differentiation factors in the culture medium, cells were passed twice a week until they reach a density of 2 × 106 cells/ml. At each passage, an aliquot of the cell culture was centrifuged, the supernatant was discarded and the cell pellet was suspended in fresh medium at a density of 200 × 103 cells/ml.
Statistical analysis
Two-tailed student’s t-test was used to determine the statistical significance. P<0·05 was considered statistically significant.
Reagents
Percoll was obtained from Amersham Pharmacia (Uppsala, Sweden) and HBSS was from Life Technologies AB (Grand Island, NY). May-Grünwald and Giemsa solutions were from Histolab (Göteborg, Sweden). HRP was from Boehringer Mannheim (Mannheim, Germany). The hexapeptide Trp-Lys-Tyr-Met-Val-D-Met-NH2 (WKYMVm) and the L-form peptide WKYMVM were synthesized and high-pressure liquid chromatography purified by Alta Bioscience (University of Birmingham, Birmingham, UK), dissolved in dimethyl sulphoxide and stored at −70° before use. Isoluminol and uric acid were from Sigma Chemical Co. (St Louis, MO). Arg-Trp-Trp-Trp-Trp-CONH2 (WRW4) was from GenScript Corp (Piscataway, NJ) and cyclosporine H was kindly provided by Novartis Pharma (Basel, Switzerland). Recombinant mouse TNF-α and keratinocyte-derived chemokine (KC) were from R&D Systems (Abingdon, UK); they were diluted in PBS containing 1% of bovine serum albumin (BSA) and stored at −70°. All subsequent dilutions of reagents were made in KRG prior to use. FITC-conjugated anti-mouse CR3 (CD11b) antibody was from BD Pharmingen (San Diego, CA).
Results
The chemotactic peptide WKYMVm triggers ROS release from murine neutrophils
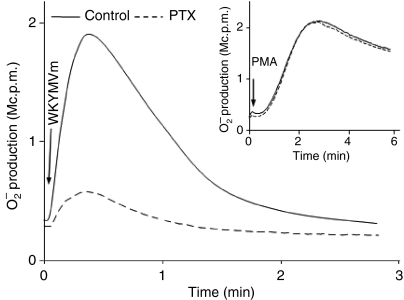
Murine neutrophils were isolated from mouse bone marrow and the NADPH-oxidase response induced by WKYMVm was studied by measuring the production of superoxide using an isoluminol amplified CL assay system. The stimulation with WKYMVm triggered a rapid release of superoxide from neutrophils with a peak response at around 0·5 min (Fig. 1). The kinetics of the response are typical for a response mediated through the G-protein coupled chemoattractant receptor (GPCR) (Fig. 1). In accordance with this, we found that preincubation of freshly isolated cells with pertussis toxin at 37° for 2 hr prior to WKYMVm stimulation abolished the ROS production (Fig. 1). The phorbol 12-myristate 13-acetate (PMA) response (a response that bypasses the surface receptor) was unaffected following treatment with 2-hr pertussis toxin (Fig. 1 inset), indicating that the cells after pertussis toxin treatment were still alive and capable of producing ROS. Similar to the pattern of NADPH-oxidase response induced by other chemoattractants, WKYMVm induced extracellular ROS release exclusively and no intracellular production of ROS was detected (data not shown). Taken together, these findings suggests that WKYMVm is a potent activator of the murine neutrophil NADPH-oxidase.
Figure 1.
WKYMVm induced superoxide release from murine neutrophils. Murine neutrophils (5 × 105 cells) were isolated from bone marrow and incubated with chemiluminescence (CL) reagents, including isoluminol and horseradish peroxidase, at 37° for 2 hr either alone (control) or with pertussis toxin (PTX) (500 ng/ml final concentration), after which reactive oxygen species (ROS) production was triggered by the addition of WKYMVm (100 nm) or phorbol 12-myristate 13-acetate (PMA) (50 nm; inset). The extracellular superoxide release was measured using an isoluminol-amplified CL technique. Representative kinetics of the CL response from three experiments are shown. Abscissa, time of CL recording (min); ordinate, the CL response (arbitrary units).
WKYMVm is a potent agonist for the receptor encoded by murine Fpr-rs2
Murine phagocytes express three Fpr family members, Fpr, Fpr-rs1 and Fpr-rs2. In order to characterize the receptor WKYMVm engaged to trigger ROS release in murine neutrophils, we used HL60 cells over-expressing Fpr-rs2 and measured the transient calcium increase in these undifferentiated cells. We observed a dose-dependent rise in intracellular calcium when the cells were triggered with WKYMVm (Fig. 2a). Non-transfected HL60 cells (control cells) did not respond to WKYMVm (data not shown). This shows that WKYMVm is a potent agonist for Fpr-rs2. Based on the fact that WKYMVm is a potent agonist for FPR and FPRL1 on human cells, but with much higher affinity for FPRL1 in human neutrophils,23 we suggest that Fpr-rs2 is the murine orthologue of FPRL1. In support of this suggestion, we found that a previously described FPRL1-specific agonist, peptide WKYMVM, in which the terminal D-methionine in WKYMVm has been replaced by the L-form of this amino acid, also strongly induced calcium mobilization in Fpr-rs2 over-expressing HL60 cells (Fig. 2b). The WKYMVm peptide has previously been shown to induce calcium influx and chemotaxis in cell lines transfected with murine Fpr, the mouse orthologue of FPR.16 These findings suggest that, on murine cells also, WKYMVm can bind two closely related receptors, one being Fpr and the other being Fpr-rs2. To further confirm that Fpr-rs2 is the orthologue of FPRL1, we studied another FPRL1 agonist, the L-conformer of hexapeptide WKYMVM, and found that this peptide also induced a robust calcium signal in HL60 cells over-expressing Fpr-rs2, whereas the FPR-specific agonist fMLF failed to induce any calcium influx (even at micromolar concentrations; data not shown). These data indicate that Fpr-rs2 displays a similar agonist preference to FPRL1. We previously showed that the peptide WRW4 is a potent antagonist for FPRL1, whereas cyclosporine H is a specific antagonist for FPR.27 We next examined the effect of these antagonists on the WKYMVm-induced calcium signal. The presence of WRW4 during the pre-warming prior to WKYMVm stimulation completely inhibited the calcium response in Fpr-rs2 over-expressing HL60 cells, whereas the presence of cyclosporine H had no effect (Fig. 2c). It was not possible to overcome the lack of effects of cyclosporin H by increasing the concentrations (up to 3 μm; data not shown). These data strengthen the relationship between Fpr-rs2 and FPRL1 in that the two receptors share antagonist sensitivity.
Figure 2.
WKYMVm induces an increase in calcium in murine formyl peptide receptor-related sequence 2 (Fpr-rs2) over-expressing HL60 cells. Undifferentiated HL60 cells transfected with Fpr-rs2 were loaded with Fura-2, and an intracellular calcium increase was triggered by the addition of WKYMVm at various final concentrations. (a) The percentage of the maximum calcium response as calculated from the peak fluorescence (340 nm) for each concentration of WKYMVm and the maximum response induced by 1 μm WKYMVm. (b) A representative calcium response induced by the L-form of WKYMVm, WKYMVM (100 nm). (c) The specific FPR antagonist cyclosporin H (1 μm; dotted line), the FPRL1 antagonist WRW4 (0·25 μm; dashed line) or Krebs–Ringer phosphate buffer (KRG) (control; solid line) was preincubated with cells during the 5-min pre-warming period before the addition of WKYMVm (10 nm). Changes in the level of cytosolic calcium were determined by measurement of the light emitted at 509 nm during excitation at 340 and 380 nm. The results are presented as a ratio between the fluorescence intensities at 340 and 380 nm from one representative experiment of six independent experiments.
Given that WKYMVm was a potent agonist for Fpr-rs2, and that WRW4 could inhibit the WKYMVm-triggered calcium response, we went on to study the effect of the antagonist on the WKYMVm-triggered ROS production in primary murine neutrophils. Because of the relatively low ROS response generated from murine cells in comparison to that from human neutrophils upon WKYMVm stimulation, and in order to improve the resolution of the system for investigating the dose-dependent inhibitory effect of WRW4, we maximized the WKYMVm response by using TNF-α primed cells before the addition of receptor antagonist. A dramatic reduction of WKYMVm-induced ROS release was observed when the FPRL1-specific antagonist WRW4 was included during the pre-warming [with an effective concentration (EC50) of 2·5 × 10−7m WRW4] and no inhibition was induced by cyclosporine H (up to 10 μm) (Fig. 3). Taken together, our results show that WKYMVm is a high-affinity agonist for Fpr-rs2 and we propose that this is the murine orthologue of human FPRL1.
Figure 3.
Effects of WRW4 and cyclosporine H on WKYMVm-induced superoxide anion production. Murine neutrophils (5 × 105 cells) were isolated from bone marrow and primed with tumour necrosis factor (TNF)-α at 37° for 30 min before stimulation with WKYMVm (5 nm). The indicated concentrations of the specific formyl peptide receptor (FPR) antagonist cyclosporine H (open boxes) or the FPRL1 antagonist WRW4 (closed boxes) were included in the 5-min pre-warming period before the addition of WKYMVm. Superoxide release upon WKYMVm stimulation was continuously recorded using the chemiluminescence (CL) technique, and the results are expressed as percentage inhibition compared with samples without any antagonist. The data presented are from one representative experiment of three.
The WKYMVm response in murine neutrophils can be primed by TNF-α and the receptor involved in the priming process is TNFRI
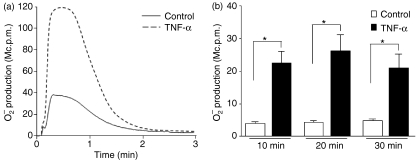
It is known that the WKYMVm response with respect to ROS production in human neutrophils can be greatly enhanced by preincubation of the neutrophils with a priming agent such as TNF-α.28 Pretreatment of human neutrophils with TNF-α for 20–30 min can fully transform these cells into a primed state, i.e. hypersensitive for producing ROS upon subsequent activation. Because of the lack of potent ROS activators in murine neutrophils, very little has been documented about the priming phenomenon/process in murine cells. We now used WKYMVm, a fairly well-characterized agonist for human cells and a potent agonist for murine cells, to investigate the priming process in murine neutrophils. Pretreatment of murine neutrophils isolated from bone marrow with a recombinant mouse TNF-α significantly enhanced the WKYMVm-triggered ROS release (Fig. 4a). Furthermore, we found that priming was a rather rapid process, as illustrated by the fact that a 10-min incubation of cells with TNF-α was sufficient to obtain significant priming (Fig. 4b).
Figure 4.
Time-dependent priming effect of the WKYMVm response by tumour necrosis factor (TNF)-α. (a) Mouse neutrophils were either left untreated (solid line) or primed with mouse recombinant TNF-α (50 ng/ml; dashed line) at 37° for 30 min and the superoxide release triggered by WKYMVm (100 nm) was recorded. (b) The same type of experiment was performed but the time for priming with TNF-α (50 ng/ml) was varied from 10 to 30 min (closed boxes), and the peak values of the response (superoxide release) were determined by chemiluminescence (CL) technique and compared with the corresponding control values obtained with cells that received no cytokine (control; open boxes). The data represent mean values (± standard deviation) from six independent experiments and a P value < 0·05 (*) was regarded as significant.
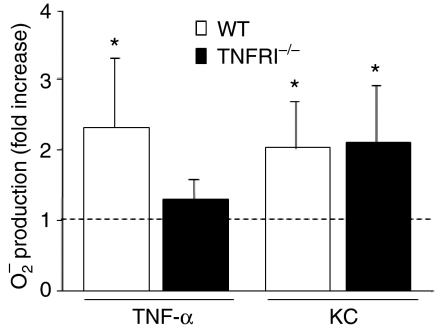
Despite the fact that TNF-α is such a pivotal cytokine, being involved in the pathogenesis of inflammatory diseases and modulation of the immune response, it is not clear whether TNFRI or TNFRII is the receptor mediating neutrophil priming. To shed light on this question, we isolated neutrophils from TNFRI−/− mice on a pure C57BL/6 background and studied the priming effect of TNF-α on the WKYMVm response in these TNFR−/− cells. Pretreatment of TNFRI−/− cells with TNF-α for 30 min at 37° did not transform these cells into a primed state; i.e. the ratio between ROS production in primed and non-primed cells was 1 when they were challenged with WKYMVm (Fig. 5). This indicates that TNFRI is the major receptor type used by TNF-α in mediating its priming effect. To confirm that the lack of priming in the TNFRI−/− neutrophils is indeed caused by the lack of TNFRI and not by a general defect in the priming process, we studied the priming process in TNFR−/− cells with an alternative priming agent, the chemokine KC, a homologue of the human growth-regulated oncogene (GRO)/melanoma growth-stimulatory activity family which signals through the chemokine receptor CXCR2 in murine neutrophils.29 Pretreatment of either cells deficient in TNFRI−/− or wild-type control cells with KC led to enhanced ROS production upon subsequent WKYMVm stimulation (Fig. 5). KC alone did not induce any ROS release (data not shown). The data presented thus clearly demonstrate an essential role of TNFRI in mediating TNF-α priming.
Figure 5.
No priming induced by tumour necrosis factor (TNF)-α in TNF receptor type I (TNFRI)-deficient (TNFRI−/−) cells. Bone marrow neutrophils were isolated from wild-type (WT) animals (strain C57B/6; open bars) or mice deficient in TNFRI on a pure C57B/6 background (TNFRI−/−, filled bars). The cells were primed with TNF-α (50 ng/ml) or keratinocyte-derived chemokine (KC) (50 ng/ml) at 37° for 30 min, and then activated with WKYMVm (100 nm final concentration). Superoxide release was measured and the peak values from primed and non-primed animals were compared and expressed as a fold increase. The data are expressed as mean ± standard deviation (n = 6) and a P value < 0·05 (*) was regarded as significant.
TNF-α-primed neutrophils show increased cell surface CR3 expression
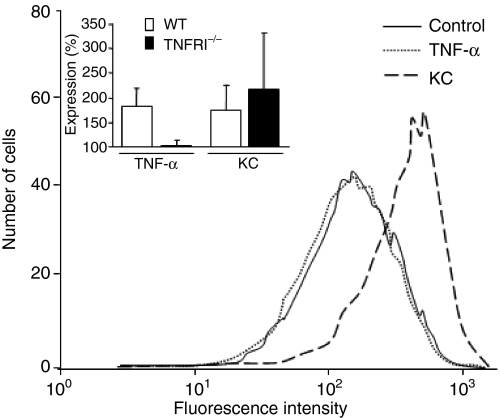
Neutrophil priming has been extensively studied in human neutrophils, and several mechanisms have been proposed as the basis of the priming phenomenon. Yet, little has been published regarding the priming process in murine neutrophils. Our data showing that murine bone marrow-derived neutrophils can be significantly primed by TNF-α with respect to the WKYMVm response led us to further evaluate the underlying mechanism. We hypothesized that our earlier proposed priming mechanism for human neutrophils, that up-regulation of the surface receptor plays a key role in mediating priming, would also apply to murine neutrophils. Because of the lack of specific antibodies against Fpr-rs2, we were not able to follow the surface expression of Fpr-rs2. To bypass this problem, we instead used CR3 (a surface marker in murine neutrophils that has previously been shown to be up-regulated after in vivo extravasation) to monitor the process of granule mobilization and receptor up-regulation in murine neutrophils. Upon TNF-α priming, an almost threefold increase in surface CR3 expression was observed on the primed cells in comparison to untreated control cells, suggesting that our earlier proposed mechanism for priming in human neutrophils indeed applies to murine neutrophils (Fig. 6 inset). The proposal of an association between granule mobilization and murine neutrophil priming gained further support from results obtained using TNFRI−/− cells which showed no change in the expression of surface CR3 upon TNF-α treatment (Fig. 6). In contrast, the surface level of CR3 expression was increased when KC replaced TNF-α as the priming agent (Fig. 6). Taken together, our results show that murine neutrophils can be primed and the granule mobilization resulted surface receptor up-regulation is an event also involved in priming of murine neutrophils.
Figure 6.
No change in surface complement receptor 3 (CR3) expression upon tumour necrosis factor (TNF)-α priming in TNF receptor type I (TNFRI)-deficient (TNFRI−/−) cells. Neutrophils were isolated from a bone marrow TNFRI−/− mouse and primed with either TNF-α (dotted line) or keratinocyte-derived chemokine (KC) (dashed line), or left untreated (control; solid line), followed by fixation in 2% paraformaldehyde. The CR3 expression was examined by flow cytometry with a fluorescein isothiocyanate (FITC)-conjugated rat anti-mouse CR3 antibody. A representative histogram from six independent experiments is shown. The inset shows the percentage of CR3 up-regulation in wild-type (WT) cells and TNFRI−/− cells upon TNF-α and KC priming in comparison to the non-primed cells. Data are expressed as mean values ± standard deviation (n = 6).
Discussion
The FPR, a member of a large family of GPCRs, and its prototype agonist fMLF, derived from bacteria, have served as an excellent model over the last few decades in attempts to understand phagocyte functions. Consequently, much information has been generated from this model system with respect to receptor function and the structural/functional relationships of this agonist/receptor pair.30,31 Much less is known about the function of the closely related FPR-like receptors, i.e. the human receptors FPRL1 and FPRL2 and their orthologues in other mammalian species. We show in this study that the potent peptide activator of human phagocytes, WKYMVm,23 is also a potent activator of murine neutrophils. This peptide is the only ligand identified so far that can potently trigger ROS production in murine neutrophils, and we have found that Fpr-rs2 is the murine receptor triggered by WKYMVm. In human neutrophils, this peptide binds to both FPR and FPRL1, with the highest binding affinity for FPRL1.23 To determine whether the murine Fpr-rs2 can be triggered by WKYMVm, we determined the activity induced in HL60 cells over-expressing this receptor, and we found that WKYMVm induces a potent calcium response in these cells. This suggests that Fpr-rs2 is a functional receptor for WKYMVm in addition to the previously described Fpr.16 Orthologue relationships between murine and human FPRs have been speculated upon, with Fpr being suggested as the human FPR orthologue. This suggestion was primarily based on the high sequence similarity between the two receptors. The FPRL1 orthologue in the mouse has not been clearly identified and characterized. Based on the results presented here we propose that Fpr-rs2 is the murine othologue of human FPRL1. Our suggestion is further supported by the finding that the potent FPRL1 agonist WKYMVM is indeed a strong inducer of calcium mobilization in HL60 cells over-expressing Fpr-rs2 (14 and our own observation). Among the eight known murine Fprs, Fpr-rs2 shares the greatest sequence similarity with Fpr-rs1, originally identified as a lipoxin A4-binding receptor that has also been suggested to bind human FPRL1.32 We previously questioned whether lipoxin A4 really employs FPRL1 as a receptor,33 and the fact that WKYMVm fails to induce internalization of receptor–ligand complexes in Chinese hamster ovary (CHO) cells stably expressing Fpr-rs1 also argues against the theory that this receptor is the mouse orthologue of FPRL1 (F. Boulay, unpublished data). Taken together, our results show that WKYMVm activates Fpr-rs2 in murine cells, and we propose that Fpr-rs2 is the murine orthologue of human FPRL1. We also found that the WKYMVm-induced response is sensitive to an FPRL1-specific antagonist but not to an FPR-specific antagonist, suggesting that the peptide preferentially activates the cells through Fpr-rs2 (the proposed human FPRL1 orthologue) even if it has the potential also to bind Fpr.
Our study also addressed the priming process in murine neutrophils using WKYMVm as a triggering stimulus. The priming phenomenon in human neutrophils has been well documented and studied using both in vivo exudated cells and in vitro primed cells. The molecular mechanisms involved in the priming process are still not clear, despite the fact that there is a strong association of neutrophil priming with the pathogenesis of several diseases, including multisystem organ failure after sepsis and trauma as well as neutrophil-mediated tissue damage.18 In this study we used TNF-α, a well-known and potent priming agent produced by activated monocytes/macrophages at very early stages of inflammation,34 and, in order to gain more insight into TNF-α-mediated priming, we transferred our priming protocols from the human to a murine system. We have shown that TNF-α-treated murine neutrophils also produce much more superoxide than non-primed control cells upon stimulation with WKYMVm. This suggests that murine cells have also evolved the priming machinery, triggered by one or other of the TNF-α receptors. The cytokine exerts its biological functions through two known receptors, namely TNFRI and TNFRII.19,35 TNFRI is the best characterized and many biological functions of TNF-α have been attributed solely to TNFRI.20 Regarding the precise receptor type(s) involved in mediating TNF-α priming, numerous studies using neutralizing antibodies against one or both receptors in human neutrophils have generated data that are inconclusive.34,35 In this study, we used model cells in which TNFRI is deleted by gene targeting and studied the effect of TNF-α priming in cells lacking TNFRI. We found that TNF-α-mediated priming is completely abolished in these cells, suggesting an essential role of TNFRI in transforming neutrophils into a primed state. However, we cannot at present exclude a possible involvement also of TNFRII in the priming process. Future studies using neutrophils deficient in TNFRII will provide solid data to resolve this issue.
The potent priming effect we observe when using murine bone marrow-derived neutrophils suggests that this is a good model cell type for research aimed at increasing our understanding of the molecular mechanism underlying priming. We have previously proposed that one key mechanism for transforming human neutrophils into the primed state is mobilization of storage organelles/granules leading to increased exposure of new receptors on the cell surface. In support of this proposal, we observed a strong association between enhanced neutrophil response and increased surface expression of CR3, an abundantly granule-stored marker in human neutrophils, as a result of the process of granule mobilization.21,36 In contrast to the well-characterized granular proteins in human neutrophils, virtually nothing is known about the localization and nature of granular proteins in murine neutrophils. Accordingly, nothing is known about the subcellular localization of the murine Fprs. This is mainly a consequence of the difficulty of obtaining sufficient numbers of murine neutrophils for subcellular fractionation and subsequent biochemical analysis. However, as FPRs are localized in the same compartments as CR3, which can be mobilized during priming in human neutrophils,36 we hypothesize that the murine Fprs are also stored in the mobilizable granules. Because antibodies against Fpr and Fpr-rs2 are currently not available, to test this hypothesis we investigated whether receptor up-regulation through granule mobilization is also a mechanism for priming of WKYMVm responses in murine neutrophils by examining the surface expression of murine CR3. We found that CR3 was mobilized in parallel with the priming effect, indicating that subcellular granule mobilization and surface receptor up-regulation are also involved in the priming of murine neutrophils. In further support of this mechanism for mediating priming, some direct in vivo links and associations between receptor mobilization and priming have been previously documented both in human neutrophils and in murine neutrophils. Exudated human neutrophils recovered from skin chambers have been shown to be primed concomitant with increased surface receptor exposure.37–40 Murine neutrophils recovered from the peritoneal cavity of animals pretreated with an intraperitoneal injection of uric acid were primed and exposed increasing numbers of CR3 molecules on their surface.17 In conclusion, we have shown that murine neutrophils can be primed and that a close association between priming and granule mobilization is also found in murine neutrophils.
Acknowledgments
This work was supported by the Swedish Medical Research Council, the King Gustaf V 80-Year Foundation, the Swedish Society for Medical Research (SSMF) and the Swedish Government under the ALF agreement.
References
- 1.Roos D, van Bruggen R, Meischl C. Oxidative killing of microbes by neutrophils. Microbes Infect. 2003;5:1307–15. doi: 10.1016/j.micinf.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 2.Nauseef WM. How human neutrophils kill and degrade microbes: an integrated view. Immunol Rev. 2007;219:88–102. doi: 10.1111/j.1600-065X.2007.00550.x. [DOI] [PubMed] [Google Scholar]
- 3.Babior BM. Phagocytes and oxidative stress. Am J Med. 2000;109:33–44. doi: 10.1016/s0002-9343(00)00481-2. [DOI] [PubMed] [Google Scholar]
- 4.Betten A, Dahlgren C, Mellqvist UH, Hermodsson S, Hellstrand K. Oxygen radical-induced natural killer cell dysfunction: role of myeloperoxidase and regulation by serotonin. J Leukoc Biol. 2004;75:1111–5. doi: 10.1189/jlb.1103595. [DOI] [PubMed] [Google Scholar]
- 5.Hultqvist M, Backlund J, Bauer K, Gelderman KA, Holmdahl R. Lack of reactive oxygen species breaks T cell tolerance to collagen type II and allows development of arthritis in mice. J Immunol. 2007;179:1431–7. doi: 10.4049/jimmunol.179.3.1431. [DOI] [PubMed] [Google Scholar]
- 6.Olofsson P, Holmberg J, Tordsson J, Lu S, Akerstrom B, Holmdahl R. Positional identification of Ncf1 as a gene that regulates arthritis severity in rats. Nat Genet. 2003;33:25–32. doi: 10.1038/ng1058. [DOI] [PubMed] [Google Scholar]
- 7.Dahlgren C, Karlsson A. Respiratory burst in human neutrophils. J Immunol Methods. 1999;232:3–14. doi: 10.1016/s0022-1759(99)00146-5. [DOI] [PubMed] [Google Scholar]
- 8.Fu H, Karlsson J, Bylund J, Movitz C, Karlsson A, Dahlgren C. Ligand recognition and activation of formyl peptide receptors in neutrophils. J Leukoc Biol. 2006;79:247–56. doi: 10.1189/jlb.0905498. [DOI] [PubMed] [Google Scholar]
- 9.Migeotte I, Communi D, Parmentier M. Formyl peptide receptors: a promiscuous subfamily of G protein-coupled receptors controlling immune responses. Cytokine Growth Factor Rev. 2006;17:501–19. doi: 10.1016/j.cytogfr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 10.Ye RD, Cavanagh SL, Quehenberger O, Prossnitz ER, Cochrane CG. Isolation of a cDNA that encodes a novel granulocyte N-formyl peptide receptor. Biochem Biophys Res Commun. 1992;184:582–9. doi: 10.1016/0006-291x(92)90629-y. [DOI] [PubMed] [Google Scholar]
- 11.Gao JL, Chen H, Filie JD, Kozak CA, Murphy PM. Differential expansion of the N-formylpeptide receptor gene cluster in human and mouse. Genomics. 1998;51:270–6. doi: 10.1006/geno.1998.5376. [DOI] [PubMed] [Google Scholar]
- 12.Bylund J, Samuelsson M, Collins LV, Karlsson A. NADPH-oxidase activation in murine neutrophils via formyl peptide receptors. Exp Cell Res. 2003;282:70–7. doi: 10.1016/s0014-4827(02)00010-1. [DOI] [PubMed] [Google Scholar]
- 13.Migeotte I, Riboldi E, Franssen JD, et al. Identification and characterization of an endogenous chemotactic ligand specific for FPRL2. J Exp Med. 2005;201:83–93. doi: 10.1084/jem.20041277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao JL, Guillabert A, Hu J, et al. F2L, a peptide derived from heme-binding protein, chemoattracts mouse neutrophils by specifically activating Fpr2, the low-affinity N-formylpeptide receptor. J Immunol. 2007;178:1450–6. doi: 10.4049/jimmunol.178.3.1450. [DOI] [PubMed] [Google Scholar]
- 15.Takano T, Fiore S, Maddox JF, Brady HR, Petasis NA, Serhan CN. Aspirin-triggered 15-epi-lipoxin A4 (LXA4) and LXA4 stable analogues are potent inhibitors of acute inflammation: evidence for anti-inflammatory receptors. J Exp Med. 1997;185:1693–704. doi: 10.1084/jem.185.9.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.He R, Tan L, Browning DD, Wang JM, Ye RD. The synthetic peptide Trp-Lys-Tyr-Met-Val-D-Met is a potent chemotactic agonist for mouse formyl peptide receptor. J Immunol. 2000;165:4598–605. doi: 10.4049/jimmunol.165.8.4598. [DOI] [PubMed] [Google Scholar]
- 17.Itou T, Collins LV, Thoren FB, Dahlgren C, Karlsson A. Changes in activation states of murine polymorphonuclear leukocytes (PMN) during inflammation: a comparison of bone marrow and peritoneal exudate PMN. Clin Vaccine Immunol. 2006;13:575–83. doi: 10.1128/CVI.13.5.575-583.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hallett MB, Lloyds D. Neutrophil priming: the cellular signals that say ‘amber’ but not ‘green’. Immunol Today. 1995;16:264–8. doi: 10.1016/0167-5699(95)80178-2. [DOI] [PubMed] [Google Scholar]
- 19.Ihnatko R, Kubes M. TNF signaling: early events and phosphorylation. Gen Physiol Biophys. 2007;26:159–67. [PubMed] [Google Scholar]
- 20.Chen G, Goeddel DV. TNF-R1 signaling: a beautiful pathway. Science. 2002;296:1634–5. doi: 10.1126/science.1071924. [DOI] [PubMed] [Google Scholar]
- 21.Almkvist J, Faldt J, Dahlgren C, Leffler H, Karlsson A. Lipopolysaccharide-induced gelatinase granule mobilization primes neutrophils for activation by galectin-3 and formylmethionyl-Leu-Phe. Infect Immun. 2001;69:832–7. doi: 10.1128/IAI.69.2.832-837.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stenfeldt AL, Karlsson J, Wenneras C, Bylund J, Fu H, Dahlgren C. Cyclosporin H, Boc-MLF and Boc-FLFLF are antagonists that preferentially inhibit activity triggered through the formyl peptide receptor. Inflammation. 2007;30:224–9. doi: 10.1007/s10753-007-9040-4. [DOI] [PubMed] [Google Scholar]
- 23.Dahlgren C, Christophe T, Boulay F, Madianos PN, Rabiet MJ, Karlsson A. The synthetic chemoattractant Trp-Lys-Tyr-Met-Val-DMet activates neutrophils preferentially through the lipoxin A(4) receptor. Blood. 2000;95:1810–8. [PubMed] [Google Scholar]
- 24.Zhen L, King AA, Xiao Y, Chanock SJ, Orkin SH, Dinauer MC. Gene targeting of X chromosome-linked chronic granulomatous disease locus in a human myeloid leukemia cell line and rescue by expression of recombinant gp91phox. Proc Natl Acad Sci USA. 1993;90:9832–6. doi: 10.1073/pnas.90.21.9832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tardif M, Rabiet MJ, Christophe T, Milcent MD, Boulay F. Isolation and characterization of a variant HL60 cell line defective in the activation of the NADPH oxidase by phorbol myristate acetate. J Immunol. 1998;161:6885–95. [PubMed] [Google Scholar]
- 26.Tonetti DA, Henning-Chubb C, Yamanishi DT, Huberman E. Protein kinase C-beta is required for macrophage differentiation of human HL-60 leukemia cells. J Biol Chem. 1994;269:23230–5. [PubMed] [Google Scholar]
- 27.Karlsson J, Fu H, Boulay F, Bylund J, Dahlgren C. The peptide Trp-Lys-Tyr-Met-Val-D-Met activates neutrophils through the formyl peptide receptor only when signaling through the formylpeptide receptor like 1 is blocked. A receptor switch with implications for signal transduction studies with inhibitors and receptor antagonists. Biochem Pharmacol. 2006;71:1488–96. doi: 10.1016/j.bcp.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 28.Bylund J, Pellme S, Fu H, Mellqvist UH, Hellstrand K, Karlsson A, Dahlgren C. Cytochalasin B triggers a novel pertussis toxin sensitive pathway in TNF-alpha primed neutrophils. BMC Cell Biol. 2004;5:21. doi: 10.1186/1471-2121-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bozic CR, Kolakowski LF, Jr, Gerard NP, et al. Expression and biologic characterization of the murine chemokine KC. J Immunol. 1995;154:6048–57. [PubMed] [Google Scholar]
- 30.Seifert R, Wenzel-Seifert K. The human formyl peptide receptor as model system for constitutively active G-protein-coupled receptors. Life Sci. 2003;73:2263–80. doi: 10.1016/s0024-3205(03)00654-4. [DOI] [PubMed] [Google Scholar]
- 31.Prossnitz ER, Ye RD. The N-formyl peptide receptor: a model for the study of chemoattractant receptor structure and function. Pharmacol Ther. 1997;74:73–102. doi: 10.1016/s0163-7258(96)00203-3. [DOI] [PubMed] [Google Scholar]
- 32.Fiore S, Maddox JF, Perez HD, Serhan CN. Identification of a human cDNA encoding a functional high affinity lipoxin A4 receptor. J Exp Med. 1994;180:253–60. doi: 10.1084/jem.180.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Christophe T, Karlsson A, Rabiet MJ, Boulay F, Dahlgren C. Phagocyte activation by Trp-Lys-Tyr-Met-Val-Met, acting through FPRL1/LXA4R, is not affected by lipoxin A4. Scand J Immunol. 2002;56:470–6. doi: 10.1046/j.1365-3083.2002.01149.x. [DOI] [PubMed] [Google Scholar]
- 34.Aggarwal BB, Natarajan K. Tumor necrosis factors: developments during the last decade. Eur Cytokine Netw. 1996;7:93–124. [PubMed] [Google Scholar]
- 35.Tartaglia LA, Goeddel DV. Two TNF receptors. Immunol Today. 1992;13:151–3. doi: 10.1016/0167-5699(92)90116-O. [DOI] [PubMed] [Google Scholar]
- 36.Bylund J, Karlsson A, Boulay F, Dahlgren C. Lipopolysaccharide-induced granule mobilization and priming of the neutrophil response to Helicobacter pylori peptide Hp(2-20), which activates formyl peptide receptor-like 1. Infect Immun. 2002;70:2908–14. doi: 10.1128/IAI.70.6.2908-2914.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Follin P, Briheim G, Dahlgren C. Mechanisms in neutrophil priming: characterization of the oxidative response induced by formylmethionyl-leucyl-phenylalanine in human exudated cells. Scand J Immunol. 1991;34:317–22. doi: 10.1111/j.1365-3083.1991.tb01552.x. [DOI] [PubMed] [Google Scholar]
- 38.Follin P, Dahlgren C. Altered O2-/H2O2 production ratio by in vitro and in vivo primed human neutrophils. Biochem Biophys Res Commun. 1990;167:970–6. doi: 10.1016/0006-291x(90)90618-w. [DOI] [PubMed] [Google Scholar]
- 39.Follin P, Wymann MP, Dewald B, Ceska M, Dahlgren C. Human neutrophil migration into skin chambers is associated with production of NAP-1/IL8 and C5a. Eur J Haematol. 1991;47:71–6. doi: 10.1111/j.1600-0609.1991.tb00564.x. [DOI] [PubMed] [Google Scholar]
- 40.Karlsson A, Follin P, Leffler H, Dahlgren C. Galectin-3 activates the NADPH-oxidase in exudated but not peripheral blood neutrophils. Blood. 1998;91:3430–8. [PubMed] [Google Scholar]