Abstract
Objective
Premorbid demographic backgrounds of injured individuals are likely to reflect more accurately the status of patients with traumatic brian injury (TBI) than clinical factors. However, the concrete study about the relationship between the demographic factors and neurocognitive function in TBI patients has not been reported. The object of this study was to evaluate the effect of premorbid demographic factors on the recovery of neurocognitive function following TBI.
Methods
From July 1998 to February 2007, 293 patients (male: 228, female: 65) with a history of head injury, who had recovered from the acute phase, were selected from our hospital to include in this study. We analyzed the effect of premorbid demographic factors including age, sex, educational level and occupation on the recovery of neurocognitive function in each TBI subgroup as defined by Glasgow Coma Scale (GCS) score. Intelligence and memory are components of neurocognitive function, and the Korean Wechsler Intelligence Scale (K-WAIS) and the Korean memory assessment scale (K-MAS) were used in this study. The results were considered significant at p<0.05.
Results
The higher level of education was a good prognostic factor for intelligence regardless of GCS score and younger age group showed a better result for memory with an exception of severe TBI group. In the severe TBI group, the meaningful effect of demographic factors was not noted by the cause of influence of severe brain injury.
Conclusion
The demographic factors used in this study may be helpful for predicting the precise prognosis and developing an appropriate rehabilitation program for TBI patients.
Keywords: Traumatic brain injury, Premorbid demographic factors, Prognosis
INTRODUCTION
The outcome following traumatic brain injury (TBI) is dependent not only on clinical factors, but also on premorbid demographic factors of the injured individuals15,17,20). Clinical factors include all physical examination elements, the severity of the injury (initial GCS, duration of post-traumatic amnesia (PTA) and computer tomographic (CT) scan pathology), while premorbid demographic factors include age, gender, educational periods, occupation and premorbid intelligence6,17,20).
Early studies reported an association between clinical factors and an outcome in TBI patients2,6,10,14,16,18). These studies used early statistical methods and the independence model along with a large brain trauma database to identify combinations of acute clinical elements that are associated with patients' general outcomes5,6,8-10,21). These clinical elements included pupillary reactivity, spontaneous and reflex eye movements, age at onset and duration of coma. The outcome was measured using the Glasgow Outcome Scale (GOS)6,10). Acute clinical elements have previously been related to morbidity, mortality and prediction of early disability. In particular, PTA after TBI was regarded as an important factor for accurate outcome prediction6).
Recently, several authors have reported that these clinical factors are limited for a precious description of TBI patients, and premorbid demographic backgrounds of injured individuals are likely to reflect more accurately the status of patients with TBI17,20). Some studies have indicated that the higher level of education is related to a better outcome, but age and race are less important factors related to outcome13,17). Other authors have reported that sexual dysmorphism may play an important role in outcome following TBI1,22). The mechanism by which gender may influence the outcome following TBI is not yet clear.
With respect to the outcome following TBI from neurocognitive function, the concrete study about the relationship between the demographic factors and neurocognitive function in TBI patients has not been clearly verified. In this study, we explored premorbid demographic factors that may have influenced the recovery of neurocognitive function in TBI patients.
MATERIALS AND METHODS
Patient population
Approval for retrospective chart reviews was obtained from our hospital where this study included the data from 1998 to February 2007. The 293 patients were selected from both hospitalized and outpatient referrals (hospitalized 268, outpatient 25; male 228, female 65) on the basis of the following criteria : 1) age between 18 and 60 years; 2) patients with a history of head injury (due to a traffic accident, an industrial accident or other causes) who had recovered from the acute clinical phase; 3) the absence of documented hypoxia; 4) the absence of prior or subsequent head trauma resulting in a neurological condition or psychiatric illness, or a history of alcohol or substance abuse; 5) the absence of a premorbid intellectual deficiency; 6) the absence of prior chronic illness over the past six months that had an influence on activity; and 7) adequate cooperation with testing. Participants who met the criteria for probable or definite malingered neurocognitive dysfunction were excluded.
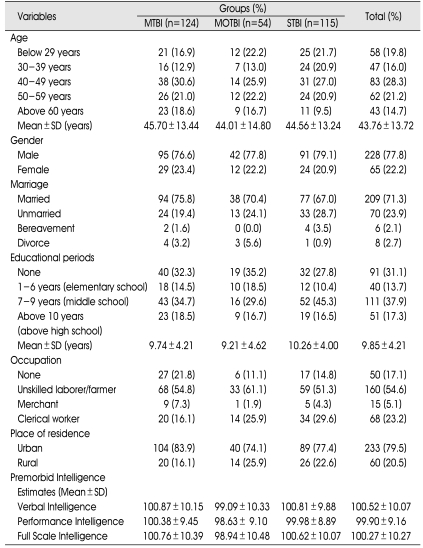
Premorbid demographic characteristics of the TBI sample groups are presented in Table 1. The TBI sample (n=293) was predominantly male (77.8%) with a mean age of 43.76±13.72 years, an average educational level of 9.85±4.21 years, and the percentage of married participants at 71.3%, and incidence of residence in an urban area at 79.5%. Occupational status at the time of injury was as follows : unskilled laborer/farmer (54.6%), clerical worker (23.2%), none (17.1%), and merchant (5.1%). The average premorbid intelligence estimates were as follows : verbal intelligence (100.52±10.07), performance intelligence (99.90±9.16) and full-scale intelligence (100.27±10.27).
Table 1.
Demographic Data
MTBI : mild traumatic brain injury (TBI) group, MOTBI : moderate TBI group, STBI : severe TBI group, SD : standard deviation
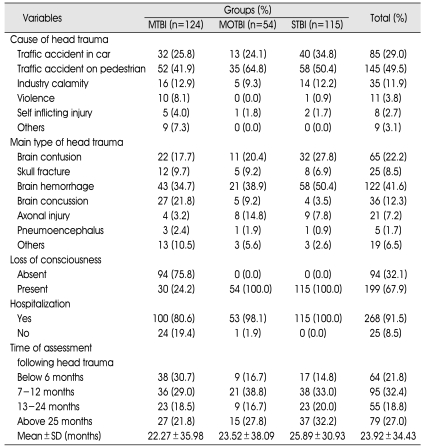
Clinical characteristics of the TBI sample groups are presented in Table 2. The causes of TBI were traffic accident as a pedestrian (49.5%), traffic accident in a car (29.0%), industry calamity (11.9%), violence (3.8%), self inflicting injury (2.7%) and others (3.1%). The types of head trauma were brain hemorrhage (41.6%), brain contusion (22.2%), brain concussion (12.3%), skull fracture (8.5%), axonal injury (7.2%), pneumoencephalus (1.7%) and others (6.5%). Incidence of loss of consciousness from the accident was 67.9%, and the incidence of hospitalization following the trauma was 91.5%.
Table 2.
Clinical Data
MTBI : mild traumatic brain injury (TBI) group, MOTBI : moderate TBI group, STBI : severe TBI group, SD : standard deviation
Procedure
The patients were divided into three subgroups based on the GCS score at the accident scene or upon initial examination following hospital arrival. The subgroups included mild TBI (GCS score 13-15), moderate TBI (GCS score 9-12) and severe TBI (GCS score 3-8) and assessed the relationship between neurocognitive function and demographic factors including age, gender, educational levels, and occupation using the Korean Wechsler Intelligence Scale (K-WAIS) and the Korean Memory Assessment Scale (K-MAS). The K-WAIS and the K-MAS were administered in a standardized manner as part of a neurocognitive evaluation. Testing was performed only when participants were medically stable and could recall meaningful information. On average, testing was assessed at 23.92±34.43 months after injury, and the assessment was performed with a review of documentation, a clinical interview and cognitive testing. Participants were given a routine warning that the tests were designed to ascertain whether they were faking or exaggerating, that cognitive impairment would be administered, and that evidence of inadequate effort would be reported.
K-WAIS
The K-WAIS is a Korean version of the Wechsler Adult Intelligence Scale-Revised (WAIS-R), and it is an intelligence test battery consisting of six verbal subsets and five performance subsets that assess three general areas of intelligence quotients : verbal intelligence quotients, performance intelligence quotients and full-scale intelligence quotients.
K-MAS
The K-MAS is a Korean version of the Memory Assessment Scale (MAS) is a memory test battery made up of 12 subsets that assess four general areas of memory function : immediate memory scale, verbal memory scale, visual memory scale and global memory scale.
Premorbid Intelligence Estimates
Premorbid intelligence estimates consider the differences between premorbid and postmorbid intelligences. The premorbid intelligence estimates can be measured by the standardized K-WAIS sample according to age and education. This sample comprises three subtypes : verbal intelligence quotients, performance intelligence quotients and full-scale intelligence quotients. We measured premorbid intelligences in three subgroups : the mild TBI group, the moderate TBI group and the severe TBI group.
Statistics
Data analysis was performed using SPSS Version 12.0. Descriptive statistics were obtained to determine the demographic variables' influence on the recovery of intelligence and memory function following TBI. One-way analysis of variances (ANOVAs) and t-test were conducted to assess the overall differences between demographic variables and the result of the K-WAIS or the K-MAS in each TBI subgroup. The results were considered significant at p<0.05.
RESULTS
Intelligence
The assessment of intelligence was performed with the three subtypes of K-WAIS including verbal intelligence, performance intelligence and full-scale intelligence. The effects of age, gender, educational period and occupation for the above intelligence subtypes in each TBI subgroup were analyzed and are presented in Tables 3-6.
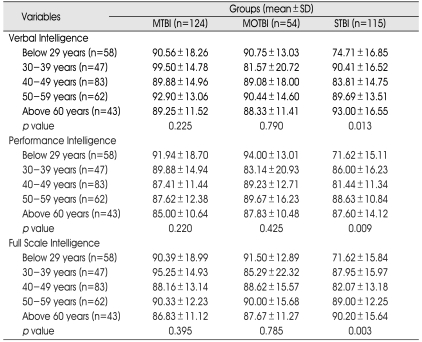
Table 3.
Age and Intelligence
MTBI : mild traumatic brain injury (TBI) group, MOTBI : moderate TBI group, STBI : severe TBI group, SD : standard deviation, p value : between age and subtype intelligence in TBI subgroups (p<0.05)
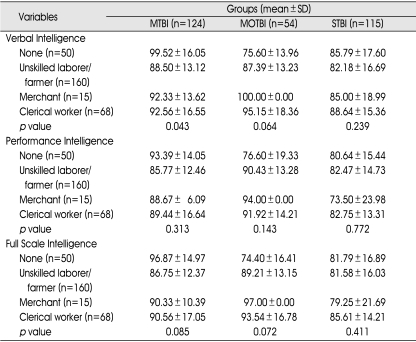
Table 6.
Occupation and Intelligence
p value : between occupation and subtype intelligence in TBI subgroups (p<0.05). MTBI : mild traumatic brain injury (TBI) group, MOTBI : moderate TBI group, STBI : severe TBI group, SD : standard deviation
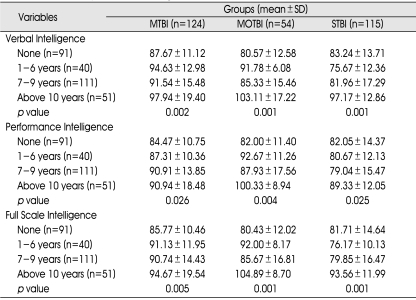
There were no statistically significant differences on any of the subtypes of intelligence among the different age groups in the mild and moderate TBI subgroups, whereas there were statistically significant differences for all intelligence subtypes among age groups in the severe TBI subgroup (Table 3).
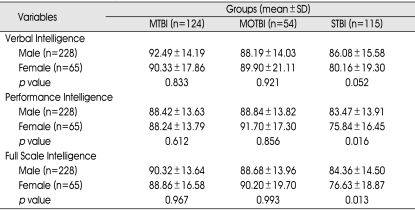
There were statistically significant differences in performance and full-scale intelligence among the gender groups in the severe TBI subgroup, whereas there were no statistically significant differences of any of the subtypes of intelligence among the gender groups in the mild and moderate TBI groups. Furthermore, verbal intelligence was not significantly different among the gender groups in the severe TBI subgroup (Table 4).
Table 4.
Gender and Intelligence
p value ; between gender and subtype intelligence in TBI subgroups (p<0.05).MTBI : mild traumatic brain injury (TBI) group, MOTBI : moderate TBI group, STBI : severe TBI group, SD : standard deviation
There were statistically significant differences in all three subtypes of intelligence according to educational periods in all TBI subgroups (Table 5).
Table 5.
Educational periods and Intelligence
p value : between educational periods and subtype intelligence in TBI subgroups (p<0.05). MTBI :mild traumatic brain injury (TBI) group, MOTBI : moderate TBI group, STBI : severe TBI group, SD : standard deviation
There were no statistically significant differences of any of the subtypes of intelligence according to occupation in any of the TBI subgroups, except verbal intelligence in the mild TBI group (Table 6).
Memory
Memory was assessed using four subtypes of K-MAS : immediate memory scale, verbal memory scale, visual memory scale and global memory scale. The effects of age, gender, educational periods and occupation on these four aspects of memory subtypes were analyzed in each TBI subgroup and are presented in Tables 7-10.
Table 7.
Age and Memory
p value ; between age and subtype memory in TBI subgroups (p<0.05). MTBI : mild traumatic brain injury (TBI) group, MOTBI : moderate TBI group, STBI : severe TBI group, SD : standard deviation
Table 10.
Occupation and Memory
p value : between occupation and subtype memory in TBI subgroups (p<0.05). MTBI : mild traumatic brain injury (TBI) group, MOTBI : moderate TBI group, STBI : severe TBI group, SD : standard deviation
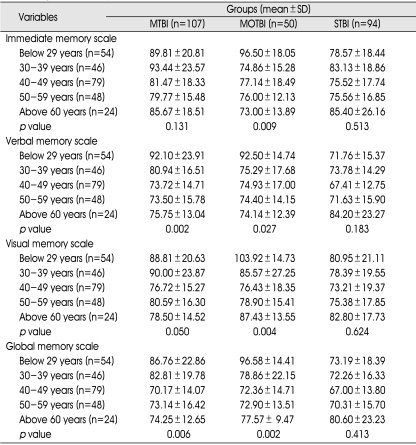
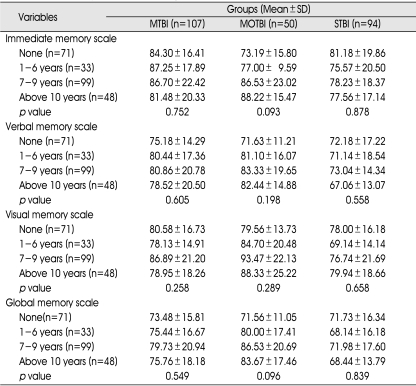
There were statistically significant differences between subtypes of memory according to age in the mild and moderate TBI subgroups, except for verbal intelligence in the mild TBI group. There were no statistically significant differences in any subtype of memory according to age in the severe TBI group (Table 7).
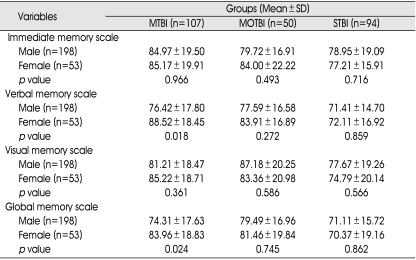
There were no statistically significant differences in subtypes of memory according to gender groups in any TBI subgroup with the exception of verbal and global memory in the mild TBI subgroup (Table 8).
Table 8.
Gender and Memory
p value : between gender and subtype memory in TBI subgroups (p<0.05). MTBI : mild traumatic brain injury (TBI) group, MOTBI : moderate TBI group, STBI :severe TBI group, SD : standard deviation
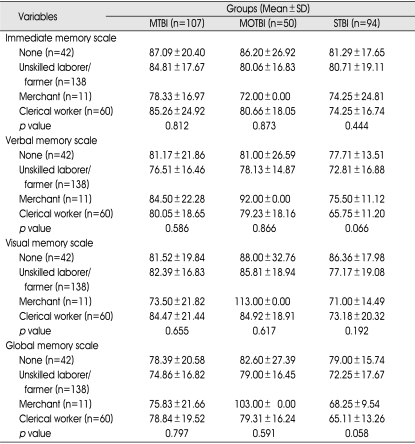
Furthermore, there were no statistically significant differences of any memory subtype according to educational period or occupation in any TBI subgroup (Table 9, 10).
Table 9.
Educational periods and Memory
p value : between educational periods and subtype memory in TBI subgroups (p<0.05). MTBI : mild traumatic brain injury (TBI) group, MOTBI : moderate TBI group, STBI : severe TBI group, SD : standard deviation
DISCUSSION
Many authors have reported that the premorbid demographic backgrounds of a traumatic brain-injured individual are likely to accurately reflect the status of patients with TBI. Outcomes following TBI were determined by evaluating the neurocognitive function of patients, and we investigated the effect of pre-morbid demographic factors on the recovery of neurocognitive function. Intelligence and memory of the neurocognitive function were assessed using K-WAIS and K-MAS, respectively, in light of the fact that several authors have reported a significant "dose response" relationship between TBI severity and intelligence-memory discrepancies12). We analyzed these outcomes with intelligence and memory divided them into subtypes.
Cognitive intelligence and memory reserves are consistent with the prevailing clinical assumption that greater premorbid intellectual functioning is related to better outcome following TBI11). This theory suggests that higher education level and intelligence may preserve functional capacity regardless of injury or disease severity11).
Some studies have demonstrated that individuals with fewer years of education may be more vulnerable to the functional impact of TBI on intelligence quotient (IQ) outcome, regardless of the injury severity or degree of structural brain damage11,13).
Verbal fluency tasks are considered to be amongst the most sensitive indicators of cerebral dysfunction3,19). Previous research has noted that individuals with above average IQs who have sustained brain impairment obtain higher verbal fluency scores than do below-average control subjects3). Despite having sustained a brain injury, many individuals with higher verbal intelligence scores have better brain function than do individuals with lower verbal intelligence3). Education has consistently been shown to influence on verbal performance, while the impacts of gender and age have been less clear3,4,7).
This report describes the relationship between these premorbid demographic factors and neurocognitive outcome following TBI. The neurocognitive outcome was evaluated via intelligence and memory assessments that included each subtype (intelligence: verbal, performance and full-scale, memory : immediate, verbal, visual and global). Subgroups with mild TBI, moderate TBI and severe TBI that were divided according to GCS were compared according to the influence of pre-morbid factors (including age, gender, educational periods and occupation) on neurocognitive function. In the severe TBI group, despite each demographic factor's effect, the severity of injury (GCS) was the predominant prognostic factor. In other words, for the patients that are initially more severely injured, neurocognitive outcomes (intelligence and memory) do not tend to be affected by demographic factors.
Educational level was an important prognostic factor for all subtypes of intelligence regardless of the severity of TBI. In particular, patients with more than 10 years of educational period had higher intelligence than other patients. However, we could not explain the appropriate cause of the other outcomes with statistical significance in intelligence, which is thought to be influenced by the severity of TBI or unknown other factors. As for memory outcome, being in a younger age group tended to be a better prognostic factor for all subtypes of memory in the mild and moderate TBI groups. We believe that the decrease of memory function as a consequence of aging is a causative factor for this finding. The female group with mild TBI presented better verbal and global memory function following TBI than the male group. Several authors have suggested that female hormones may have a neuroprotective effect and that these may be associated with females' tendency to have better memory function22). In the severe TBI group, demographic factors had no effect on the recovery of neurocognitive function due to the dominant influence of severe brain injury.
CONCLUSION
A higher educational level in the intelligence and being in a young age group in the memory are found to be good prognostic factors for the recovery of neurocognitive function following TBI. We speculate that the outcome of TBI is associated with the recovery of neurocognitive function following TBI, and consideration of the aforementioned prognostic premorbid demographic factors may be helpful for the prediction of precise prognosis and the application of appropriate rehabilitative programs in TBI patients.
Acknowledgements
"This research was supported by the Yeungnam University research grants in 2004."
References
- 1.Bayir H, Marion DW, Puccio AM, Wisniewski SR, Janesko KL, Clark RS, et al. Marked gender effect on lipid peroxidation after severe traumatic brain injury in adult patients. J Neurotrauma. 2004;21:1–8. doi: 10.1089/089771504772695896. [DOI] [PubMed] [Google Scholar]
- 2.Becker DP, Miller JD, Ward JD, Greenberg RP, Young HF, Sakalas R. The outcome from severe head injury with early diagnosis and intensive management. J Neurosurg. 1977;47:491–502. doi: 10.3171/jns.1977.47.4.0491. [DOI] [PubMed] [Google Scholar]
- 3.Bittner RM, Crowe SF. The relationship between working memory, processing speed, verbal comprehension and FAS performance following traumatic brain injury. Brain Inj. 2007;21:709–719. doi: 10.1080/02699050701468917. [DOI] [PubMed] [Google Scholar]
- 4.Bolla KI, Lindgren KN, Bonaccorsy C, Bleecker ML. Predictors of verbal fluency (FAS) in the healthy elderly. J Clin Psychol. 1990;46:623–628. doi: 10.1002/1097-4679(199009)46:5<623::aid-jclp2270460513>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 5.Braakman R, Gelpke GJ, Habbema JD, Maas AI, Minderhoud JM. Systematic selection of prognostic features in patients with severe head injury. Neurosurgery. 1980;6:362–370. [PubMed] [Google Scholar]
- 6.Brown AW, Malec JF, McClelland RL, Diehl NN, Englander J, Cifu DX. Clinical elements that predict outcome after traumatic brain injury : a prospective multicenter recursive partitioning (decision-tree) analysis. J Neurotrauma. 2005;22:1040–1051. doi: 10.1089/neu.2005.22.1040. [DOI] [PubMed] [Google Scholar]
- 7.Gladsjo JA, Schuman CC, Evans JD, Peavy GM, Miller SW, Heaton RK. Norms for letter and category fluency : demographic corrections for age, education, and ethnicity. Assessment. 1999;6:147–178. doi: 10.1177/107319119900600204. [DOI] [PubMed] [Google Scholar]
- 8.Jennett B, Teasdale G, Braakman R, Minderhoud J, Heiden J, Kurze T. Prognosis of patients with severe head injury. Neurosurgery. 1979;4:283–289. doi: 10.1227/00006123-197904000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Jennett B, Teasdale G, Braakman R, Minderhoud J, Knill-Jones R. Predicting outcome in individual patients after severe head injury. Lancet. 1976;1:1031–1034. doi: 10.1016/s0140-6736(76)92215-7. [DOI] [PubMed] [Google Scholar]
- 10.Jennett B, Teasdale G, Galbraith S, Pickard J, Grant H, Braakman R, et al. Severe head injuries in three countries. J Neurol Neurosurg Psychiatry. 1977;40:291–298. doi: 10.1136/jnnp.40.3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kesler SR, Adams HF, Blasey CM, Bigler ED. Premorbid intellectual functioning, education, and brain size in traumatic brain injury : an investigation of the cognitive reserve hypothesis. Appl Neuropsychol. 2003;10:153–162. doi: 10.1207/S15324826AN1003_04. [DOI] [PubMed] [Google Scholar]
- 12.Langeluddecke PM, Lucas SK. Wechsler Adult Intelligence Scale-Third Edition findings in relation to severity of brain injury in litigants. Clin Neuropsychol. 2003;17:273–284. doi: 10.1076/clin.17.2.273.16499. [DOI] [PubMed] [Google Scholar]
- 13.Leckliter IN, Matarazzo JD. The influence of age, education, IQ, gender, and alcohol abuse on Halstead-Reitan Neuropsychological Test Battery performance. J Clin Psychol. 1989;45:484–512. doi: 10.1002/1097-4679(198907)45:4<484::aid-jclp2270450402>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 14.Levati A, Farina ML, Vecchi G, Rossanda M, Marrubini MB. Prognosis of severe head injuries. J Neurosurg. 1982;57:779–783. doi: 10.3171/jns.1982.57.6.0779. [DOI] [PubMed] [Google Scholar]
- 15.Marmarou A, Lu J, Butcher I, McHugh GS, Mushkudiani NA, Murray GD, et al. IMPACT database of traumatic brain injury : design and description. J Neurotrauma. 2007;24:239–250. doi: 10.1089/neu.2006.0036. [DOI] [PubMed] [Google Scholar]
- 16.Miller JD, Butterworth JF, Gudeman SK, Faulkner JE, Choi SC, Selhorst JB, et al. Further experience in the management of severe head injury. J Neurosurg. 1981;54:289–299. doi: 10.3171/jns.1981.54.3.0289. [DOI] [PubMed] [Google Scholar]
- 17.Mushkudiani NA, Engel DC, Steyerberg EW, Butcher I, Lu J, Marmarou A, et al. Prognostic value of demographic characteristics in traumatic brain injury : results from the IMPACT study. J Neurotrauma. 2007;24:259–269. doi: 10.1089/neu.2006.0028. [DOI] [PubMed] [Google Scholar]
- 18.Overgaard J, Hvid-Hansen O, Land AM, Pedersen KK, Christensen S, Haase J, et al. Prognosis after head injury based on early clinical examination. Lancet. 1973;2:631–635. doi: 10.1016/s0140-6736(73)92477-x. [DOI] [PubMed] [Google Scholar]
- 19.Ruff RM, Light RH, Parker SB, Levin HS. The psychological construct of word fluency. Brain Lang. 1997;57:394–405. doi: 10.1006/brln.1997.1755. [DOI] [PubMed] [Google Scholar]
- 20.Sherrill-Pattison S, Donders J, Thompson E. Influence of demographic variables on neuropsychological test performance after traumatic brain injury. Clin Neuropsychol. 2000;14:496–503. doi: 10.1076/clin.14.4.496.7196. [DOI] [PubMed] [Google Scholar]
- 21.Teasdale G, Parker L, Murray G, Knill-Jones R, Jennett B. Predicting the outcome of individual patients in the first week after severe head injury. Acta Neurochir Suppl (Wien) 1979;28:161–164. doi: 10.1007/978-3-7091-4088-8_39. [DOI] [PubMed] [Google Scholar]
- 22.Wagner AK, Bayir H, Ren D, Puccio A, Zafonte RD, Kochanek PM. Relationships between cerebrospinal fluid markers of excitotoxicity, ischemia, and oxidative damage after severe TBI : the impact of gender, age, and hypothermia. J Neurotrauma. 2004;21:125–136. doi: 10.1089/089771504322778596. [DOI] [PubMed] [Google Scholar]