Abstract
Objective
For patients with giant or dissecting aneurysm, multimodal treatment consisting extracranial-intracranial bypass surgery plus clip or coil for parent artery occlusion may be necessary. In this study, the safety and efficacy of multimodal treatment in 15 patients with complex aneurysms were evaluated retrospectively.
Methods
From January 1995 to June 2007, the authors treated 15 complex aneurysms that were unable to be clipped or coiled. Among them, nine patitents had unruptured aneurysms and 6 had ruptured aneurysms. Aneurysms were located in the internal cerebral artery (ICA) in 11 patients (4 in the dorsal wall, 4 in the terminal ICA, 1 in the paraclinoid, and 2 in the cavernous ICA), in the middle cerebral artery (MCA) in 2, and in the posterior circulation in two patients
Results
Fifteen patients with complex aneurysms were treated with bypass surgery previously. Thirteen patients were treated with external carotid middle cerebral artery (ECA-MCA) anastomosis, and one patient with superficial temporal to posterior cerebral artery (STA-PCA) and another patient with occipital artery to posterior inferior cerebellar artery (OA-PICA) anastomosis. Parent artery occlusion was then performed with a clip in 9 patients, with a coil in 4, with balloon plus coil in one patient. All 15 aneurysms were successfully treated with clip or coil combined with bypass surgery. Follow-up angiograms showed good patency of anastomotic site in 10 out of 11 patients, and perfusion study showed sufficient perfusion in 6 out of 9 patients.
Conclusion
These findings indicate that for patients with complex aneurysms, clip or coil for parent vessel occlusion with additive bypass surgery can successfully exclude the aneurysm from the neurovascular circulatory system.
Keywords: Aneurysm, Clip, Coil, Bypass
INTRODUCTION
Recent advances in endovascular treatment have increased safety and efficacy for treating patients with aneurysms, but neither this modality alone nor microsurgical clipping alone is sufficient for patients with complex aneurysm such as giant or dissecting aneurysms. A neurovascular, multimodal, team approach is necessary to select the optimal management strategy for patients with complex aneurysms. In this study, we describe here our experiences from using a multimodal approach in patients with complex aneurysms. Our goals were to identify the types of complex aneurysms for which such an approach is recommended and to evaluate efficacy and outcome of these multimodal approaches.
MATERIALS AND METHODS
A review of our medical records identified fifteen patients who underwent multimodal treatment for complex aneurysms between January 1995 and June 2007. Patients undergoing clip treatment for the recurrence of a previously treated aneurysm with a coil or coil occlusion with bypass surgery were included. Location of aneurysm, types of endovascular and surgical treatment, types of bypass surgery, follow-up results, and treatment-associated complications were evaluated.
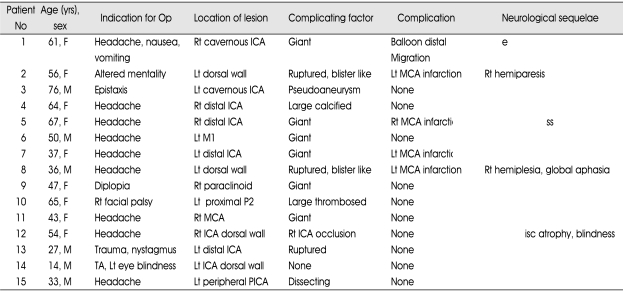
Patients characteristics
Among fifteen patients, six were male and nine were female with mean age of 48.7 years (ranginf from 17 to 76 years) (Table 1). Nine patients had unruptured aneurysms and 6 had ruptured aneurysms. Of the nine patients with unruptured aneurysms, eight presented with symptoms related to mass effects of aneurysm, and one patient who had multiple traumatic dissecting aneurysms of the ICA showed distal anterior cerebral artery (ACA) territory infarction. Of the six patients with ruptured aneurysms, five presented with subarachnoid hemorrhage (SAH), 4 with dorsal wall aneurysms of the internal cerebral artery (ICA), and 1 with dissection of the posterior inferior cerebellar artery (PICA). One patient had massive nasal bleeding because of a pseudoaneurysm of the cavernous ICA.
Table 1.
Clinical summary of 15 patients with aneurysms who underwent combined treatment approaches
Characteristics of the complex aneurysms
Of fifteen aneurysms, thirteen aneurysms were located in the anterior circulation and two were located in the posterior circulation. In the anterior circulation, four lesions involved dorsal wall of ICA, four lesions involved terminal ICA, one lesion involved paraclinoid ICA, two lesions involved cavernous ICA, and two lesions were located in middle cerebral artery (MCA). In the posterior circulation, one involved posterior cerebral artery (PCA) P2 portion, and one involved in peripheral PICA. Six types of the lesion complexity in our study were the following : 1) giant aneurysm (n=6) , 2) ICA dorsal wall aneurysm (n=5) , 3) psedoaneurysm (n=1), 4) large calcified aneurysm (n=1), 5) serpentine thromosed giant aneurysm (n=1), and 6) dissecting aneurysm (n=1).
Indications for bypass
The balloon occlusion test (BTO) represents the main criteria for choosing an appropriate bypass conduit. During the study, three different conditions were evaluated : 1) good clinical tolerance during the occlusion test, no sign of hypoperfusion at Diamox single positron emission computed tomography (SPECT); 2) good clinical tolerance, appearance of hypoperfusion at SPECT; 3) poor clinical tolerance. The ICA can easily be occluded without bypass in the first condition. It is a routine to perform STA-MCA bypass with low flow in the second condition. It is general to perform saphenous vein graft (ECA-SVG-M2) or radial artery graft (ECA-RAG-M2) is generally performed in the third condition.
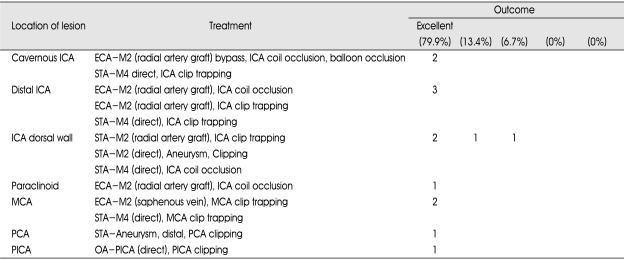
Treatment modalities
Treatment decisions were made after review by a neurovascular team composed of two cerebrovascular surgeons (B.K., J.A.) and one endovascular neurosurgeon (D.K.). Bypass surgery included low flow bypass such as superficial temporal artery to middle cerebral artery (STA-MCA) direct anastomosis in six patients and high flow bypass such as external carotid artery-radial artery graft-middle cerebral artery (ECA-RAG-MCA) bypass grafting in six patients and external carotid artery-saphenous vein graft-middle cerebral artery (ECA-SVG-MCA) bypass grafting in one patient (Table 2). Two patients had posterior circulation aneurysms. These patients were treated with occipital artery-posterior inferior cerebellar artery (OA-PICA) and STA-P2 anastomosis. Parent artery occlusion was performed with a clip in nine patients, with a coil in four, with a balloon plus coil in one patient. Distal ICA aneurysm, which may be caused by trauma, was planned for ICA trapping with clip and was performed by direct neck clipping due to aneurysm morphology based on microscopic view.
Table 2.
Locations of lesions, treatments, and outcomes in 15 patients with complex aneurysms
RESULTS
The mean follow-up period for surviving patients was 30.3 months (range, 7 to 55 months). All aneurysms were excluded from the blood flow of their respective parent arteries. Twelve patients had excellent outcomes, three patients had complications, and there was no mortality. Postoperative angiography showed that 10 out of 11 patients had good patency of anastomotic site and peripheral flow. The remaining one patient had post-surgical neurologic deficits resulting from occlusion of the bypass graft. Perfusion computed tomography (CT) showed that six of nine patients had good patency of distal blood flow, whereas three patients showed insufficiency. All 3 patients with insufficient flow on perfusion CT suffered strokes, resulting from occlusion of the bypass graft in one patient and from insufficient bypass blood flow in two patients, presented with hemiparesis with or without aphasia.
Illustrative cases
Case 1
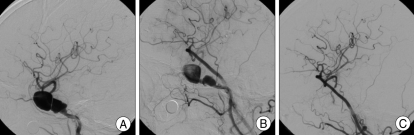
This 61-year-old woman had a history of headache and ocular pain. Magnetic resonance imaging and magnetic resonance angiography revealed a mesial temporal lobe mass and a bony erosion, and four-vessel angiography demonstrated a bilobular giant cavernous aneurysm.
Follow-up angiography after a right STA-MCA bypass with radial artery interposition graft showed good patency. Six days after surgery, a right ICA occlusion was performed with a balloon. External carotid angiography demonstrated excellent filling of the distal MCA branches through the bypass graft. Three years later, the patient reported resolution of her symptoms. CT angiography confirmed obliteration of the aneurysm and good patency of the bypass graft (Fig. 1).
Fig. 1.
Patient 1. (A) Cerebral angiograms showing a lobulating giant cavernous aneurysm before treatment, after EC-IC bypass with a radial artery graft (B), and after ICA occlusion with a balloon (C). Peripheral middle cerebral artery flow is observed to fill via the bypass graft, together with complete exclusion of the aneurysm from the circulation.
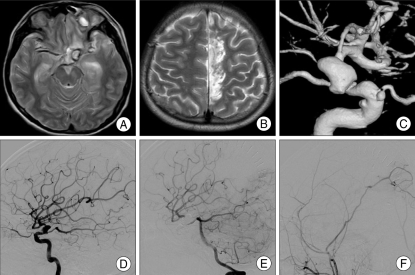
Case 14
This 14-year-ld boy had a left facial bone injury and left eye blindness resulting from a traffic accident. Brain MRI showed a small mass-like lesion with hemorrhage at the left frontal base. Three months after the head injury, he complained about right leg weakness (Fig. 2). An MRI showed a new infarction of the left distal ACA territory. CTA and transfemoral cerebral angiography (TFCA) showed a traumatic aneurysm of the left ICA dorsal wall, multiple aneurysms of the left ophthalmic artery, and cavernous ICA arising from multiple vessel wall injury.
Fig. 2.
Patient 14. Magnetic resonance (MRI) showing a small mass lesion with hemorrhage at the left frontal base (A). Three months after the injury, the patient complained of right leg weakness; MRI shows a new infarction of the left distal anterior cerebral artery territory (B). A 3D angiogram showing a traumatic pseudoaneurysm at the left ICA dorsal wall and multiple aneurysms at the left ophthalmic artery and cavernous in the internal cerebral artery (ICA) (C). Angiograms demonstrating dorsal wall aneurysm before surgery (D), after superficial temporal artery-middle cerebral artery (STA-MCA) bypass surgery (E), and after ICA occlusion with a coil (F). Distal MCA flow is observed to fill via the posterior communicating artery and anastomotic vessel.
Direct ICA occlusion was not recommended because of his relative young age and due to the perfusion defect revealed during a Diamox SPECT study. STA-M4 anastomosis was performed; seven days later, left ICA occlusion was performed with a coil. During ICA occlusion, the posterior communicating artery was saved since it was a major supplier for distal ICA flow.
The patient's postoperative outcome was good, and he was discharged without any new deficit.
DISCUSSION
The intracranial giant aneurysms (<2.5 cm) involving the ICA such as paraclinoid aneurysm, cavernous ICA aneurysm, and MCA aneurysm present several treatment difficulties. Direct surgical approach or endovascular techniques is incapable for these gaint aneurysms due to an artheroma or a calcification at the base of the aneurysm, retraction injury, and occlusion of the parent artery or adjacent perforators. The aneurysm size, the width of the neck, the outflow arteries incorporated into the aneurysm base, the blister-like aneurysm, the fusiform and dolichoectactic morphology of the sac, and the poor radiological visualization of the aneurysm and its adjacent branches are the causes of failure for both the microsurgical and endovascular treatments. Symptomatic traumatic internal carotid artery dissection or the traumatic damage of the intrapetrous ICA is impossible to be treated by direct surgical clipping or endovascular coiling. Complex aneurysms in our series included gaint ICA aneurysm, ICA dorsal wall aneurysm, large calcified aneurysm, pseudoaneurysm, serpentine thrombosed giant aneurysm, and dissecting aneurysm.
Without treatment, patients with complex aneurysms usually have poor prognosis1,4,8). Aggressive treatment is required, with the best treatment being the complete exclusion of the aneurysm from the circulation. Direct neck clipping is impossible, however, for lesions involving both the parent artery and its neighboring branches, as well as for ruptured dorsal wall aneurysms of the ICA and dissecting aneurysms. Although recent advances in endovascular treatments have increased their safety and efficacy, endovascular modalities alone are ineffective for these complex aneurysms. Rather, these complex lesions require parent artery occlusion with clip or coil with additive bypass surgery. These multimodal treatments have been found to significantly alter the natural course of these complex lesions2,3,6,9-14,18,19).
We found that multimodal treatment was both safe and effective in all fifteen patients with complex aneurysms. Multimodal treatment may be divided into four categories : 1) endovascular or surgical treatment after prior unsuccessful surgical or endovascular treatment, 2) endovascular treatment of a remote second aneurysm, 3) endovascular proximal parent artery control during surgery for clip occlusion/ decompression, and 4) combination of surgical and endovascular treatment for complex intracranial aneurysms18). All our patients fell within the last category. Our treatment modality was composed of booster bypass surgery and parent artery occlusion with clip or coil, and each procedure was performed by different neurosurgeons.
The first multimodal techniques used were within the third category and consisted of temporary ICA occlusion with a balloon during aneurysm dissection or during clip application with suction decompression3,5,7,16,17). Temporary balloon occlusion of the ICA before dissection of the unruptured aneurysm allowed proximal artery control without requiring neck dissection for proximal control of the ICA. This procedure, however, carries particular associated risks and is difficult to perform simultaneously during microsurgical clip application3,7,16). Cerebral revascularization, an adjunctive method used to treat complex aneurysms and cranial base tumors, has also been used to treat complex aneurysms, together with microsurgical clipping and endovascular coiling for parent artery occlusion3,9,11,14,18,19). Patients with giant intracranial aneurysms, in whom parent artery occlusion with clipping alone was associated with high complication rates, have also been treated by this method19).
Several trials have described the multimodal treatment of large numbers of patients,3,13-15) with the combination of endovascular and surgical methods for the initial treatment plan. These patients may be categorized into three subgroups. The first subgroup consists of patients with aneurysms involving the petrous, cavernous, or paraclinoid ICA aneurysms that were not amenable to direct surgical clip occlusion and that required a STA-MCA bypass or a saphenous vein interposition graft followed by parent-vessel occlusion. The second subgroup consists of patients with aneurysms in the posterior circulation requiring an STA-PCA or OA-PICA bypass followed by parent artery occlusion. The third subgroup is composed of patients with complex aneurysms that cannot be eliminated from the circulation and that require flow-redirection techniques.
In our cases, the most common aneurysms requiring a combined technique included ruptured dorsal wall aneurysms of the ICA and giant or large cavernous ICA aneurysms. All MCA and PCA aneurysms were symptomatic giant aneurysms. One ruptured PICA dissecting aneurysm was treated by OA-to-PICA anastomosis with simultaneous parent artery occlusion with clipping.
Dorsal wall aneurysms of the ICA with subarachnoid hemorrhages were difficult to be dissected and almost impossible to be clipped. In these patients, bypass surgery was performed first, followed by parent artery occlusion with a clip or coil. This methodology was also used in patients with giant aneurysms of the PCA and giant serpentine aneurysms of the MCA in our series.
BTOs were used to evaluate the necessity for bypass surgery in four patients with ICA aneurysms. Three of these patients showed normal clinical status and good cross-flow, but decreased perfusion reservoir by Diamox single positron emission computed tomography (SPECT).
Among four patients who underwent booster bypass surgery followed by parent artery occlusion in the same day, two patients developed complications, suggesting that parent artery occlusion should be performed after several days of primary bypass surgery, even in patients with subarachnoid hemorrhages.
CONCLUSION
Complex aneurysms in our study, those that were difficult to be treated simply by direct neck clipping or endovascular coiling, included giant aneurysms, ICA dorsal wall aneurysms, dissecting aneurysm, pseudoaneurysm, serpentine thrombosed aneurysm, and large calcified aneurysm. Combination of bypass surgery plus parent artery occlusion with a clip or coil may be recommended for these complex aneurysms.
Although these multimodal treatments are associated with complications, the high-risk nature of these complex aneurysms mandates an aggressive approach. The risks of multimodal treatment methods are likely lower than those associated with the natural progression of this subset of aneurysms.
References
- 1.Anson JA. Epidemiology and natural history of giant aneurysms. In: Awad IA, Barrow DL, editors. Giant Intracranial Aneurysms. Park Ridge: AANS; 1995. pp. 23–34. [Google Scholar]
- 2.Arnautovic KI, Al-Mefty O, Angtuaco E. A combined microsurgical skull-base and endovascular approach to giant and large paraclinoid aneurysms. Surg Neurol. 1998;50:504–518. doi: 10.1016/s0090-3019(97)80415-6. discussion 518-520. [DOI] [PubMed] [Google Scholar]
- 3.Barnett DW, Barrow DL, Joseph GJ. Combined extracranialintracranial bypass and intraoperative balloon occlusion for the treatment of intracavernous and proximal carotid artery aneurysms. Neurosurgery. 1994;35:92–97. doi: 10.1227/00006123-199407000-00014. discussion 97-98. [DOI] [PubMed] [Google Scholar]
- 4.Barrow DL, Alleyne C. Natural history of giant intracranial aneurysms and indications for intervention. Clin Neurosurg. 1995;42:214–244. [PubMed] [Google Scholar]
- 5.Batjer HH, Samson DS. Retrograde suction decompression of giant paraclinoidal aneurysms. Technical note. J Neurosurg. 1990;73:305–306. doi: 10.3171/jns.1990.73.2.0305. [DOI] [PubMed] [Google Scholar]
- 6.Drake CG. Giant intracranial aneurysms: experience with surgical treatment in 174 patients. Clin Neurosurg. 1979;26:12–95. doi: 10.1093/neurosurgery/26.cn_suppl_1.12. [DOI] [PubMed] [Google Scholar]
- 7.Eckard DA, Purdy PD, Bonte FJ. Temporary balloon occlusion of the carotid artery combined with brain blood flow imaging as a test to predict tolerance prior to permanent carotid sacrifice. AJNR Am J Neuroradiol. 1992;13:1565–1569. [PMC free article] [PubMed] [Google Scholar]
- 8.Ferguson GG, Peerless SJ, Drake CG. Natural history of intracranial aneurysms. N Engl J Med. 1981;305:99. doi: 10.1056/NEJM198107093050211. [DOI] [PubMed] [Google Scholar]
- 9.Gelber BR, Sundt TM., Jr Treatment of intracavernous and giant carotid aneurysms by combined internal carotid ligation and extra- to intracranial bypass. J Neurosurg. 1980;52:1–10. doi: 10.3171/jns.1980.52.1.0001. [DOI] [PubMed] [Google Scholar]
- 10.Hacein-Bey L, Connolly ES, Jr, Mayer SA, Young WL, Pile-Spellman J, Solomon RA. Complex intracranial aneurysms : combined operative and endovascular approaches. Neurosurgery. 1998;43:1304–1312. doi: 10.1097/00006123-199812000-00020. discussion 1312-1313. [DOI] [PubMed] [Google Scholar]
- 11.Heros RC, Ameri AM. Rupture of a giant basilar aneurysm after saphenous vein interposition graft to the posterior cerebral artery. Case report. J Neurosurg. 1984;61:387–390. doi: 10.3171/jns.1984.61.2.0387. [DOI] [PubMed] [Google Scholar]
- 12.Heros RC, Nelson PB, Ojemann RG, Crowell RM, DeBrun G. Large and giant paraclinoid aneurysms : surgical techniques, complications, and results. Neurosurgery. 1983;12:153–163. doi: 10.1227/00006123-198302000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Hoh BL, Carter BS, Budzik RF, Putman CM, Ogilvy CS. Results after surgical and endovascular treatment of paraclinoid aneurysms by a combined neurovascular team. Neurosurgery. 2001;48:78–89. doi: 10.1097/00006123-200101000-00014. discussion 87-90. [DOI] [PubMed] [Google Scholar]
- 14.Hoh BL, Putman CM, Budzik RF, Carter BS, Ogilvy CS. Combined surgical and endovascular techniques of flow alteration to treat fusiform and complex wide-necked intracranial aneurysms that are unsuitable for clipping or coil embolization. J Neurosurg. 2001;95:24–35. doi: 10.3171/jns.2001.95.1.0024. [DOI] [PubMed] [Google Scholar]
- 15.Jafar JJ, Russell SM, Woo HH. Treatment of giant intracranial aneurysms with saphenous vein extracranial-to-intracranial bypass grafting : indications, operative technique, and results in 29 patients. Neurosurgery. 2002;51:138–144. doi: 10.1097/00006123-200207000-00021. discussion 144-146. [DOI] [PubMed] [Google Scholar]
- 16.Mathis JM, Barr JD, Jungreis CA, Yonas H, Sekhar LN, Vincent D, et al. Temporary balloon test occlusion of the internal carotid artery : experience in 500 cases. AJNR Am J Neuroradiol. 1995;16:749–754. [PMC free article] [PubMed] [Google Scholar]
- 17.Mizoi K, Takahashi A, Yoshimoto T, Fujiwara S, Koshu K. Combined endovascular and neurosurgical approach for paraclinoid internal carotid artery aneurysms. Neurosurgery. 1993;33:986–992. doi: 10.1227/00006123-199312000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Ponce FA, Albuquerque FC, McDougall CG, Han PP, Zabramski JM, Spetzler RF. Combined endovascular and microsurgical management of giant and complex unruptured aneurysms. Neurosurg Focus. 2004;17:E11. doi: 10.3171/foc.2004.17.5.11. [DOI] [PubMed] [Google Scholar]
- 19.Serbinenko FA, Filatov JM, Spallone A, Tchurilov MV, Lazarev VA. Management of giant intracranial ICA aneurysms with combined extracranial-intracranial anastomosis and endovascular occlusion. J Neurosurg. 1990;73:57–63. doi: 10.3171/jns.1990.73.1.0057. [DOI] [PubMed] [Google Scholar]