Abstract
Objective
Jugular foramen schwannomas are uncommon pathological conditions. This article is constituted for screening these tumors in a wide perspective.
Materials
One-hundred-and-ninty-nine patients published in 19 articles between 1984 to 2007 years was collected from Medline/Index Medicus.
Results
The series consist of 83 male and 98 female. The mean age of 199 operated patients was 40.4 years. The lesion located on the right side in 32 patients and on the left side in 60 patients. The most common presenting clinical symptoms were hearing loss, tinnitus, disphagia, ataxia, and hoarseness. Complete tumor removal was achieved in 159 patients. In fourteen patients tumor reappeared unexpectedly. The tumor was thought to originate from the glossopharyngeal nerve in forty seven cases; vagal nerve in twenty six cases; and cranial accessory nerve in eleven cases. The most common postoperative complications were lower cranial nerve palsy and facial nerve palsy. Cerebrospinal fluid leakage, meningitis, aspiration pneumonia and mastoiditis were seen as other complications.
Conclusion
This review shows that jugular foramen schwannomas still have prominently high morbidity and those complications caused by postoperative lower cranial nerve injury are life threat.
Keywords: Cranial nerve, Schwannoma, Skull base tumors, Surgery, Jugular foramen
INTRODUCTION
Jugular foramen (JF) tumors are uncommon pathological conditions. Histopathologically, paraganglioma, schwannoma and meningioma are the most commonly seen masses at this location. Cranial nerve (CN) sheath tumors constitute 5-10% of all intracranial neoplasms5). Jugular foramen schwannomas arising from cranial nerves IX, X, XI (as means glossopharyngeal nerve, vagal nerve and cranial accessory nerve) are rare and they constitute approximately 2.9% to 4% of all intracranial schwannomas18). Jugular foramen schwannomas represent 10-30% of all tumors observed around the jugular foramen16,21). There has been rising in the number of reports, although the most of these articles present case reports and no large series exist in the medical literature. This article contained 204 patients reported before in the Medline/Index Medicus is constituted for screening the jugular foramen schwannomas in a wide perspective.
MATERIALS AND METHODS
Demographic data
Collected data was analyzed for number of patient, age, sex, side of tumor, exact location and extent of tumor as determined on basis of radiologic and operative findings, operative procedures, range of surgical resection, tumor origin of cranial nerve and postoperative outcome.
Clinical staging
Franklin et al.6) classified these tumors into Class A, B, C based on the categories used for glomus jugulare tumors. Kaye et al.9) developed similar but easier grading system; and Pellet et al.15) modified this grading system as follows.
Type A : primarily intracranial : with minimal extension into the jugular foramen.
Type B : primarily within the bone : with or without an intracranial component.
Type C : primarily extracranial : with only a minor extension into the jugular foramen or into the posterior fossa.
Type D : saddlebag- or dumbbell-shaped intra- and extracranial extension.
Surgical approaches
Various surgical approaches, depending on tumor extension and location, have been described before. They include posterior fossa craniectomy, suboccipital, infratemporal in various type, retrosigmoid, transcervical, transmastoid, cervicomastoid, petrooccipital transsigmoid, trans-/ retro-/ infra-labyrinthine, transcodylar, combined extradural-posterior petrous- suboccipital approach.
RESULTS
Demographic data
One-hundred-and-ninety-nine of 204 patients published in 19 articles in Medline/Index Medicus between 1984 to 2007 years were operated one or two stages. Five patients were not operated : two of five patients who had normal lower cranial nerve function were managed with watchful expectancy; and the other one's medical data was not enough, the fourth patient who had no tissue biopsy underwent radiosurgery and the last one had cerebral embolic infarct after the procedure of the angiography preoperatively5,10,14,18,23).
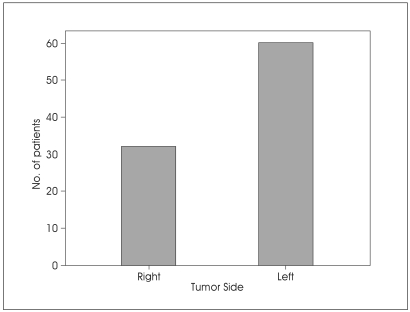
Except of one article, patients in the study group ranged in age from 13 to 73 years (mean was 40.4 years). The series consist of 83 male and 98 female (male/female ratio is 0,85). The lesion located on the right side in 32 patients (34.8%) and on the left side in 60 patients (65.2%) (Fig. 1). All remaining patients' tumor side, sex and age discriminations were not given in published series (Table 1).
Fig. 1.
Bar graph showing the location of jugular foramen schwannomas.
Table 1.
Summary of published series of jugular foramen schwannomas
N : total number of the patients, n : number of the operated patients, M : male gender, F : female gender, R : right, L : left, CN : cranial nerve, ITFA : infratemporal fossa approach, POTS : petrooccipital transsigmoid approach, PFC : posterior fossa approach, MTCA : modified transcochlear approach, GTR : gross total resection, NTR : near total resection, STR : subtotal resection, FN : facial nerve, CSF : cerebrospinal fluid, IX : glossopharyngeal nerve, X : vagal nerve, XI : cranial accessory nervePFC glossopharyngeal nerve; X: vagal nerve; XI: cranial accessory nerve
Clinical manifestation
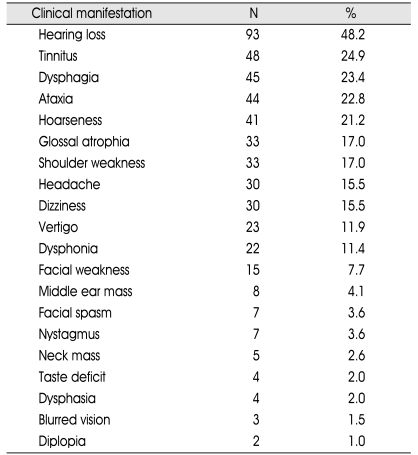
The most common presenting clinical symptoms and signs in 192 patients were hearing loss (48.2%), tinnitus (24.9%); dysphagia (23.4%); ataxia (23.4%); and hoarseness (21.2%) (Table 2).
Table 2.
The details of the clinical signs and symptoms of the patients with the jugular foramen schwannoma
N : number of the patient
Tumor origin
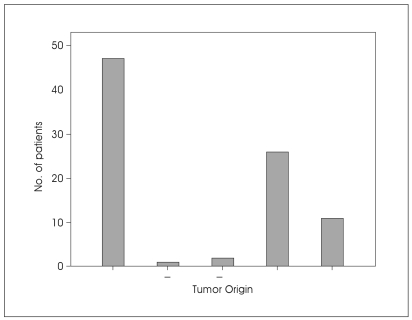
At surgery, the tumor was thought to originate from the glossopharyngeal nerve in 47 cases (23.6%). In two patients, the side of origin was either the glossopharyngeal or cranial accessory nerve and one patient the side of origin was either the glossopharyngeal or vagal nerve. In twenty six cases (13%), the tumor originated from vagal nerve and in eleven cases (5.5%), the tumor originated from cranial accessory nerve; whereas in the remainder, the origin of the tumor remained unknown (Fig. 2).
Fig. 2.
Bar graph demonstrating the tumor origin of jugular foramen schwannomas.
Tumor type
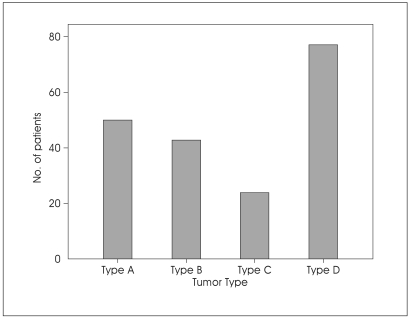
According to Kaye and Pellet's classification, 50 tumors (25.7%) were type A; 43 (22.1%) were type B; 24 (12.3%) were type C and 77 (39.7%) were type D (Fig. 3).
Fig. 3.
Bar graph showing tumor type of jugular foramen schwannomas.
Surgical techniques
Various surgical approaches, depending on tumor extension and location, have been described in this data. They include infratemporal fossa (n : 42), petrooccipital transsigmoid (n : 32), retrosigmoid (n : 32), posterior fossa craniectomy (n : 23), suboccipital (n : 22), transcervical (n : 20), translabyrinthine (n : 20), transcondylar (n : 13), infralabyrinthine (n : 12), retromastoid (n : 10), retrolabyrinthine (n : 9), transmastoid (n : 8), transcochlear (n : 7), suprajugular (n : 6), far lateral (n : 4), transjugular (n : 3), jugulopetrosectomy (n : 3), transotic (n : 2), transsigmoid (n : 1), modified transcochlear (n : 1) and transparotide approach (n : 1) (Table 1).
Tumor removal
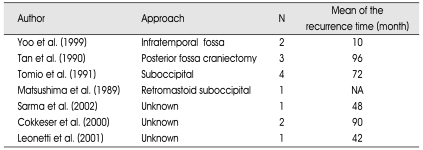
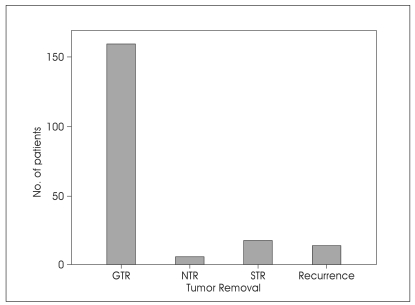
Complete tumor removal was achieved in 159 patients (86.9%). Near total tumor resection was achieved in 6 patients (3.3%) and subtotal tumor removal was accomplished in 18 patients (9.8%). In fourteen patients (7.6%) tumor reappeared unexpectedly; but their reoperations were not detailed enough in related articles. The mean of recurrence time was 59.6 months (Table 3, Fig. 4).
Table 3.
The number of the recurrent tumor with their first surgical approaches in order to the authors
N : number of the patient, NA : not available
Fig. 4.
Bar graph showing the degree of tumor removal in jugular foramen schwannomas. GTR : gross total resection, NTR; near total resection, STR : subtotal resection.
Postoperative complications
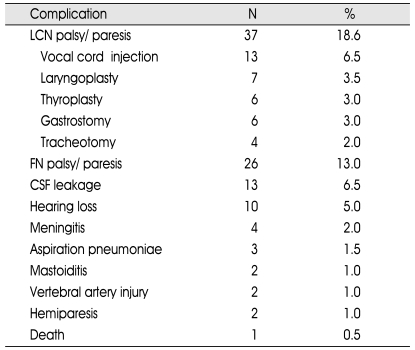
The most common postoperative complications were lower cranial nerve palsy (18.6%), facial nerve palsy (trancient or temporary) (13.0%), and hearing loss (5.0%). Vocal cord injection (6.5%), laryngoplasty (3.5%), thyroplasty (3.0%), gastrostomy (3.0%), and tracheotomy (2.0%) were performed for lower cranial nerve palsy postoperatively. CSF leakage (6.5%), meningitis (2.0%), aspiration pneumonia (1.5%) and mastoiditis (1%) were seen rare conditions. Vertebral artery injury was developed in two patients (1.0%). Hemiparesis was seen in two patients (1.0%) and one patient (0.5%) died after the complicated CSF leakage followed by meningitis (Table 4).
Table 4.
Postoperative complications
N : number of the patient, CSF : cerebrospinal fluid, LCN : lower cranial nerve, FN : facial nerve
DISCUSSION
Anatomy
Jugular foramen is a canal coursing anteriorly, inferiorly, and laterally from an intracranial to an extracranial opening. The glossopharyngeal, vagal and cranial accessory nerves emerge in a path from the medulla oblongata to the jugular foramen, where they leave the posterior fossa. A fibrous or bony septum separates the pars nervosa (anteromedial compartment), containing the inferior petrosal sinus, vena canaliculi cochlea and IXth CN from the pars vascularis (posterolateral compartment), including the Xth and XIth CNs with the proximal part of the jugular bulb. Xth and XIth CNs have a common dural sheath, however, the glossopharygeal nerve has a separate dural covering as it passes through the foramen17,20). The vertical portion of the facial nerve stradles the jugular bulb3). The meningeal branches of the occipital and ascending pharyngeal arteries are responsible for the vascular supply to tumors in this region17).
Demographic data
Jugular foramen schwannomas commonly occur between third and sixth decades of life, mainly in women and there seems to be no tumor predilection for the left or right side17,24). Sex and age discriminations of this study were as follows: 98 of 181 patients were women, and except of one article their mean of age was 40.4 years (minimum 13 maximum 73 years). However, this study points that tumor was found at the left side in sixty patients, and at the right side in thirty-two patients. Schwannomas arise from purely motor neurons and it has been hypothesized that jugular foramen schwannomas may develop from ganglia of IXth and Xth CNs, however the nerve of origin for the tumor is often identified only during surgery and the exact nerve of origin remains for most part unknown18). In fact this study shows that the nerve of origin for tumor is identified in just 87 of 199 patients, and all remain was not determined. Additionally, in 47 of 87 patients, tumor originated from the glossopharyngeal nerve; in 26 patients from the vagal nerve; and in 11 patients from accessory nerve. In 70-90% of normal population, the left internal jugular vein is dominant, and it could be hypothesized that one of the cause which triggered the oncogenic transmission might be microtraumas originated from jugular bulb pulsation; but exact reason is not known yet12).
Tumor type
These tumors may originate from cisternal, foraminal, or extracranial portion of the nerves. Tumors originating from cisternal part grow toward the intracranial space and cerebellopontine angle superiorly; tumors originating from the foraminal portion predominantly expand the bone and jugular foramen; and tumors originating from the extracranial part present with major extracranial growth, and may extend to the parapharyngeal space inferiorly. Large lesions may also extend to the vertical carotid canal and petrous apex2,8,9,18,19).
At least two types of classification have been developed for jugular foramen schwannomas, depending on the side and extension of the tumor radiologically18,22). These help the surgeons pre- and intra-operatively to decide on the surgical approach9,17,18). Today, most populary used classification system is developed by Kaye and Pellet9,15). Intracranial and foraminal tumors have the longer duration as opposed to extracranial tumors, because of their slow insidious growth pattern. Therefore, symptoms do not manifest until the jugular foramen schwannoma reaches in large size17). Tan et al.20) noted the range of symptoms to be 4 months to 10 years. Actually in this study, the type A, B and D tumors which primarily grows intracranially and/or foraminally were much more seen than type C; and this result support the idea that these tumors have slow insidious growth pattern.
Clinical manifestation
The presenting symptoms of these tumors vary, and because of a slow growth pattern, the diagnosis according to clinical findings is difficult. Symptoms do not manifest until the tumor reaches a large size. If the tumor arises more proximally, it will extend intracranially, presenting as a posterior fossa or cerebellopontin angle tumor. The distal lesions appear as extracranial, cervical, or skull base masses. Tumors arising in the midregion will expand the temporal bone. But, because the JF is so small that all cranial nerves may be affected at the same time and the symptoms may usually admix with upper cranial nerve schwannoma signs17). The most common initial symptoms are hearing loss, tinnitus, ataxia, vertigo. Thus most of patients showed eighth cranial nerve symptoms. They also cause spesific symptoms such as dysphagia, hoarseness, dysphonia, shoulder weakness, glossal atrophia; but these symptoms are usually tolerated by their opposite nerve functions. Some of the patients present headache, blurred vision or nystagmus because of increased intracranial pressure. Kaye et al.9) reported that presenting symptoms varied according to the growth pattern of tumors; but Yoo et al.24) had not found statistical correlation between tumor type and clinical presentation. Syndromes associated with space-occupying lesions around the JF are described as follows : Vernet's syndrome; Collet-Sicard's syndrome; Villaret's syndrome; Avellis' syndrome; Jackson's syndrome21).
In this study the most of clinical signs and symptoms are associated with otolaryngologic deficits (such as hearing loss, tinnitus, hoarseness, vertigo and swallowing difficulties); and with cerebellar disturbances (such as ataxia, nystagmus, dizziness). Because of nonspecific signs and symptoms of the disease, otolaryngologic evaluation of these patients is certain essential for differential diagnosis and for pre- and post-operative surgical outcome, and it must include a complete history and physical examination with special attention to all cranial nerves11).
Differential diagnosis
Today, preoperative magnetic resonance (MR) imaging and computed tomography (CT) scaning are imperative for diagnosis, classify the tumor and plan the best surgical approach8). Radiologically, the differential diagnosis of these tumors include jugular paragangliomas, vestibular schwannomas and other cranial nerve sheath tumors, meningiomas, hypoglossal neurinomas, metastatic tumors, ependymomas, other tumors involving of the cerebellopontine angle (namely meningiomas, choroid plexus papillomas, chordomas, exophytic pontine gliomas, chondromas, cerebellar hemangioblastomas, myxofibrosarcomas, epidermoid cysts, carcinomas of the tympanic cavity, lymphomas, salivary glands tumors, squamous cell carcinoma), infectious lesions (from middle ear cavity, mastoid air cells or external auditory canal) and pseudomasses such as normal vascular asymmetry, a high jugular bulb or jugular diverticulum and aneurysms of the petrous part of carotid artery1,17,18,24). Thin slice CT scans provide adequate data about the enlargement of the jugular canal. On CT scan without contrast enhancement, schwannoma appears isodense or sligthly hyperdense, and usually causes enlargement of the jugular foramen with smooth well defined bony margins without bone infiltration. After administration of contrast medium, schwannoma shows moderate enhancement, however meningioma or glomus jugulare tumors show marked enhancement with contrast medium. Meningiomas frequently invade and destruct the bone, producing hyperostosis and thickness of the jugular tubercule. Glomus tumors usually erode and destroy the bone, particularly the jugular spine and tubercule. Because of the absence of bony or air artifact, MR imaging and MR angiography help management of the tumor, and also demonstrate tumor extention, the soft tissue details, vascular supply of the tumor, relationship of major vessels, patency of jugular bulb, and other cranial nerve position or involvement17). The imaging characteristics of jugular foramen schwannomas are usually iso- or hypointense on T1 weighted images (WI) and iso- or hyperintense on T2 WI and enhance with gadolinium-DTPA. On MR scans, paraganglioma appears hypointense on T1 WI and isointense on T2 WI; and shows their characteristic pattern namely "salt and pepper" by their nonhomogenious enhancement and multiple high-velocity flow-voids within the lesion. On the other hand, meningiomas are iso- or hypointense on T1 WI and hyperintense on T2 WI. They can be distinguished from schwannomas by their irregular borders, enhancing more intensely, prolonged contrast retention into the venous phase and their pathognomonic sign namely "dura tail" with gadolinium-DTPA. Schwannomas tend to compress to the jugular bulb, however paragangliomas and meningiomas tend to invade the vessel8,13,18,23). Vestibular schwannomas may extend to the jugular foramen and they can be differentiated from jugular foramen schwannomas by widening of the internal acoustic canal on MR or CT scans. In metastatic tumors, irregular and aggressive bony destruction, heterogenous enhancement of the lesion and internal jugular vein invasion are important findings to distinguish from jugular foramen schwannomas. Epidermoid cysts, ependymomas of the cervicomedullary junction and cisternal vestibular schwannomas without enlargement of internal acoustic canal may be confused with cystic schwannomas, even on MR images. Another common MR scans abnormal images of the JF is a pseudomass. Turbulent venous flow within the JF may mimic a schwannoma at this region and cause to misdiagnosis23,24).
Surgical procedures
The choice of surgical approach depends on tumor size, tumor location and presenting symptoms especially preoperative hearing23). Mentioned all authors at the above accept that the ideal surgical approach should obtain a wide access to the whole tumor with minimal brain manuplation with minimal morbidity related to additional CN deficits and if possible, single stage total tumor removal is attempted. They also suggest that achievement of the complete tumor extirpation, the surgical procedures should be well planned, because repeated operation increases the risk of lower cranial nerve injuries and incomplete removal involves inevitable recurrence. The choice of surgical approach, there is problems include :
Adequate exposure (these tumors spread in three different paths of least resistance : intracranial, intraosseous or intrapetrous, extracranial);
Close contiguity to the facial nerve and jugular bulb;
Potential injury to the other cranial nerves18).
Thin CT scan provides adequate data about the enlargement of the jugular canal and demonstrates either bone infiltration. MR scan with gadolinium enhancement can demonstrate the soft tissue details, vascular supply to the tumors, relationship of major vasculatures, patency of the internal jugular vein and its bulb17). The surgical management of these tumors should include control of the great vessels and adjacent lower cranial nerves11). Carotid and vertebral angiography and/or MR angiography is helpful to determine the vascularity of the tumor and it can evaluate the displacement of adjacent blood vessels and carotid system involvement. During arteriography, arterial embolization may also helpful to decrease operative blood loss7). Additionally, for planning of the surgical procedures it can demonstrate contralateral sigmoid sinus dominancy and development of venous plexus10).
There had been many approaches identified to this region before but no general consensus has been reached on the choice of surgical approach. Also, some authors may choose a single-stage operation but others may constitute two step operation with otorhinolaryngologist4,6,24). Most of authors prefer infratemporal fossa approach (ITFA) which provides the greatest exposure of the jugular foramen, carotid artery, and anterior temporal bone and it allows direct access to the posterior fossa3,4,10,11,18,23,24). It can be combined with translabyrinthine, retrosigmoid, transcochlear approaches for a large type B or D tumor which have anterior intracranial extension10). In contrast, single translabyrinthine approach gives access to reach to the posterior fossa and partially to the jugular foramen. The transcondylar and the far lateral approaches are used for the primarily extracranial extension of the tumor which located inferior of the jugular bulb. But, for these approaches the vertebral artery mobilization requires and it might be injured23). Sanna et al.18) and Mazzoni et al.14) prefered petrooccipital-transsigmoid approach for preservation of hearing and facial nerve functions, and they reported no recurrence of the tumor. Sigmoid sinus transsection gives optimal visualisation of the cerebellopontin angle with opening jugular bulb which provides control of jugular foramen. In patient with unserviceable preoperative hearing, the approach includes the labyrinth10). Samii et al.17) suggested that the retrosigmoid suboccipital approach is suitable for removal of the type A tumors, however type B to D tumors are removed totally using retrosigmoid approach combined with cervical-transmastoid approach; and they did not recommend any approach which destroys the inner ear causing loss of hearing. Also, Kadri and Al-Mefty8) did not favor any approach obtain sacrifice of the labyrinthine because of given a chance to improvement of the hearing, they performed transcondylar/suprajugular approach to the patients and they prefered to reserve radiosurgery for the rare patients who had single functioning ipsilateral venous drainage. On the other hand Kadri and Al-Mefty8) and Sanna et al.18) believe that the retrosigmoid approach does not provide access to the jugular foramen and neck in type D tumors. Tomio et al.22) also suggested to perform suboccipital approach with opening the jugular foramen to type B and C tumors, because their four patients with no opened juguler foramen had tumor recurrence.
Surgical outcome
According to Carvalho et al.2) schwannomas usually do not infiltrate the cranial nerves, therefore microsurgical techniques permit radical tumor removal with minimal or no complications. They suggested that for solid schwannomas, tumor consistency allows surgeon to hold the tumor capsule and dissect the arachnoidal plane easily away from the tumor, to preserve the surrounding neurovascular tissues; however for cystic schwannomas with thin cyst wall, the arachnoid plane may not be preserved after opening cyst and gives to surgeon difficulties like to pull the tumor with a forceps and then peel it away, especially from the brain stem and cranial nerves. Therefore, to prevent the arachnoid plane and other neurovascular structures, surgeons should begin by identifying the interface between tumor and its surrounding structures and avoid the opening of the cyst. They also found that postoperative morbidity of cystic schwannomas are seen less frequent than solid tumors.
Because the close relationship between facial nerve and jugular foramen schwannomas, morbidity of the facial nerve usually result from the surgical retraction to reach the tumor. Çokkeser et al.3) maintained that if the tumor involves the hypotympanium or the middle ear, anterior transposition of the facial nerve is important for safe tumor removal from the carotid artery. They reported that after the surgery facial nerve paresis of fourteen patients was House-Brackmann grade I, of two patients was grade II, of one patient was grade III and of one patient was grade V.
In this study, complete tumor removal was achieved in 151 patients (87.2%), near total tumor resection was achieved in 6 patients (3.4%) and subtotal tumor removal was accomplished in 16 patients (9.2%). In fourteen patients (7.6%), tumor reappeared; but their re-operations were not detailed enough in related articles. The most common postoperative complications were lower cranial nerve palsies (18.6%) which consist of vocal cord dysfunctions and pharyngeal disturbances; and transient or temporary facial nerve paresis (13.0%). Wilson et al.23) printed that new cranial nerve palsy rate of 15-16% in jugular foramen schwannomas. These deficits appear either as a result of the intentional sacrifice while performs total tumor extirpation or functional loss despite anatomic relationships. But, with long term follow up, most patients tolerate these deficits well by the help of speech and swallowing rehabilitation, and shoulder physical therapy. In some patients, speech and swallowing benefit from vocal cord injection, laryngoplasty and gastrostomy18). On the other hand, the surgeon should be carefull that the acute development of postoperative deficits can not be compensated in patients who had no deficits preoperatively, and this condition could be life threat. The surgeon also should choice adequate surgical approach which is defined by tumor's location and extension for preservation of the surrounding neurovascular tissues, because repeated surgery increases the risk of injury to the lower cranial nerves8,17). Additionally, surgeon could decrease the incidence of postoperative complications using electrophysiological monitoring and new microneurosurgical and skull base techniques.
In this study, other postoperative complications were CSF leakage (6.5%), meningitis (2.0%), aspiration pneumonia (1.5%) and mastoiditis (1%). One patient (0.5%) died after the complicated CSF leakage followed by meningitis.
CONCLUSION
This review could not answer the questions as following: why the jugular foramen schwannomas were originated mostly from the left side of the patient and why the tumor origin mostly glossopharyngeal nerve. On the other hand this review shows that jugular foramen schwannomas still have prominently high morbidity and those complications caused by postoperative lower cranial nerve injury are life threat especially if the patient had no deficits preoperatively. Therefore, the surgeon should carefully collect the detailed data about the tumor, and consult the patient with otolaryngologist preoperatively for lower cranial nerve functions (i.e. speech, swallowing, hearing). Overall, successful treatment of jugular foramen schwannomas includes complete tumor removal without creating additional neurologic deficits.
References
- 1.Caldemeyer KS, Mathews VP, Azzarelli B, Smith RR. The jugular foramen : a review of anatomy, masses, and imaging characteristics. Radiographics. 1997;17:1123–1139. doi: 10.1148/radiographics.17.5.9308106. [DOI] [PubMed] [Google Scholar]
- 2.Carvalho GA, Tatagiba M, Samii M. Cystic schwannomas of the jugular foramen : clinical and surgical remarks. Neurosurgery. 2000;46:560–566. doi: 10.1097/00006123-200003000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Cokkeser Y, Brackmann DE, Fayad JN. Conservative facial nerve management in jugular foramen schwannomas. Am J Otol. 2000;21:270–274. doi: 10.1016/s0196-0709(00)80021-6. [DOI] [PubMed] [Google Scholar]
- 4.Crumley RL, Wilson C. Schwannomas of the jugular foramen. Laryngoscope. 1984;94:772–778. doi: 10.1288/00005537-198406000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Eldevik OP, Gabrielsen TO, Jacobsen EA. Imaging findings in schwannomas of the jugular foramen. AJNR Am J Neuroradiol. 2000;21:1139–1144. [PMC free article] [PubMed] [Google Scholar]
- 6.Franklin DJ, Moore GF, Fisch U. Jugular foramen peripheral nerve sheath tumors. Laryngoscope. 1989;99:1081–1087. doi: 10.1288/00005537-198210000-00020. [DOI] [PubMed] [Google Scholar]
- 7.Horn KL, House WF, Hitselberger WE. Schwannomas of the jugular foramen. Laryngoscope. 1985;95:761–765. [PubMed] [Google Scholar]
- 8.Kadri PA, Al-Mefty O. Surgical treatment of dumbbell-shaped jugular foramen schwannomas. Neurosurg Focus. 2004;17:E9. doi: 10.3171/foc.2004.17.2.9. [DOI] [PubMed] [Google Scholar]
- 9.Kaye AH, Hahn JF, Kinney SE, Hardy RW, Jr, Bay JW. Jugular foramen schwannomas. J Neurosurg. 1984;60:1045–1053. doi: 10.3171/jns.1984.60.5.1045. [DOI] [PubMed] [Google Scholar]
- 10.Lee SK, Park K, Kong DS, Cho YS, Baek CH, Nam DH, et al. Surgical tactics and outcome of treatment in jugular foramen schwannomas. J Clin Neurosci. 2001;8(Suppl 1):32–39. doi: 10.1054/jocn.2001.0874. [DOI] [PubMed] [Google Scholar]
- 11.Leonetti JP, Wachter B, Marzo SJ, Petruzzelli G. Extracranial lower cranial nerve sheath tumors. Otolaryngol Head Neck Surg. 2001;125:640–644. doi: 10.1067/mhn.2001.119865. [DOI] [PubMed] [Google Scholar]
- 12.Lichtenstein D, Saïfi R, Augarde R, Prin S, Schmitt JM, Page B, et al. The Internal jugular veins are asymmetric. Usefulness of ultrasound before catheterization. Intensive Care Med. 2001;27:301–305. doi: 10.1007/s001340000792. [DOI] [PubMed] [Google Scholar]
- 13.Matsushima T, Hasuo K, Yasumori K, Yoshida K, Hirakata R, Fukui M, et al. Magnetic resonance imaging of jugular foramen neurinomas. Acta Neurochir (Wien) 1989;96:83–87. doi: 10.1007/BF01456163. [DOI] [PubMed] [Google Scholar]
- 14.Mazzoni A, Sanna M, Saleh E, Achilli V. Lower cranial nerve schwannomas involving the jugular foramen. Ann Otol Rhinol Laryngol. 1997;106:370–379. doi: 10.1177/000348949710600503. [DOI] [PubMed] [Google Scholar]
- 15.Pellet W, Cannoni M, Pech A. The widened transcochlear approach to jugular foramen tumors. J Neurosurg. 1988;69:887–894. doi: 10.3171/jns.1988.69.6.0887. [DOI] [PubMed] [Google Scholar]
- 16.Ramina R, Maniglia JJ, Fernandes YB, Paschoal JR, Pfeilsticker LN, Coelho Neto M. Tumors of the jugular foramen : diagnosis and management. Neurosurgery. 2005;57(Suppl 1):59–68. doi: 10.1227/01.neu.0000163483.44754.47. discussion 59-68. [DOI] [PubMed] [Google Scholar]
- 17.Samii M, Babu RP, Tatagiba M, Sepehrnia A. Surgical treatment of jugular foramen schwannomas. J Neurosurg. 1995;82:924–932. doi: 10.3171/jns.1995.82.6.0924. [DOI] [PubMed] [Google Scholar]
- 18.Sanna M, Bacciu A, Falcioni M, Taibah A. Surgical management of jugular foramen schwannomas with hearing and facial nerve function preservation : a series of 23 cases and review of the literature. Laryngoscope. 2006;116:2191–2204. doi: 10.1097/01.mlg.0000246193.84319.e5. [DOI] [PubMed] [Google Scholar]
- 19.Sarma S, Sekhar LN, Schessel DA. Nonvestibular schwannomas of the brain : a 7-year experience. Neurosurgery. 2002;50:437–438. doi: 10.1097/00006123-200203000-00002. discussion 438-439. [DOI] [PubMed] [Google Scholar]
- 20.Tan LC, Bordi L, Symon L, Cheesman AD. Jugular foramen neuromas : a review of 14 cases. Surg Neurol. 1990;34:205–211. doi: 10.1016/0090-3019(90)90130-h. [DOI] [PubMed] [Google Scholar]
- 21.Tekkök IH, Ozcan OE, Turan E, Onol B. Jugular foramen meningioma. Report of a case and review of the literature. J Neurosurg Sci. 1997;41:283–292. [PubMed] [Google Scholar]
- 22.Tomio S, Takakura K. Twelve cases of jugular foramen neurinoma. Skull Base Surg. 1991;1:152–160. doi: 10.1055/s-2008-1056998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson MA, Hillman TA, Wiggins RH, Shelton C. Jugular foramen schwannomas : diagnosis, management, and outcomes. Laryngoscope. 2005;115:1486–1492. doi: 10.1097/01.mlg.0000172196.76865.a1. [DOI] [PubMed] [Google Scholar]
- 24.Yoo H, Jung HW, Yang HJ. Jugular foramen schwannomas : surgical approaches and outcome of treatment. Skull Base Surg. 1999;9:243–252. doi: 10.1055/s-2008-1058133. [DOI] [PMC free article] [PubMed] [Google Scholar]