Abstract
The CD4+ CD25+ regulatory population of T cells (Treg cells), which expresses the forkhead family transcription factor (Foxp3), is the key component of the peripheral tolerance mechanism that protects us from a variety of autoimmune diseases. Experimental evidence shows that Treg cells recognize a wide range of antigenic specificities with increased reactivity to self antigens, although the affinity of these interactions remains to be further defined. The Treg repertoire is highly diverse with a distinct set of T-cell receptors (TCRs), and yet is overlapping to some extent with the repertoire of conventional T cells (Tconv cells). The majority of Treg cells are generated in the thymus. However, the role of the TCR specificity in directing thymic precursors to become Treg or Tconv cells remains unclear. On the one hand, the higher self reactivity of Treg cells and utilization of different TCRs in Treg and Tconv repertoires suggest that in TCR interactions an initial decision is made about the ‘suitability’ of a developing thymocyte to become a Treg cell. On the other hand, as Treg cells can recognize a wide range of foreign antigens, have a diverse TCR repertoire, and show some degree of overlap with Tconv cells, the signals through the TCR may be complementary to the TCR-independent process that generates precursors of Treg cells. In this review, we discuss how different features of the Treg repertoire influence our understanding of Treg specificities and the role of self reactivity in the generation of this population.
Keywords: MHC/HLA, regulatory T cell, T-cell receptor, T cells, thymus
Introduction
The basis of immune tolerance is associated with the ability of the immune system to purge autoreactive T cells in the thymus (central tolerance) and impose unresponsiveness in the periphery (peripheral tolerance). During thymic development of T cells, immature thymocytes express a highly diverse repertoire of αβ T-cell receptors (TCRs), which is a critical factor in differentiation and activation of T cells.1 Theoretically, the combinatorial and junctional diversity of TCRs is sufficient for recognition of more antigens than the number of T cells present at any time in an individual.2 When TCRs and major histocompatibility complex (MHC) molecules presenting self antigens interact, this highly diverse set of TCRs is shaped into a repertoire of TCRs that is devoid of high-affinity autoreactive clones (negative selection) but that can interact weakly with MHC/self-peptide complexes (positive selection), ensuring the ability of the immune system to respond to different pathogens in the context of self MHC molecules.1,3,4 Unfortunately, some autoreactive T cells escape thymic negative selection and enter the periphery, where peripheral tolerance is implemented by T-cell anergy, deletion or active suppression.5 Failure in the effectiveness of these processes leads to the development of many autoimmune diseases. One of the major components of peripheral tolerance is a suppression mechanism carried out by Treg cells.6,7 Although Treg cells can be recruited to this lineage in the periphery from conventional CD4+ T cells (adaptive/induced Treg cells), the majority of the Treg repertoire is generated during the normal process of maturation in the thymus and plays a central role in the control of autoimmunity.6–11 In this review, we will focus on the TCR repertoire of natural Treg cells that is shaped in the thymus.
The existence of an immunosuppressor subset within the CD4+ T-cell population was indicated by the rescue of thymectomized rodents from developing autoimmune disease by inoculation with the total T-cell population or the CD4+ T-cell subset.12–15 Sakaguchi et al. proposed the CD25 molecule [the interleukin (IL)-2 receptor α-chain] to be a phenotypical marker of regulatory/suppressor cells.16 CD4+ CD25+ T cells constitute 5–10% of peripheral CD4+ T cells in normal naïve mice and humans, and they are associated with inhibition of the effector functions of autoreactive T cells.16–18 However, the most accurate definition of Treg cells came with identification of the forkhead family transcription factor (Foxp3), allowing more precise classification of the regulatory population.19–21 Foxp3 does not act as a master switch for Treg cell lineage commitment but rather stabilizes the phenotype and suppressive function of anergic thymocytes by altering the wide array of Treg cell-specific genes.22–25 Despite advances in our understanding of Treg cell development and function, we still do not know how these cells are generated and what the role of specificity of the TCR is in diverting thymocytes into the conventional or regulatory lineage of T cells.
Specificity of the Treg repertoire
At the basis of our understanding of Treg cell identity lies the role of their TCR, which seems to be involved during both the developmental and the functional phases of Treg cells. The importance of the TCR is highlighted by the fact that Treg cells do not develop spontaneously in αβTCR transgenic mice deficient in recombination activating gene (RAG−/−), and the number of thymocytes is reduced in αβTCR and βTCR transgenic mice, suggesting that only certain TCRs can be responsible for Treg cell thymic development.26,27 Also, both in vitro and in vivo experiments show that activation of the suppressor functions of Treg cells is TCR dependent and that this activation relies on recognition of a cognate antigen.28–31 However, once activated, the Treg cells exhibit a TCR-non-specific bystander suppression of other T cells.32
Indications that Treg cells may preferentially express TCRs directed towards self antigens come from a few sources. Neonatal thymectomy at around day 3 after birth (d3tx) induces organ-specific autoimmune diseases such as gastritis, thyroiditis, dacryoadenitis and oophoritis.33–35 d3tx eliminates CD4+ CD25+ regulatory T cells from the periphery; however, adoptive transfer of these cells can rescue thymectomized mice from an autoimmune phenotype, leading to the conclusion that this population is involved in peripheral tolerance to self antigens.17,36 Experiments with double transgenic mice for TCR and its ligand showed that high-affinity interactions with a neo-autoantigen expressed on radio-resistant cells in the thymus can induce TCR transgenic thymocytes to undergo selection and to become Treg cells.30,37 Similar observations were made in other TCR transgenic systems where selection or conversion of regulatory T cells was achieved by ectopic expression of cognate antigen or by crossing transgenic TCR mice with mice expressing a particular ligand.38–41 Interestingly, thymocytes expressing lower affinity TCR could not differentiate into Treg cells in the presence of different levels of transgene-encoded antigen, suggesting that a high-affinity interaction is required for development of Treg cells.37 Taken together, these findings form the basis for the opinion that TCRs expressed on Treg cells can recognize self antigens with high affinity and that these high-affinity interactions are required for Treg cell lineage commitment.
A competing view is that an increase in the size of the Treg population is attributable not to instructive selection of thymocytes by high-affinity antigens, but rather to the ability of Foxp3-positive cells to resist negative selection coinciding with the massive deletion of Tconv cells.42–44 In experiments where a transgenic TCR was confronted with different levels of expression of tetracycline-induced neo-self antigen, the proportion of Treg cells increased without a change in absolute numbers.42 Similar observations were made in other TCR transgenic models with Aire-dependent ectopic expression of hen egg lysozyme under insulin promoter or injection of ovalbumin (OVA) peptide-loaded dendritic cells.43,44 The numbers of CD25+ T cells in the thymus did not change, although there was an efficient deletion of TCRhi CD25− T cells, resulting in an increased proportion of CD25+ regulatory T cells.
The proposed intrinsic self reactivity of Treg cells is also obscured by the fact that Treg cells can recognize exogenous antigens derived from bacteria, viruses and parasites, neo-antigens and allo-antigens, and can suppress graft versus host disease.45–52 The ability of Treg cells to control different types of immunity prompted experiments to determine the contribution of self versus non-self specificities within this population. Unfortunately, Treg cells have an inherent inability to proliferate upon TCR stimulation, making them difficult to study in vitro. A temporal break of anergy is achieved by anti-TCR stimulation in the presence of exogenous recombinant IL-2.26,29 A comparison of in vitro responses of CD25+ versus CD25− CD4+ T cells in the presence of IL-2 and/or IL-15 showed that Treg cells can respond to allogeneic, but not syngeneic, antigen-presenting cells (APCs) in both mice and humans.53–55 However, when limiting dilutions were used to compare the precursor frequencies of self- and allo-specificities within Tconv and Treg cells, the precursor frequency of self-specific TCRs was found to be two to three times higher than the precursor frequency of allo-specific TCRs in the C57Bl/6 Treg repertoire.56 This suggested enrichment, but not dominance, of self-reactive specificities of Treg cells. To directly address the specificity of the Treg cells in vivo and in vitro, Hsieh et al.‘disconnected’ the TCRs from anergic parental Treg cells by retroviral transduction of their TCRs into conventional CD4+ T cells using TCRβ transgenic mice.57 These transductants proliferate more rapidly than transductants expressing TCRs derived from Tconv cells upon adoptive transfer into lymphopenic hosts or in co-cultures with autologous APCs. Moreover, when these TCRβ transgenic mice were crossed with Foxp3-deficient mice that are prone to autoimmunity, the Treg and autoreactive Foxp3− CD25+ cells, but not the Foxp3− CD25− cells, expressed TCRs with overlapping receptors.58 These results implied that Treg cells express TCRs with increased affinity to self, higher than that required for homeostatic expansion, and that these TCRs overlap with TCRs expressed by pathogenic, self-reactive T cells. Surprisingly, analysis of T-cell hybridomas derived from Treg and Tconv cells bearing TCRs representing the most dominant TCRs in each of the unmanipulated populations did not reveal increased reactivity of Treg cells towards self.59 Taken together, these results demonstrate that the frequency of high-affinity, self-reactive TCRs in the Treg population is very low, even if it is higher than the frequency in Tconv cells. They also indicate that further analysis is required to determine at what levels of avidity this increased self reactivity of Treg cells ‘operates’ and how it correlates with the avidity range of self reactivity of Tconv cells.
Diversity of the Treg repertoire
Treg cells are only a fraction of the whole CD4 T-cell population and can respond not only to self but also to foreign antigens, and this raises the question of how such a small population can control so many different specificities and how this diversity of TCRs is achieved.
Early analyses of αβTCR diversity on CD4+ CD25+ T cells showed that their repertoire is diverse, which contrasts with the canonical or semi-diverse repertoires of TCRs expressed on other minor thymic subpopulations of CD4+ T cells expressing natural killer cells antigen (NK1.1) or being selected on CD1 molecules.27,28 The comparison of the usage of TCR variable region segments Vβ (and/or Vα) on Tconv and Treg cells showed similar contributions of variable subfamilies to the two subpopulations of T cells in both mice and humans.28,54,56,60,61 Further analysis of complementarity-determining region 3 (CDR3) size distribution by spectratyping showed that the diversity of CDR3β was also very similar between CD4+ CD25+ and CD4+ CD25− T-cell subsets and that there was no skewing of the CDR3β repertoire of CD4+ CD25+ T cells.60,61 The conclusion drawn from these analyses was that the repertoire of Treg cells is very diverse. Unfortunately, the global analysis of millions of different TCRs62,63 using only a limited number of parameters based on TCRV region usage and/or CDR3 length distribution cannot determine the identity of individual TCRs and can show differences only when there is a clonotypic, oligoclonal response.64,65 A more detailed approach to identify TCR repertoires is to compare sequences of individual Tconv and Treg cells by single-cell reverse transcription–polymerase chain reaction (RT-PCR).57,58,66,67 Such an analysis was performed in mice expressing a transgenic TCRβ and/or a miniature repertoire of TCRs in which all T cells express one TCRβ chain and various TCRα chains. Therefore, the CDR3α region could be used as a molecular marker of individual T-cell clones. The comparison of thymic and peripheral TCR repertoires of Tconv and Treg cells confirmed that both populations have very diverse TCR repertoires with a substantial fraction of unique TCRs. As the thymic positive selection of Tconv cells is very promiscuous, because of weak avidity interactions between TCRs and self MHC/peptide complexes, the high diversity of the Treg repertoire suggests a similar promiscuity in the selection of Treg cells. This is even more evident in ‘single-peptide’ mice, where MHC class II molecules (Ab) are loaded exclusively with one peptide, covalently bound Ea52-68 (Ep, fragment of alpha chain of class II Ed molecule) peptide (AbEp mice) or class II-associated invariant chain (CLIP) peptide (H2-DM-deficient mice), but the diversity of Treg repertoires remains as high as in Tconv cells.66,67 This is consistent with a previous report that, in mice with a different diversity and level of expression of the MHC class II/peptide complexes in the thymus, the total number of selected Treg cells remains proportional to the numbers of Tconv cells.54 Moreover, as Tconv and Treg cells express distinct TCR repertoires, there is a noticeable overlap, ranging from 10 to 42%. In mice expressing a polyclonal population of self antigens on MHC class II molecules (Abwt) the overlap is estimated to be approximately 10–20% (Morisita Horn index of 0·1–0·2).57,58,66 In ‘single-peptide’ mice, the estimated overlap increases to 38% (AbEp mice) and 42% (H2-DM-deficient mice).66,67 This correlation of increasing overlap with decreasing diversity of selecting ligands suggests that selection and possibly commitment of the Treg cells in the thymus is not directly linked (hardwired) to the high self reactivity of TCRs. Another possibility is that selected TCRs in ‘single-peptide’ mice are enriched in specificities towards the MHC frame rather than a peptide.68 This would suggest that shared TCRs between Tconv and Treg cells are enriched in more promiscuous TCRs, which are characterized by shorter CDR3 lengths,66,67 as is characteristic for cross-reactive TCRs.68 Nevertheless, the expression of distinct TCRs by Tconv and Treg cells might be a result of different selection niches of the two populations. In this scenario, the increase in diversity of selecting ligands does not increase the relative diversity of Treg versus Tconv cells but rather allows selection/survival of TCR repertoires that are more exclusive for each of the two lineages.
Thymic localization of Treg lineage commitment
Development of Treg cells relies not only on signals through the TCR, but also on cytokine signalling or accessory molecules interacting with thymic stromal cells [IL-2, CD28, CD40 and glucocorticoid-induced tumour necrosis factor receptor (GITR)].69–73 This different requirement for additional signals might be characterized by different accessory cell types expressing MHC II molecules or by different timing of self-antigen recognition. As the selection of Treg cells occurs in the thymus with a primary role for thymic epithelial cells,26,74 the spatial and temporal localization of factors involved in this process is of great importance to understanding how the Treg repertoire is generated.
During thymic development, low-avidity TCR/MHC interactions provide a survival signal to CD4+ CD8+ TCR+ thymocytes, whereas high-avidity interactions (coinciding with the transition of thymocytes from the thymic cortex to the medulla) lead to a deletion of potentially harmful specificities. However, for Treg cells it was reported that, when BALB/c mice were infected with mouse mammary tumour virus (MMTV) SW, a subset of Vβ6-bearing CD4+ CD25+ T cells appeared to be resistant to apoptosis mediated by superantigens.75 A similar observation was made for the C57Bl/6 background, where Vβ5+ CD4+ CD25+ T cells were roughly three times more frequent than Vβ5+ CD4+ CD25− T cells,54 probably as a result of endogenous expression of mammary tumour virus locus 9 (Mtv-9).76 In contrast, when self antigen was encoded by abundantly expressed covalent Ab/peptide complex (AbEp63K), both regulatory and conventional thymocytes bearing Vβ14 TCRs were negatively selected. The AbEp63K complexes are expressed in both the thymic cortex and the medulla, while expression of Mtv-9-encoded superantigen was not found in the thymic cortex.77 As the expression of MHC II on the thymic cortex was shown to promote selection and differentiation of Treg cells,78 we suggested that, while the CD4+ CD25+ Treg cells were first detectable in the thymic medulla,26 their functional commitment must have occurred in the thymic cortex.54 More extensive analysis of superantigen-mediated deletion of thymocytes showed that superantigens can efficiently induce deletion of Treg precursors from the thymic population in DBA/2 mice, where both the thymic epithelium and APCs of bone marrow origin are present.56 However, when superantigens were present only on the thymic epithelium, negative selection of Treg cells was impaired, and enhanced development of Treg cells was observed.79 The difference between C57Bl/6 and DBA/2 mice was attributed to the difference in avidity strength between I-Ed/superantigen (high-avidity) and I-Ab/superantigen (low-avidity), suggesting that Treg cells with TCRs of low avidity to self may escape negative selection. Moreover, expression of cognate antigen on cortical epithelial cells may contribute to positive selection of Treg cells.80 This conclusion is consistent with previous findings that thymocytes that interact with cognate self antigen presented on thymic epithelial cells may become Treg cells.74,78 The importance of cortical induction of Treg cells as a dominant mechanism of Treg cell generation is also supported by experiments in CCR7-deficient mice, which have impaired thymocyte trafficking from the cortex to the medulla and in which mature T cells are exported to the periphery directly from the cortex.81
However, there is also published evidence supporting the view that thymic commitment (or even positive selection) of Treg cells occurs in the thymic medulla. Most of the Foxp3+ thymocytes are found in the thymic medulla, and, based on the tracking of Treg precursors within the thymus during ontogeny, it has been proposed that signals associated with the medulla are responsible for commitment to the Treg lineage.22,82 Similarly, in humans it has been proposed that Hassall’s corpuscles instruct thymic dendritic cells to induce expression of Foxp3 on medium- to high-affinity, self-reactive T cells, leading to the generation of Treg cells.83 Additionally, interaction with a cognate antigen presented by a subset of Aire+ medullary thymic epithelial cells (mTECs) leads to the differentiation of Foxp3+ Treg cells in mice.84 This last conclusion relies on the access of developing thymocytes to Aire+ mTECs being limited; however, recently it has been shown that Aire+ mTECs undergo rapid turnover.85 This ‘speedy’ apoptosis promotes cross-presentation of the tissue antigens on other types of Aire-negative cells. Interestingly, in Aire-deficient mice, where expression of tissue-specific antigens is reduced, there is still efficient maturation of Treg cells.86 Nevertheless, identification of the CD25+ Foxp3− population as precursors of CD25+ Foxp3+ Treg cells argues in favour of medullary conditioning of Treg precursors leading to induction of Foxp3 expression.87,88 The most recent study by Liston et al. addresses the question of cortical versus medullary commitment of Treg cells and concludes that the thymic cortex is fully capable of supporting Treg cell differentiation.89 However, these data do not exclude the possibility that the thymic medulla also contributes to the process of Treg cell selection.89
The contrasting conclusions from the experimental evidence may be explained by the fact that Treg cells represent a set of T cells that reach their functional identity through different thymic developmental pathways, including even the MHC-independent pathway.90
Implications for the model of Treg repertoire selection
Our understanding of the requirements for the differentiation of Treg cells and the shaping of their TCR repertoires has evolved with time, but unfortunately confusion remains as to where, in the thymus, commitment to this lineage occurs and what the role of the TCR signal of a particular strength is in this process. The vague description of self reactivity of Treg cells adds to the confusion. TCRs on Treg cells have been described as recognizing self ligands with high affinity, increased or moderate avidity, avidity between positively and negatively selecting signals, a certain increased affinity range, or an overlap with pathogenic autoreactive T cells. Also, in vitro data show that, although cognate specificities of Treg cells can be defined, it is very difficult to identify the range of self reactivities, especially with respect to a lower limit, using polyclonal TCR repertoires of Treg cells. Therefore we cannot completely exclude the possibility that even TCRs with weak affinities to self will be included in the Treg repertoire. This uncertainty makes it difficult to define the avidity range and its role in sequential processes leading to the selection of Treg cells.
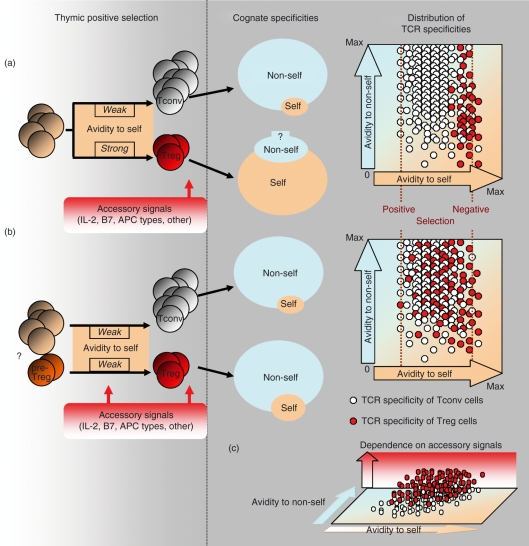
Experiments with TCR transgenic mice suggest an instructive model of Treg cell development where ‘higher’ avidity interactions with the selecting ligand drive the lineage commitment of these cells.30,37–41 With this in mind, a two-step model has been proposed that is reinforced by recent data, where the thymic selection of Treg cells relies on TCR-dependent signals followed by TCR-independent IL-2Rβ–signal transducer and activator of transcription 5 (Stat5) signals.87,88,91 In this model, developing thymocytes with potentially autoreactive TCRs receive signals upon recognition of self antigens, which in turn up-regulates expression of the CD25 molecule. The CD4+ CD8− CD25+ Foxp3− precursors of Treg cells are then rescued by an IL-2 signal in the thymic medulla. However, the contribution of medullary Foxp3− precursors to the Treg lineage competes with the cortical population of CD4+ CD8+ Foxp3+ CD69+ CCR7+ precursors identified by Liston et al., where the cortex is sufficient to generate Foxp3 thymocytes, and their medullary localization is associated with rapid CCR7-dependent migration following cortical induction of Foxp3.89 Nevertheless, if we assume sequential events in the two-step model, where TCR instruction occurs prior to the second ‘survival/conditioning’ signal, then we have to consider that a certain ‘cut-off’ avidity will define the separation between the receptors used by Tconv and Treg cells. As TCRs with an avidity below the negative selection signal are effectively selected into the Tconv lineage, this cut-off avidity would have to be quite close to negative selection to be able to separate TCRs used by Tconv and Treg cells. One would expect, in this case, a limited diversity of TCRs on Treg cells with a dominant reactivity to self and avidity in a range close to negative selection (Fig. 1a). However, that is not the case as the diversity of the Treg repertoire is huge, with a wide range of different specificities.
Figure 1.
Generation of specificities of the regulatory T (Treg) repertoire. (a) Commitment of Treg cells is driven by higher affinity interactions between the T-cell receptor (TCR) and major histocompatibility complex (MHC)/self-peptide complexes. These interactions induce the Treg phenotype and forkhead family transcription factor (Foxp3) expression and skew the specificity of the TCR repertoire towards self antigens. Consequently, conventional T cells (Tconv cells) and Treg cells express different sets of TCRs that recognize non-self or self antigens, respectively. The size of the sets of cognate specificities represents a number of different specificities, not the total number of cells. The distribution of TCR specificities does not account for the clonal frequency of individual specificities. (b) Currently unknown mechanisms generate precursors of Treg cells prior to the TCR selection. Treg precursors are subjected to TCR-dependent and -independent signals. Positive selection of both lineages proceeds on the same pool of self ligands testing the unbiased distribution of TCRs. Both Tconv and Treg repertoires have cognate specificities primarily directed to recognition of non-self antigens with small fractions of high-affinity self-reactive TCRs. (c) The functional status of pre-Treg cells allows a separation of TCRs between Tconv and Treg cells in combination with different accessory signals [interleukin (IL)-2/IL-2R, CD28/B7, CD40/CD40L and antigen-presenting cell (APC) types].
Alternatively, we can assume that the separating ‘cut-off’ avidity represents avidity that is close to a positively selecting signal. After all, every T cell has a TCR with avidity to ‘self’ above the threshold for positive selection (although not all T cells are autoreactive). This is evident from the partial phosphorylation of a TCR-ζ chain in Tconv cells, which allows them to maintain their sensitivity to a low density of foreign peptides in the periphery.92 This partial phosphorylation is elevated in peripheral Treg cells, suggesting that they exist in a partially activated state.93 This partially activated state could be the consequence of a requirement for a continuous homeostatic/survival signal through the TCR to sustain responsiveness of Treg cells to IL-2, as is the case for Tconv cells, which require to sustain responsiveness to IL-7 (discussed in Sprent & Cho).94 It would also be consistent with a ‘quantal theory’ where a summation of signals through the TCR and IL-2R may determine distinct fates such as positive selection (survival), the differentiation to Treg cells (anergy), and negative selection (apoptosis).95 However, if we set our cut-off avidity close to positively selecting avidity, then it is no longer a ‘cut-off’ but rather is a lower limit of the selection range for Treg cells that overlaps substantially with a selection range used by Tconv cells. This would also reduce the importance of ‘instruction’ through a TCR-dependent signal and put the emphasis on the functional status of precursors of Tconv and Treg cells. In this case, the recognition of MHC/self-peptide complexes would contribute to signals required for Treg cells to survive in the thymus and periphery. Even in the two-step model, if we change the timing of TCR-dependent signals and assume that they coincide with TCR-independent signals, then conceptually the model is similar to that where precursors of Treg cells are pre-committed and their functional status changes the ‘interpretation’ of the signal through the TCR.
The idea of pre-commitment of Treg precursors is supported by findings that the expression of Foxp3 can occur in pre-selected CD4− CD8− thymocytes, where these cells develop in the absence of trans-conditioning by CD4+ CD8+ thymocytes.96 Also, thymocytes with ‘disabled’ Foxp3 expression appear anergic, demonstrating that some of the key features of Treg cells are acquired independently of Foxp3 expression and TCR specificity to self antigens.23,97 Similarly, in humans, developing CD4− CD8− thymocytes express Foxp3 in the absence of TCR-mediated signals.98 All this suggests the alternative, stochastic-survival model as a selection process for the majority of Treg cells (Fig. 1b). In this model, commitment of Treg precursors (pre-Treg) occurs prior to thymic selection by the TCR, based on the stochastic process. The pre-Treg cells have a unique physiological status and exist as a fraction of the cortical CD4+ CD8+ or late double-negative thymocytes. This ensures that pre-Treg cells have a chance to rearrange and test every possible TCR. These seeds of the TCR repertoires for both Tconv and Treg populations might already be partially differentiated, based on several TCR-dependent and TCR-independent factors, including different proliferative kinetics of precursors, accumulation of subthreshold positive selecting signals, last-minute rescue from death by neglect, temporary arrest in the cell cycle or a currently unknown signal(s) delivered upon contact of thymocytes with other thymocytes or thymic stromal cells. The final Treg repertoire can be enriched in self-reactive TCRs as a result of increased resistance to negative selection, possibly not influencing reactivity to ‘non-self’. The proposed Stat5-dependent signalling would contribute to the survival of thymic precursors, together with other accessory signals coming from different cellular sources.99
The role of TCR-dependent signals may be complementary rather than dominant in the selection phase of the process. This would also explain results obtained from the analyses of ‘single-peptide’ mice, where a decrease in diversity of TCR-dependent signals (all MHC II molecules occupied by one peptide) forces an increase in the overlap of TCR repertoires between Tconv and Treg cells without compromising the relative diversity of TCRs on both populations. And finally, if we treat TCRs on Treg cells as we treat TCRs on Tconv cells, meaning that every TCR has dual specificity, to self and non-self, then it would not be a surprise that Treg cells can recognize both self and non-self antigens even if the self reactivity of Treg cells is somewhat increased (Fig. 1).6,59
Acknowledgments
The authors thank Leszek Ignatowicz for critical reading of the manuscript and valuable suggestions.
References
- 1.Hogquist KA, Baldwin TA, Jameson SC. Central tolerance: learning self-control in the thymus. Nat Rev Immunol. 2005;5:772–82. doi: 10.1038/nri1707. [DOI] [PubMed] [Google Scholar]
- 2.Davis MM. T cell receptor gene diversity and selection. Annu Rev Biochem. 1990;59:475–96. doi: 10.1146/annurev.bi.59.070190.002355. [DOI] [PubMed] [Google Scholar]
- 3.Kappler JW, Roehm N, Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987;49:273–80. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- 4.Kisielow P, Bluthmann H, Staerz UD, Steinmetz M, von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988;333:742–6. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- 5.Stockinger B. T lymphocyte tolerance: from thymic deletion to peripheral control mechanisms. Adv Immunol. 1999;71:229–65. doi: 10.1016/s0065-2776(08)60404-6. [DOI] [PubMed] [Google Scholar]
- 6.Piccirillo CA, Shevach EM. Naturally-occurring CD4+CD25+ immunoregulatory T cells: central players in the arena of peripheral tolerance. Semin Immunol. 2004;16:81–8. doi: 10.1016/j.smim.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–62. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 8.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+ J Exp Med. 2003;198:1875–86. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walker MR, Kasprowicz DJ, Gersuk VH, Benard A, Van Landeghen M, Buckner JH, Ziegler SF. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+ J Clin Invest. 2003;112:1437–43. doi: 10.1172/JCI19441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3:253–7. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 11.Chatila T. Molecular mechanisms of regulatory T cell development. J Clin Immunol. 2008 doi: 10.1007/s10875-008-9241-0. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Nishizuka Y, Sakakura T. Thymus and reproduction: sex-linked dysgenesia of the gonad after neonatal thymectomy in mice. Science. 1969;166:753–5. doi: 10.1126/science.166.3906.753. [DOI] [PubMed] [Google Scholar]
- 13.Penhale WJ, Farmer A, McKenna RP, Irvine WJ. Spontaneous thyroiditis in thymectomized and irradiated Wistar rats. Clin Exp Immunol. 1973;15:225–36. [PMC free article] [PubMed] [Google Scholar]
- 14.Penhale WJ, Irvine WJ, Inglis JR, Farmer A. Thyroiditis in T cell-depleted rats: suppression of the autoallergic response by reconstitution with normal lymphoid cells. Clin Exp Immunol. 1976;25:6–16. [PMC free article] [PubMed] [Google Scholar]
- 15.Sakaguchi S, Takahashi T, Nishizuka Y. Study on cellular events in post-thymectomy autoimmune oophoritis in mice. II. Requirement of Lyt-1 cells in normal female mice for the prevention of oophoritis. J Exp Med. 1982;156:1577–86. doi: 10.1084/jem.156.6.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- 17.Suri-Payer E, Amar AZ, Thornton AM, Shevach EM. CD4+CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J Immunol. 1998;160:1212–8. [PubMed] [Google Scholar]
- 18.Shevach EM. Certified professionals: CD4(+)CD25(+) suppressor T cells. J Exp Med. 2001;193:F41–6. doi: 10.1084/jem.193.11.f41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 20.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 21.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–42. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 22.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–41. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 23.Gavin MA, Rasmussen JP, Fontenot JD, Vasta V, Manganiello VC, Beavo JA, Rudensky AY. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445:771–5. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- 24.Wan YY, Flavell RA. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 2007;445:766–70. doi: 10.1038/nature05479. [DOI] [PubMed] [Google Scholar]
- 25.Zheng Y, Rudensky AY. Foxp3 in control of the regulatory T cell lineage. Nat Immunol. 2007;8:457–62. doi: 10.1038/ni1455. [DOI] [PubMed] [Google Scholar]
- 26.Itoh M, Takahashi T, Sakaguchi N, Kuniyasu Y, Shimizu J, Otsuka F, Sakaguchi S. Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J Immunol. 1999;162:5317–26. [PubMed] [Google Scholar]
- 27.Olivares-Villagomez D, Wensky AK, Wang Y, Lafaille JJ. Repertoire requirements of CD4+ T cells that prevent spontaneous autoimmune encephalomyelitis. J Immunol. 2000;164:5499–507. doi: 10.4049/jimmunol.164.10.5499. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi T, Kuniyasu Y, Toda M, Sakaguchi N, Itoh M, Iwata M, Shimizu J, Sakaguchi S. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cells: induction of autoimmune disease by breaking their anergic/suppressive state. Int Immunol. 1998;10:1969–80. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- 29.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–96. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Apostolou I, Sarukhan A, Klein L, von Boehmer H. Origin of regulatory T cells with known specificity for antigen. Nat Immunol. 2002;3:756–63. doi: 10.1038/ni816. [DOI] [PubMed] [Google Scholar]
- 31.Klein L, Khazaie K, von Boehmer H. In vivo dynamics of antigen-specific regulatory T cells not predicted from behavior in vitro. Proc Natl Acad Sci USA. 2003;100:8886–91. doi: 10.1073/pnas.1533365100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thornton AM, Shevach EM. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J Immunol. 2000;164:183–90. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- 33.Kojima A, Taguchi O, Nishizuka Y. Experimental production of possible autoimmune gastritis followed by macrocytic anemia in athymic nude mice. Lab Invest. 1980;42:387–95. [PubMed] [Google Scholar]
- 34.Kojima A, Prehn RT. Genetic susceptibility to post-thymectomy autoimmune diseases in mice. Immunogenetics. 1981;14:15–27. doi: 10.1007/BF00344296. [DOI] [PubMed] [Google Scholar]
- 35.Tung KS, Smith S, Teuscher C, Cook C, Anderson RE. Murine autoimmune oophoritis, epididymoorchitis, and gastritis induced by day 3 thymectomy. Immunopathology. Am J Pathol. 1987;126:293–302. [PMC free article] [PubMed] [Google Scholar]
- 36.Asano M, Toda M, Sakaguchi N, Sakaguchi S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J Exp Med. 1996;184:387–96. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, Naji A, Caton AJ. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2:301–6. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 38.Kawahata K, Misaki Y, Yamauchi M, Tsunekawa S, Setoguchi K, Miyazaki JI, Yamamoto K. Generation of CD4+CD25+ regulatory T cells from autoreactive T cells simultaneously with their negative selection in the thymus and from nonautoreactive T cells by endogenous TCR expression. J Immunol. 2002;168:4399–405. doi: 10.4049/jimmunol.168.9.4399. [DOI] [PubMed] [Google Scholar]
- 39.Walker LS, Chodos A, Eggena M, Dooms H, Abbas AK. Antigen-dependent proliferation of CD4+ CD25+ regulatory T cells in vivo. J Exp Med. 2003;198:249–58. doi: 10.1084/jem.20030315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lohr J, Knoechel B, Jiang S, Sharpe AH, Abbas AK. The inhibitory function of B7 costimulators in T cell responses to foreign and self-antigens. Nat Immunol. 2003;4:664–9. doi: 10.1038/ni939. [DOI] [PubMed] [Google Scholar]
- 41.D’Cruz LM, Klein L. Development and function of agonist-induced CD25+Foxp3+ regulatory T cells in the absence of interleukin 2 signaling. Nat Immunol. 2005;6:1152–9. doi: 10.1038/ni1264. [DOI] [PubMed] [Google Scholar]
- 42.van Santen HM, Benoist C, Mathis D. Number of T reg cells that differentiate does not increase upon encounter of agonist ligand on thymic epithelial cells. J Exp Med. 2004;200:1221–30. doi: 10.1084/jem.20041022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liston A, Lesage S, Wilson J, Peltonen L, Goodnow CC. Aire regulates negative selection of organ-specific T cells. Nat Immunol. 2003;4:350–4. doi: 10.1038/ni906. [DOI] [PubMed] [Google Scholar]
- 44.Bonasio R, Scimone ML, Schaerli P, Grabie N, Lichtman AH, von Andrian UH. Clonal deletion of thymocytes by circulating dendritic cells homing to the thymus. Nat Immunol. 2006;7:1092–100. doi: 10.1038/ni1385. [DOI] [PubMed] [Google Scholar]
- 45.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420:502–7. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 46.Cohen JL, Trenado A, Vasey D, Klatzmann D, Salomon BL. CD4(+)CD25(+) immunoregulatory T Cells: new therapeutics for graft-versus-host disease. J Exp Med. 2002;196:401–6. doi: 10.1084/jem.20020090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S. Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J Exp Med. 2002;196:389–99. doi: 10.1084/jem.20020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taylor PA, Lees CJ, Blazar BR. The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood. 2002;99:3493–9. doi: 10.1182/blood.v99.10.3493. [DOI] [PubMed] [Google Scholar]
- 49.Suvas S, Kumaraguru U, Pack CD, Lee S, Rouse BT. CD4+CD25+ T cells regulate virus-specific primary and memory CD8+ T cell responses. J Exp Med. 2003;198:889–901. doi: 10.1084/jem.20030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Edinger M, Hoffmann P, Ermann J, Drago K, Fathman CG, Strober S, Negrin RS. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med. 2003;9:1144–50. doi: 10.1038/nm915. [DOI] [PubMed] [Google Scholar]
- 51.Lerman MA, Larkin J, III, Cozzo C, Jordan MS, Caton AJ. CD4+ CD25+ regulatory T cell repertoire formation in response to varying expression of a neo-self-antigen. J Immunol. 2004;173:236–44. doi: 10.4049/jimmunol.173.1.236. [DOI] [PubMed] [Google Scholar]
- 52.Ochando JC, Yopp AC, Yang Y, et al. Lymph node occupancy is required for the peripheral development of alloantigen-specific Foxp3+ regulatory T cells. J Immunol. 2005;174:6993–7005. doi: 10.4049/jimmunol.174.11.6993. [DOI] [PubMed] [Google Scholar]
- 53.Dieckmann D, Plottner H, Berchtold S, Berger T, Schuler G. Ex vivo isolation and characterization of CD4+CD25+ T cells with regulatory properties from human blood. J Exp Med. 2001;193:1303–10. doi: 10.1084/jem.193.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pacholczyk R, Kraj P, Ignatowicz L. Peptide specificity of thymic selection of CD4+CD25+ T cells. J Immunol. 2002;168:613–20. doi: 10.4049/jimmunol.168.2.613. [DOI] [PubMed] [Google Scholar]
- 55.Jiang S, Camara N, Lombardi G, Lechler RI. Induction of allopeptide-specific human CD4+CD25+ regulatory T cells ex vivo. Blood. 2003;102:2180–6. doi: 10.1182/blood-2003-04-1164. [DOI] [PubMed] [Google Scholar]
- 56.Romagnoli P, Hudrisier D, van Meerwijk JP. Preferential recognition of self antigens despite normal thymic deletion of CD4(+)CD25(+) regulatory T cells. J Immunol. 2002;168:1644–8. doi: 10.4049/jimmunol.168.4.1644. [DOI] [PubMed] [Google Scholar]
- 57.Hsieh CS, Liang Y, Tyznik AJ, Self SG, Liggitt D, Rudensky AY. Recognition of the peripheral self by naturally arising CD25+ CD4+ T cell receptors. Immunity. 2004;21:267–77. doi: 10.1016/j.immuni.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 58.Hsieh CS, Zheng Y, Liang Y, Fontenot JD, Rudensky AY. An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nat Immunol. 2006;7:401–10. doi: 10.1038/ni1318. [DOI] [PubMed] [Google Scholar]
- 59.Pacholczyk R, Kern J, Singh N, Iwashima M, Kraj P, Ignatowicz L. Nonself-antigens are the cognate specificities of Foxp3+ regulatory T cells. Immunity. 2007;27:493–504. doi: 10.1016/j.immuni.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kasow KA, Chen X, Knowles J, Wichlan D, Handgretinger R, Riberdy JM. Human CD4+CD25+ regulatory T cells share equally complex and comparable repertoires with CD4+CD25− counterparts. J Immunol. 2004;172:6123–8. doi: 10.4049/jimmunol.172.10.6123. [DOI] [PubMed] [Google Scholar]
- 61.Fujishima M, Hirokawa M, Fujishima N, Sawada K. TCRalphabeta repertoire diversity of human naturally occurring CD4+CD25+ regulatory T cells. Immunol Lett. 2005;99:193–7. doi: 10.1016/j.imlet.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 62.Arstila TP, Casrouge A, Baron V, Even J, Kanellopoulos J, Kourilsky P. A direct estimate of the human alphabeta T cell receptor diversity. Science. 1999;286:958–61. doi: 10.1126/science.286.5441.958. [DOI] [PubMed] [Google Scholar]
- 63.Casrouge A, Beaudoing E, Dalle S, Pannetier C, Kanellopoulos J, Kourilsky P. Size estimate of the alpha beta TCR repertoire of naive mouse splenocytes. J Immunol. 2000;164:5782–7. doi: 10.4049/jimmunol.164.11.5782. [DOI] [PubMed] [Google Scholar]
- 64.Pannetier C, Cochet M, Darche S, Casrouge A, Zoller M, Kourilsky P. The sizes of the CDR3 hypervariable regions of the murine T-cell receptor beta chains vary as a function of the recombined germ-line segments. Proc Natl Acad Sci USA. 1993;90:4319–23. doi: 10.1073/pnas.90.9.4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miqueu P, Guillet M, Degauque N, Dore JC, Soulillou JP, Brouard S. Statistical analysis of CDR3 length distributions for the assessment of T and B cell repertoire biases. Mol Immunol. 2006;44:1057–64. doi: 10.1016/j.molimm.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 66.Pacholczyk R, Ignatowicz H, Kraj P, Ignatowicz L. Origin and T cell receptor diversity of Foxp3+CD4+CD25+ T cells. Immunity. 2006;25:249–59. doi: 10.1016/j.immuni.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 67.Wong J, Obst R, Correia-Neves M, Losyev G, Mathis D, Benoist C. Adaptation of TCR repertoires to self-peptides in regulatory and nonregulatory CD4+ T cells. J Immunol. 2007;178:7032–41. doi: 10.4049/jimmunol.178.11.7032. [DOI] [PubMed] [Google Scholar]
- 68.Huseby ES, Kappler JW, Marrack P. Thymic selection stifles TCR reactivity with the main chain structure of MHC and forces interactions with the peptide side chains. Mol Immunol. 2008;45:599–606. doi: 10.1016/j.molimm.2006.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Malek TR, Porter BO, Codias EK, Scibelli P, Yu A. Normal lymphoid homeostasis and lack of lethal autoimmunity in mice containing mature T cells with severely impaired IL-2 receptors. J Immunol. 2000;164:2905–14. doi: 10.4049/jimmunol.164.6.2905. [DOI] [PubMed] [Google Scholar]
- 70.Malek TR, Yu A, Vincek V, Scibelli P, Kong L. CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rbeta-deficient mice. Implications for the nonredundant function of IL-2. Immunity. 2002;17:167–78. doi: 10.1016/s1074-7613(02)00367-9. [DOI] [PubMed] [Google Scholar]
- 71.Tang Q, Henriksen KJ, Boden EK, et al. Cutting edge: CD28 controls peripheral homeostasis of CD4+CD25+ regulatory T cells. J Immunol. 2003;171:3348–52. doi: 10.4049/jimmunol.171.7.3348. [DOI] [PubMed] [Google Scholar]
- 72.Tai X, Cowan M, Feigenbaum L, Singer A. CD28 costimulation of developing thymocytes induces Foxp3 expression and regulatory T cell differentiation independently of interleukin 2. Nat Immunol. 2005;6:152–62. doi: 10.1038/ni1160. [DOI] [PubMed] [Google Scholar]
- 73.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–52. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 74.Modigliani Y, Coutinho A, Pereira P, Le Douarin N, Thomas-Vaslin V, Burlen-Defranoux O, Salaun J, Bandeira A. Establishment of tissue-specific tolerance is driven by regulatory T cells selected by thymic epithelium. Eur J Immunol. 1996;26:1807–15. doi: 10.1002/eji.1830260822. [DOI] [PubMed] [Google Scholar]
- 75.Papiernik M, de Moraes ML, Pontoux C, Vasseur F, Penit C. Regulatory CD4 T cells: expression of IL-2R alpha chain, resistance to clonal deletion and IL-2 dependency. Int Immunol. 1998;10:371–8. doi: 10.1093/intimm/10.4.371. [DOI] [PubMed] [Google Scholar]
- 76.Scherer MT, Ignatowicz L, Winslow GM, Kappler JW, Marrack P. Superantigens: bacterial and viral proteins that manipulate the immune system. Annu Rev Cell Biol. 1993;9:101–28. doi: 10.1146/annurev.cb.09.110193.000533. [DOI] [PubMed] [Google Scholar]
- 77.Moore NC, Anderson G, McLoughlin DE, Owen JJ, Jenkinson EJ. Differential expression of Mtv loci in MHC class II-positive thymic stromal cells. J Immunol. 1994;152:4826–31. [PubMed] [Google Scholar]
- 78.Bensinger SJ, Bandeira A, Jordan MS, Caton AJ, Laufer TM. Major histocompatibility complex class II-positive cortical epithelium mediates the selection of CD4(+)25(+) immunoregulatory T cells. J Exp Med. 2001;194:427–38. doi: 10.1084/jem.194.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ribot J, Romagnoli P, van Meerwijk JP. Agonist ligands expressed by thymic epithelium enhance positive selection of regulatory T lymphocytes from precursors with a normally diverse TCR repertoire. J Immunol. 2006;177:1101–7. doi: 10.4049/jimmunol.177.2.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ribot J, Enault G, Pilipenko S, Huchenq A, Calise M, Hudrisier D, Romagnoli P, van Meerwijk JP. Shaping of the autoreactive regulatory T cell repertoire by thymic cortical positive selection. J Immunol. 2007;179:6741–8. doi: 10.4049/jimmunol.179.10.6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kurobe H, Liu C, Ueno T, et al. CCR7-dependent cortex-to-medulla migration of positively selected thymocytes is essential for establishing central tolerance. Immunity. 2006;24:165–77. doi: 10.1016/j.immuni.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 82.Fontenot JD, Dooley JL, Farr AG, Rudensky AY. Developmental regulation of Foxp3 expression during ontogeny. J Exp Med. 2005;202:901–6. doi: 10.1084/jem.20050784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Watanabe N, Wang YH, Lee HK, Ito T, Wang YH, Cao W, Liu YJ. Hassall’s corpuscles instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus. Nature. 2005;436:1181–5. doi: 10.1038/nature03886. [DOI] [PubMed] [Google Scholar]
- 84.Aschenbrenner K, D’Cruz LM, Vollmann EH, Hinterberger M, Emmerich J, Swee LK, Rolink A, Klein L. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat Immunol. 2007;8:351–8. doi: 10.1038/ni1444. [DOI] [PubMed] [Google Scholar]
- 85.Gray D, Abramson J, Benoist C, Mathis D. Proliferative arrest and rapid turnover of thymic epithelial cells expressing Aire. J Exp Med. 2007;204:2521–8. doi: 10.1084/jem.20070795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Anderson MS, Venanzi ES, Chen Z, Berzins SP, Benoist C, Mathis D. The cellular mechanism of Aire control of T cell tolerance. Immunity. 2005;23:227–39. doi: 10.1016/j.immuni.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 87.Burchill MA, Yang J, Vang KB, et al. Linked T cell receptor and cytokine signaling govern the development of the regulatory T cell repertoire. Immunity. 2008;28:112–21. doi: 10.1016/j.immuni.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lio CWJ, Hsieh CS. A two-step process for thymic regulatory T cell development. Immunity. 2008;28:100–11. doi: 10.1016/j.immuni.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liston A, Nutsch KM, Farr AG, Lund JM, Rasmussen JP, Koni PA, Rudensky AY. Differentiation of regulatory Foxp3+ T cells in the thymic cortex. Proc Natl Acad Sci USA. 2008;105:11903–8. doi: 10.1073/pnas.0801506105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stephens GL, Andersson J, Shevach EM. Distinct subsets of FoxP3+ regulatory T cells participate in the control of immune responses. J Immunol. 2007;178:6901–11. doi: 10.4049/jimmunol.178.11.6901. [DOI] [PubMed] [Google Scholar]
- 91.Liston A, Rudensky AY. Thymic development and peripheral homeostasis of regulatory T cells. Curr Opin Immunol. 2007;19:176–85. doi: 10.1016/j.coi.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 92.Stefanova I, Dorfman JR, Germain RN. Self-recognition promotes the foreign antigen sensitivity of naive T lymphocytes. Nature. 2002;420:429–34. doi: 10.1038/nature01146. [DOI] [PubMed] [Google Scholar]
- 93.Andersson J, Stefanova I, Stephens GL, Shevach EM. CD4+CD25+ regulatory T cells are activated in vivo by recognition of self. Int Immunol. 2007;19:557–66. doi: 10.1093/intimm/dxm021. [DOI] [PubMed] [Google Scholar]
- 94.Sprent J, Cho JH. Self/non-self discrimination and the problem of keeping T cells alive. Immunol Cell Biol. 2008;86:54–6. doi: 10.1038/sj.icb.7100139. [DOI] [PubMed] [Google Scholar]
- 95.Smith KA. The quantal theory of how the immune system discriminates between “self and non-self”. Med Immunol. 2004;3:3. doi: 10.1186/1476-9433-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pennington DJ, Silva-Santos B, Silberzahn T, et al. Early events in the thymus affect the balance of effector and regulatory T cells. Nature. 2006;444:1073–7. doi: 10.1038/nature06051. [DOI] [PubMed] [Google Scholar]
- 97.Lin W, Haribhai D, Relland LM, Truong N, Carlson MR, Williams CB, Chatila TA. Regulatory T cell development in the absence of functional Foxp3. Nat Immunol. 2007;8:359–68. doi: 10.1038/ni1445. [DOI] [PubMed] [Google Scholar]
- 98.Tuovinen H, Kekalainen E, Rossi LH, Puntila J, Arstila TP. Cutting edge: human CD4-CD8- thymocytes express FOXP3 in the absence of a TCR. J Immunol. 2008;180:3651–4. doi: 10.4049/jimmunol.180.6.3651. [DOI] [PubMed] [Google Scholar]
- 99.Spence PJ, Green EA. Foxp3+ regulatory T cells promiscuously accept thymic signals critical for their development. Proc Natl Acad Sci USA. 2008;105:973–8. doi: 10.1073/pnas.0709071105. [DOI] [PMC free article] [PubMed] [Google Scholar]