Abstract
Objective
The serum S100 protein has been known to reflect the severity of neuronal damage. The purpose of this study was to assess the prognostic value of the serum S100 protein by Elecsys S100 immunoassay in patients with subarachnoid hemorrhage (SAH) and intracerebral hemorrhage (ICH) and to establish reference value for this new method.
Methods
Serum S100 protein value was measured at admission, day 3 and 7 after bleeding in 42 consecutive patients (SAH : 20, ICH : 22) and 74 healthy controls, prospectively. Admission Glasgow coma scale (GCS) score, Hunt & Hess grade and Fisher grade for SAH, presence of intraventricular hemorrhage, ICH volume, and outcome at discharge were evaluated. Degrees of serum S100 elevation and their effect on outcomes were compared between two groups.
Results
Median S100 levels in SAH and ICH groups were elevated at admission (0.092 versus 0.283 µg/L) and at day 3 (0.110 versus 0.099 µg/L) compared to healthy controls (0.05 µg/L; p<0001). At day 7, however, these levels were normalized in both groups. Time course of S100 level in SAH patient was relatively steady at least during the first 3 days, whereas in ICH patient it showed abrupt S100 surge on admission and then decreased rapidly during the next 7 days, suggesting severe brain damage at the time of bleeding. In ICH patient, S100 level on admission correlated well with GCS score (r=-0.859; p=0.0001) and ICH volume (r=0.663; p=0.001). A baseline S100 level more than 0.199 µg/L predicted poor outcome with 92% sensitivity and 90% specificity. Logistic regression analyses showed Ln (S100) on admission as the only independent predictor of poor outcome (odd ratio 36.1; 95% CI, 1.98 to 656.3).
Conclusion
Brain damage in ICH patient seems to develop immediately after bleeding, whereas in SAH patients it seems to be sustained for few days. Degree of brain damage is more severe in ICH compared to SAH group based on the S100 level. S100 level is considered an independent predictor of poor outcome in patient with spontaneous ICH, but not in SAH. Further study with large population is required to confirm this result.
Keywords: S100 protein, Prognosis, Subarachnoid hemorrhage, Elecsys S100 immunoassay, Intracerebral hemorrhage
INTRODUCTION
The serum S100 protein was named by its biochemical characteristics of 100% solubility in saturated ammonium sulfate14). It has two subunits of alpha and beta. S100 beta or S100B has been known to be released from the damaged astrocyte in various conditions, such as cerebral infarction, head injury, less frequently spontaneous subarachnoid hemorrhage (SAH) and intracerebral hemorrhage (ICH) and reflect the severity of neuronal damage5,6,9,11,12,19,22). Normally, S100 is present in the cytosol or bound to the membranes of astroglial cells, mostly of the central nervous system8). If these cells are structurally or ischemically damaged, S100 is rapidly released leaking into the cerebrospinal fluid (CSF) and secondarily across the blood-brain barrier (BBB) into the circulation. An early concentration peak of S100 in serum 20 minutes after brain damage seems to reflect both cellular damage and increased permeability of the BBB2).
Several sensitive assays for the detection of serum S100B have become available1,15,16,20,22). Of these, Liaison Sangtec 100 method and Liamat method were commonly used for detection of S100B15,22). However, recently, commercially available Elecsys S100 immunoassay was introduced. It has an advantage of rapid measurement of serum S100 level for 18 min over previous LIA-mat S100 system, which requires over 2 hours6). There have been few reports regarding serum S100 protein and outcome by Elecsys S100 immunoassay in patient with head injury, subarachnoid hemorrhage and carotid stenosis1,16,18).
Spontaneous ICH is a bleeding from small perforating artery, which is usually caused by hypertension. It is a destructive focal lesion which expands brain parenchyma along the white matter as the hematoma volume is enlarged. But, SAH is a hemorrhage outside the pia matter even though it can be very dangerous in some cases, so it can cause minimal brain damage if it is not associated with uncontrolled intracranial hypertension or large amount of ICH. Thus, authors hypothesized that S100 protein elevation would be marked in ICH group compared to SAH group. The aim of study is to evaluate the degree of S100 elevation and its effect on outcome in SAH and ICH groups. Additionally, the authors have tried to establish normal serum value of S100 by Elecsys S100 immunoassay.
MATERIALS AND METHODS
Patient population
This prospective study was performed in 42 consecutive patients (SAH : 20, ICH : 22) who were admitted to our hospital within 24 hours after hemorrhage and in 74 healthy controls who visited our hospital for regular health checkup for three months from January 2007. Blood samplings for S100 protein measurement were performed at admission, day 3 and 7 after onset of hemorrhage. Admission Glasgow coma scale (GCS) score, Hunt & Hess grade and Fisher grade for SAH, presence of intraventricular hemorrhage (IVH), ICH volume and outcome at discharge were measured. To determine the reference value for S100, measurement of S100 level in 74 healthy controls were also performed. Factors affecting outcome at discharge were determined.
Patient management
For SAH patients, initial clinical condition was measured by Hunt & Hess grade, and severity of SAH was measured with Fisher grade. All patient except two who had angiography negative SAH, had ruptured aneurysm. Of the 18 patients who had ruptured aneurysm, two had multiple aneurysms which were occluded with combined approach of coiling and clipping in both cases. One patient who had very small aneurysm on anterior communicating artery was managed conservatively. Of the 19 aneurysms in 17 patients, 13 aneurysms were surgically clipped, whereas remaining 6 were obliterated with endovascular coil embolization (Table 1). All surgical or endovascular procedures were performed within 24 hours after admission. Thus, the day 3 samples were obtained after exclusion of aneurysm from the systemic circulation. Nimodipine therapy was started after admission and continued for two weeks with intravenous treatment and then switched to oral medication for two weeks. Triple H therapy was initiated if patient showed symptoms of clinical vasospasm or increased flow velocity more than 120 cm/sec on Transcranial Doppler examinations.
Table 1.
Clinical characteristics of 42 spontaneous hemorrhage patients
*Two patient who had multiple aneurysms were treated with coil embolization and surgical clipping for different aneurysms. SAH : subarachnoid hemorrhage, ICH : intracerebral hemorrhage, GCS : Glasgow coma scale, IVH : intraventricular hemorrhage, ACA : anterior cerebral aretery, MCA : middle cerebral artery, ICA : internal carotid artery
For ICH patient, clinical condition was measured by GCS score and surgical decision was made based on the hematoma volume, midline shift and clinical status of the patients. Hematoma volume was measured by the formula of A×B×C/27). Basal ganglia and thalamus were the most common locations of ICH. All patients underwent intravenous mannitol therapy. Mean hematoma volume was 41.6±34.8 cc (range from 0.5 to 122 cc). Mean hematoma volume of open surgery group and conservative treatment group were 54.9±29.2 cc and 28.4±36 cc, respectively (p=0.045). Fifty percent of the patients received open craniotomy and hematoma evacuation, whereas remaining 11 patients underwent conservative treatment. IVH was accompanied in 30% of SAH group whereas in 45.5% of ICH group (Table 1).
Outcome was assessed at discharge according to Glasgow Outcome Scale (GOS) score. To compare the outcome statistically, outcome was dichotomized to good or poor. Good recovery and moderate disability were allocated to good outcome group and severe disability and vegetative state/death were allocated to poor outcome group. Clinical variables and outcomes are listed in Table 1.
S100 measurement
For S100 measurements, venous blood samples (5 cc) were collected at hospital admission, day 3 and 7 after bleeding. Blood samples were allowed to clot for 20 to 30 minutes at room temperature, then were centrifuged (2500G, 10 minutes) and separated. Most of the samples were immediately measured for S100, and some samples were stored at 4℃ less then 2 days until analysis was done. The S100 proteins in serum were measured by full automated modular analytic E170 system using electrochemoluminometric immunoassay (Elecsys S100® ; Roche Diagnostics, Penzberg, Germany). Detection ranges for S100 were between 0.005 µg/L and 39 µg/L with a within-run coefficient of variation from 0.7% to 1.8%.
Statistical analysis
Data are expressed as median [interquartile range] for continuous variables. Data were analyzed with statistical package SPSS software for windows, version 11.0.1 (SPSS, Inc., Chicago, IL). Significance for intergroup differences was assessed by Fisher's exact test for categorical variables and Mann-Whitney U test for continuous variables. To study correlations between continuous variables, Speaman rank correlation coefficients (r) were used.
Receiver operating characteristics (ROC) curves were configured to establish the cut-off points of S100 with optimal sensitivity and specificity predicting poor outcome. Logistic regression analyses were performed to determine the factors that could be considered as independent predictors of poor outcome, using the forward stepwise method by likelihood ratio test. A p value of <0.05 was considered significant.
RESULTS
Poor outcomes were achieved in 10% (2/20) of SAH and 54.5% (12/22) of ICH groups.
The median S100 level for healthy controls was 0.05 [0.036 to 0.066] µg/L (reference value for 95 percentile : 0.12 µg/L). Median serum S100 level in SAH and ICH groups on admission (0.092 [0.052 to 0.163] µg/L versus 0.283 [0.070 to 0.567] µg/L), and at day 3 (0.110 [0.072 to 0.176] µg/L and 0.099 [0.069 to 0.329] µg/L) were significantly higher than those of healthy controls (p<0001). But, at day 7, these levels were normalized in both groups (0.050 [0.038 to 0.100] µg/L versus 0.058 [0.048 to 0.091] µg/L). Statistically significant differences between groups were identified only on admission samples (p=0.042) (Fig. 1).
Fig. 1.
Box plot showing serum S100 protein time course concentrations in patients with subarachnoid hemorrhage (SAH) (open box) and intracerebral hemorrhage (ICH) (hatched box). The ends of the box marked with open diamond and open circle are the 25th and 75th quartiles, respectively. The line across the middle of the box identifies the median sample value. S100 protein concentration is higher than control group at day 1 and 3 measurement in both groups. But, at day 7, S100 protein level is not different compare to control group. Difference of serum S100 level on admission is statistically significant between SAH and ICH group (*p=0.042). S100 protein levels measured at day 3 or day 7 were not different between groups (Mann-Whitney U test). Extreme value of S100 (5.76) in ICH group at day 1 measurement is omitted to express the differences effectively.
SAH group
Time course of S100 level in SAH patient was relatively steady at least during the first 3 days after bleeding and then decreased to normal range at day 7, suggesting sustained brain damage for few days after SAH. Even though admission S100 protein level did not correlate with initial clinical status measured by Hunt & Hess grade, it had a tendency to correlate with admission GCS score (r=-0.393; p=0.087). However, admission S100 concentration did not influence on the outcome at discharge in 20 SAH patients. This result is probably due to the small sample size and deviation of the patients with good clinical grade (all but one patient was HH grade II or III).
According to Fisher grade, S100 level was higher as the Fisher grade increased, but the difference was not statistically significant. In a subgroup of SAH patients who accompanied by ICH (n=4, 4-51 cc), admission S100 level was the highest (1.11 µg/L) in a patient with ICH of 51 cc.
ICH group
Time course of S100 level in ICH patient showed abrupt S100 surge on admission and then decreased rapidly during the next 7 days. Admission GCS score was negatively correlated with serum S100 level at admission (r=-0.859; p=0.0001), day 3 (r=-0.838; p=0.0001) and day 7 (r=-0.746; p=0.021).
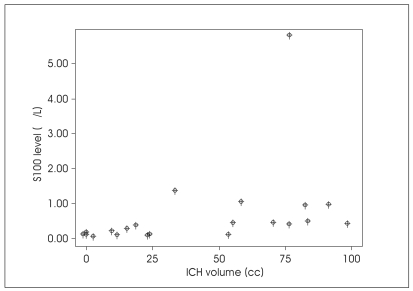
There were positive correlations between ICH volume and serum S100 level at admission (r=0.663; p=0.001)(Fig. 2) and also at day 3 (r=0.609; p=0.021), but there showed no correlation at day 7 measurement.
Fig. 2.
Scatter plot showing positive correlation of intracerebral hematoma volume with admission S100 level (r=0.663, p=0.001).
Those patients who had intraventricular extension of ICH had higher S100 level than those who did not at admission (0.396 [0.353 to 0.949] versus 0.07 [0.058 to 0.212] µg/L, p=0.005), at day 3 (0.329 [0.158 to 0.639] versus 0.076 [0.055 to 0.162] µg/L, p=0.048), and at day 7 (0.11 [0.098 to 0.112] versus 0.057 [0.04 to 0.069] µg/L, p=0.04) (Fig. 3).
Fig. 3.
Box plots showing S100 protein time course concentrations according to intraventricular extension of intracerebral hemorrhage (ICH) in 22 ICH patients. S100 concentrations are significantly higher in patients who have intraventricular extension of ICH (hatched box) than those who don't (open box) at day 1 (p=0.005), day 3 (p=0.048) and day 7 measurement (p=0.040). The ends of the box marked with open diamond and open circle are the 25th and 75th quartiles. The line across the middle of the box identifies the median sample value. Outliers beyond 1.5 times of interquartile range are omitted.
Serum S100 protein concentrations at admission and day 3 were correlated well with outcome at discharge measured by GOS score (r=-0.738; p=0.0001, r=-0.723; p=0.003, respectively, GOS 1=dead). To compare the median S100 level, dichotomized outcomes to good and poor were used. Poor outcome group had significantly higher S100 protein level than that of good outcome group in all three measurements (Fig. 4).
Fig. 4.
Box plots showing S100 protein level according to outcome in 22 intracerebral hemorrhage patients. Open and hatched boxes indicate good and poor outcome group, respectively. Poor outcome group demonstrates significantly higher S100 levels than those of good outcome group at admission (p=0.0001), day 3 (p=0.003) and day 7 measurements (p=0.038). The ends of the box marked with open diamond and open circle are the 25th and 75th quartiles, respectively. The line across the middle of the box identifies the median sample value. Outliers beyond 1.5 times of interquartile range are omitted.
Using ROC curve, a baseline S100 level more than 0.199 µg/L predicted poor outcome with 0.92 sensitivity and 0.90 specificity (Fig. 5). Logistic regression analyses selected Ln (S100) at admission as the only independent predictor of poor outcome (odd ratio 36.1; 95% CI, 1.98 to 656.3) in our sample.
Fig. 5.
Receiver operating characteristics curve of the serum S100 protein measurement on admission for poor outcome. A cutoff level of admission S100 protein of 0.199 µg/L predicted poor outcome with 92% sensitivity and 90% specificity. AUC=0.983 (95% CI, 0.815-1.000; p=0.0001).
DISCUSSION
Even though the prognostic value of S100 protein has been extensively studied in severe head injury, acute ischemic stroke and cardiac arrest, S100 protein in patient with SAH was less frequently studied2,3,6,13,16,17,19,21-23). Furthermore, the role of S100 protein in patient with ICH is lacking5). Thus, authors evaluated and compared the degrees of S100 elevation between SAH and ICH groups.
Time course of S100 protein release into serum in patient with hemorrhagic stroke is different from that of ischemic stroke. It is well known that S100B values increase in ischemic stroke, reaching maximum levels 2 to 4 days after onset, correlating with infarct size4,6,13). S100 protein level fell significantly thereafter, but still above the level seen in the control population at 3 months after stroke, suggesting partial restoration of glial cell integrity3).
In ICH patient, serum S100 level was the highest at day 1 after bleeding, suggesting that hemorrhage into the brain parenchyma seemed to cause maximum brain damage immediately after hemorrhage onset due to direct brain laceration, compression and distortion. Furthermore, the degree of S100 increase in ICH group was marked compare to that of SAH group, which implicated that more severe brain damage occurred in ICH group. In SAH patient, S100 level was higher than healthy control at initial samples and sustained increase at day 3, suggesting progressive brain damage after SAH.
Elecsys S100 immunoassay is known to detect both S100ab and S100bb16,18). Because small amount of S100 protein have been found in fat tissue, skin, bone, skeletal muscle and heart muscle, as well as in melanoma cells, the S100 protein measurement by Elecsys S100 immunoassay may be false positive in severe head injury, especially in association with adipose tissue injury and long bone fracture1,2,8,10,18). Mussack et al.16) reported that S100 protein measurements using this method were not reliable for the real time detection of severe brain damage in multiple trauma patients, mainly due to the S100 release from peripheral sources. But, in case of spontaneous hemorrhage such as SAH or ICH, S100 protein level by Elecsys S100 immunoassay may reflect neuronal damage quite clearly because there is little chance of S100 release from peripheral sources.
Delgado et al.5) measured S100B by conventional ELISA method on admission sample in spontaneous ICH patients and demonstrated that increased S100B was found after ICH in association with neurological deterioration and related to initial hematoma volume. But, S100 level on admission was not independent prognostic factor for poor outcome in their study. In our study admission S100 level was correlated well with GCS score and ICH volume, as well as with outcome in ICH patients. Also, only Ln (S100) on admission was an independent prognostic factor for poor outcome in ICH patients by logistic regression analysis. This difference might be explained by the different patient characteristics between the two studies.
In our study, mean hematoma volume was larger and intraventricular extension of ICH was more frequent than those of their study (46 cc versus 17 cc, 45.5% versus 25%). Thus, the degree of brain destruction seemed to be more severe in our study patients than those of theirs. The reason why the S100 protein level was not an independent predictor of poor outcome in their study was not clear, but it was probably because of the more strong impact of ICH volume than S100 protein level on outcome by multivariate analysis.
In SAH patients, S100 protein level correlated with neurological status at admission (high Hunt & Hess grade and Fisher grade) and outcome by many authors17,21-23). But, in this study admission S100 protein level showed a tendency to correlate with initial clinical status measured by GCS score, not by Hunt & Hess grade, probably because GCS score was more sensitive to reflect the clinical status than Hunt & Hess grade.
Reference value of S100 protein using Elecsys immunoassay by Muller et al.15) was mean of 0.05 µg/L (range : 0.02-0.08, 95 percentile of 0.07) in 118 healthy controls. In our study, mean S100 level was 0.055 µg/L, which was similar to that of Muller and 95 percentile of S100 protein in healthy controls was 0.12 µg/L. Rapid measurement S100 protein using Elecsys S100 immunoassay could be a valuable tool for predicting outcome in patients with spontaneous ICH, but not in SAH.
The limitations of this study are small number of the study patients and uneven distribution of initial clinical status in SAH patients. Because most of the patients were good clinical grade on admission, the effect of S100 protein on clinical status might not be fully evaluated statistically. To evaluate clinical significance of S100 protein on initial clinical status and outcome with enough statistical power, large prospective study would be required.
CONCLUSION
Brain damage in ICH patient seems to develop immediately after bleeding, whereas in SAH patients it seems to be sustained for few days. Degree of brain damage is more severe in ICH compare to SAH group based on the S100 protein level. S100 protein level is an independent predictor of poor outcome in patient with spontaneous ICH, but not in SAH. Further study with large population is required to verify this result.
Acknowledgements
In this study, rapid measurement of serum S100 protein was performed using Elecsys S100 immunoassay test, which was sponsored by Roche Diagnostics, Korea. In addition, I appreciate very much to nurse practitioner Eun-Hye Son (RN) for data collection of the patients.
References
- 1.Alber B, Hein R, Garbe C, Caroli U, Luppa PB. Multicenter evaluation of the analytical and clinical performance of the Elecsys S100 immunoassay in patients with malignant melanoma. Clin Chem Lab Med. 2005;43:557–563. doi: 10.1515/CCLM.2005.097. [DOI] [PubMed] [Google Scholar]
- 2.Blomquist S, Johnsson P, Lührs C, Malmkvist G, Solem JO, Alling C, et al. The appearance of S-100 protein in serum during and immediately after cardiopulmonary bypass surgery : a possible marker for cerebral injury. J Cardiothorac Vasc Anesth. 1997;11:699–703. doi: 10.1016/s1053-0770(97)90160-9. [DOI] [PubMed] [Google Scholar]
- 3.Butterworth RJ, Sherwood RA, Bath PM. Serum S-100 protein in acute stroke. Stroke. 1998;29:730. doi: 10.1161/01.str.29.3.730. [DOI] [PubMed] [Google Scholar]
- 4.Buttner T, Weyers S, Postert T, Sprengelmeyer R, Kuhn W. S-100 protein : serum marker of focal brain damage after ischemic territorial MCA infarction. Stroke. 1997;28:1961–1965. doi: 10.1161/01.str.28.10.1961. [DOI] [PubMed] [Google Scholar]
- 5.Delgado P, Alvarez Sabin J, Santamarina E, Molina CA, Quintana M, Rosell A, et al. Plasma S100B level after acute spontaneous intracerebral hemorrhage. Stroke. 2006;37:2837–2839. doi: 10.1161/01.STR.0000245085.58807.ad. [DOI] [PubMed] [Google Scholar]
- 6.Foerch C, Singer OC, Neumann-Haefelin T, du Mesnil de Rochemont R, Steinmetz H, Sitzer M. Evaluation of serum S100B as a surrogate marker for long-term outcome and infarct volume in acute middle cerebral artery infarction. Arch Neurol. 2005;62:1130–1134. doi: 10.1001/archneur.62.7.1130. [DOI] [PubMed] [Google Scholar]
- 7.Gebel JM, Sila CA, Sloan MA, Granger CB, Weisenberger JP, Green CL, et al. Comparison of the ABC/2 estimation technique to computer-assisted volumetric analysis of intraparenchymal and subdural hematomas complicating the GUSTO-1 trial. Stroke. 1998;29:1799–1801. doi: 10.1161/01.str.29.9.1799. [DOI] [PubMed] [Google Scholar]
- 8.Haimoto H, Hosoda S, Kato K. Differential distribution of immunoreactive S100-alpha and S100-beta proteins in normal nonnervous human tissues. Lab Invest. 1987;57:489–498. [PubMed] [Google Scholar]
- 9.Herrmann M, Curio N, Jost S, Grubich C, Ebert AD, Fork ML, et al. Release of biochemical markers of damage to neuronal and glial brain tissue is associated with short and long term neuropsychological outcome after traumatic brain injury. J Neurol Neurosurg Psychiatry. 2001;70:95–100. doi: 10.1136/jnnp.70.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kahn HJ, Baumal R, Van Eldik LJ, Dunn RJ, Marks A. Immunoreactivity of S100 beta in heart, skeletal muscle, and kidney in chronic lung disease : possible induction by cAMP. Mod Pathol. 1991;4:698–701. [PubMed] [Google Scholar]
- 11.Kanner AA, Marchi N, Fazio V, Mayberg MR, Koltz MT, Siomin V, et al. Serum S100beta : a noninvasive marker of blood-brain barrier function and brain lesions. Cancer. 2003;97:2806–2813. doi: 10.1002/cncr.11409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korfias S, Stranjalis G, Papadimitriou A, Psachoulia C, Daskalakis G, Antsaklis A, et al. Serum S-100B protein as a biochemical marker of brain injury : a review of current concepts. Curr Med Chem. 2006;13:3719–3731. doi: 10.2174/092986706779026129. [DOI] [PubMed] [Google Scholar]
- 13.Missler U, Wiesmann M, Friedrich C, Kaps M. S-100 protein and neuron-specific enolase concentrations in blood as indicators of infarction volume and prognosis in acute ischemic stroke. Stroke. 1997;28:1956–1960. doi: 10.1161/01.str.28.10.1956. [DOI] [PubMed] [Google Scholar]
- 14.Moore BW. A soluble protein characteristic of the nervous system. Biochem Biophys Res Commun. 1965;19:739–744. doi: 10.1016/0006-291x(65)90320-7. [DOI] [PubMed] [Google Scholar]
- 15.Muller K, Elverland A, Romner B, Waterloo K, Langbakk B, Unden J, et al. Analysis of protein S-100B in serum : a methodological study. Clin Chem Lab Med. 2006;44:1111–1114. doi: 10.1515/CCLM.2006.211. [DOI] [PubMed] [Google Scholar]
- 16.Mussack T, Kirchhoff C, Buhmann S, Biberthaler P, Ladurner R, Gippner-Steppert C, et al. Significance of Elecsys S100 immunoassay for real-time assessment of traumatic brain damage in multiple trauma patients. Clin Chem Lab Med. 2006;44:1140–1145. doi: 10.1515/CCLM.2006.190. [DOI] [PubMed] [Google Scholar]
- 17.Oertel M, Schumacher U, McArthur DL, Kastner S, Boker DK. S-100B and NSE : markers of initial impact of subarachnoid haemorrhage and their relation to vasospasm and outcome. J Clin Neurosci. 2006;13:834–840. doi: 10.1016/j.jocn.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 18.Oh EJ, Kim YM, Jegal DW, Kahng J, Park YJ, Han K. Diagnostic value of Elecsys S100 as a marker of acute brain injury in the emergency department. J Clin Lab Anal. 2007;21:387–392. doi: 10.1002/jcla.20201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raabe A, Grolms C, Sorge O, Zimmermann M, Seifert V. Serum S-100B protein in severe head injury. Neurosurgery. 1999;45:477–483. doi: 10.1097/00006123-199909000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Smit LH, Korse CM, Bonfrer JM. Comparison of four different assays for determination of serum S-100B. Int J Biol Markers. 2005;20:34–42. doi: 10.1177/172460080502000106. [DOI] [PubMed] [Google Scholar]
- 21.Stranjalis G, Korfias S, Psachoulia C, Kouyialis A, Sakas DE, Mendelow AD. The prognostic value of serum S-100B protein in spontaneous subarachnoid haemorrhage. Acta Neurochir (Wien) 2007;149:231–237. doi: 10.1007/s00701-006-1106-9. discussion 237-238. [DOI] [PubMed] [Google Scholar]
- 22.Weiss N, Sanchez-Pena P, Roche S, Beaudeux JL, Colonne C, Coriat P, et al. Prognosis value of plasma S100B protein levels after subarachnoid aneurysmal hemorrhage. Anesthesiology. 2006;104:658–666. doi: 10.1097/00000542-200604000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Wiesmann M, Missler U, Hagenstrom H, Gottmann D. S-100 protein plasma levels after aneurysmal subarachnoid haemorrhage. Acta Neurochir (Wien) 1997;139:1155–1160. doi: 10.1007/BF01410976. [DOI] [PubMed] [Google Scholar]