Abstract
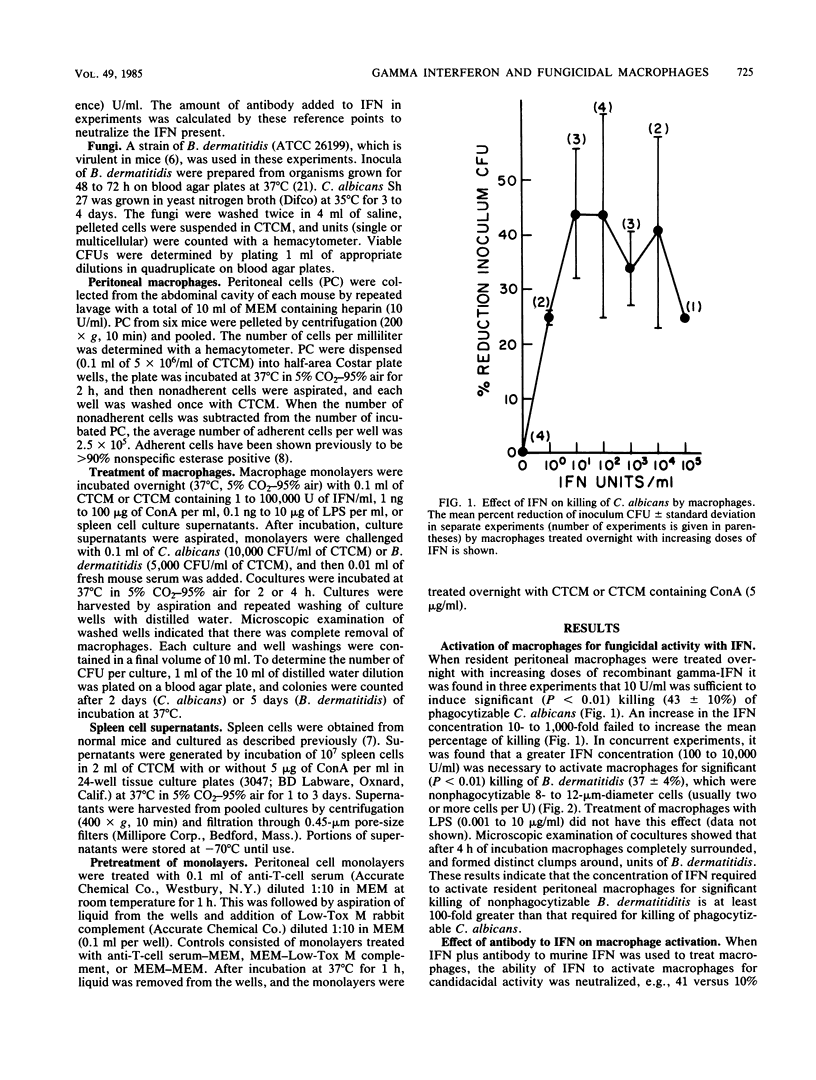
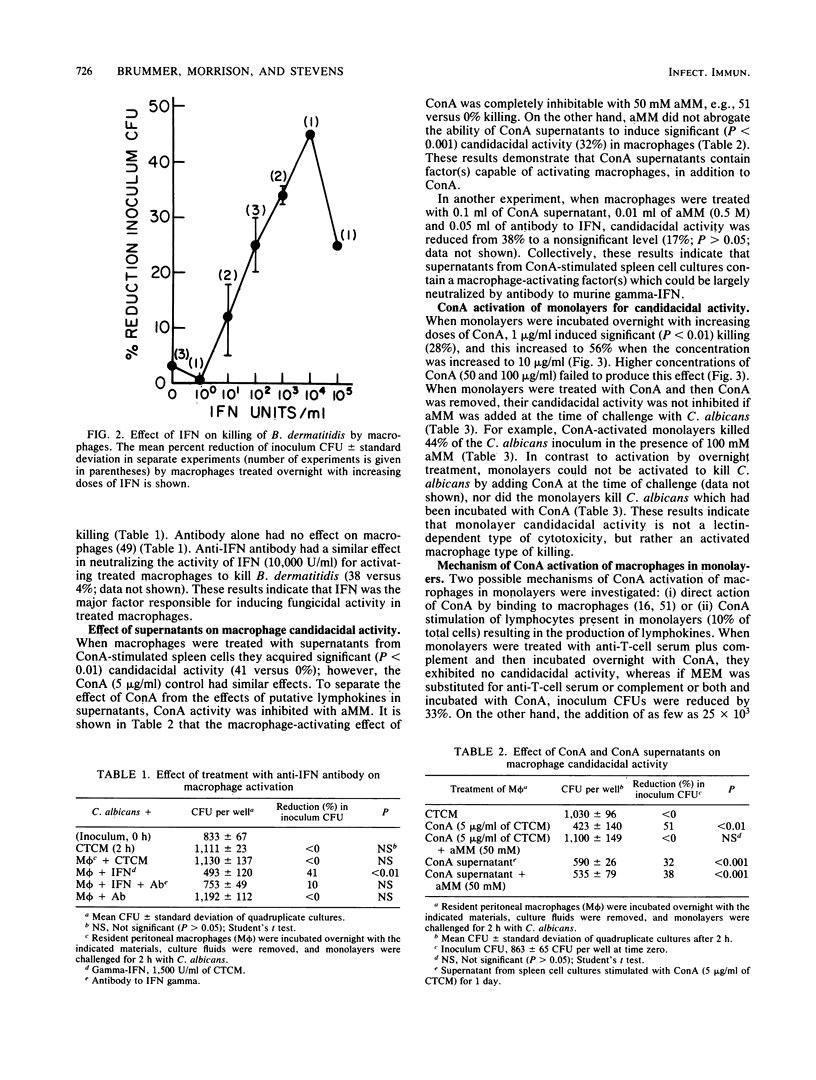
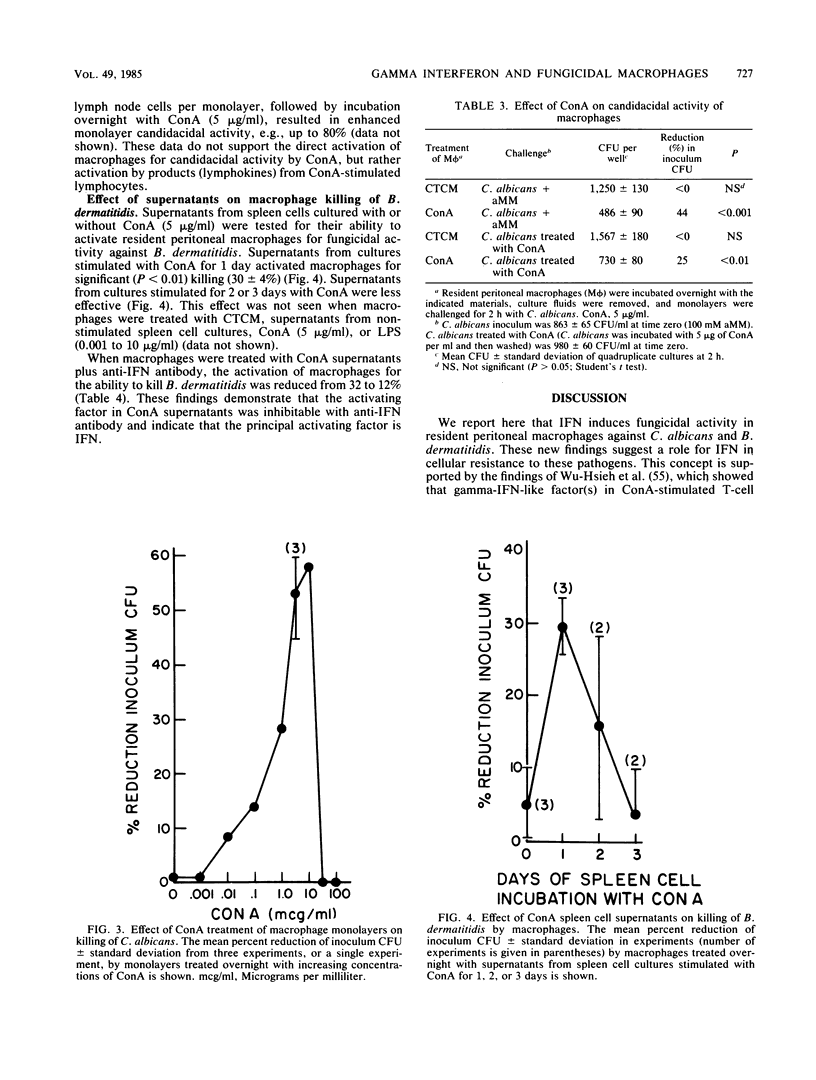
Recombinant murine gamma-interferon (IFN) and supernatants from concanavalin A (ConA)-stimulated spleen cells were tested for their ability to activate resident peritoneal macrophages (M phi) for fungicidal activity. M phi monolayers pulsed overnight with IFN exhibited significantly enhanced fungicidal activity against Candida albicans (44 +/- 12 versus 0.0%) and Blastomyces dermatitidis (34 +/- 1 versus 3 +/- 3%). The effect of IFN was dose dependent; however, less IFN (10 U/ml) was required to activate M phi to kill phagocytizable C. albicans than to kill nonphagocytizable B. dermatitidis (1,000 U/ml). ConA-stimulated spleen cell supernatants were also able to activate M phi for fungicidal activity against both fungi. The capacity of ConA-stimulated spleen cell supernatants to activate M phi for fungicidal activity was neutralized in the presence of antibody to murine IFN. ConA-treated monolayers acquired the ability to kill C. albicans, but not B. dermatitidis, which was shown to be associated with residual (10%) lymphocytes in the monolayers. Lipopolysaccharide (0.001 to 10 micrograms/ml) failed to consistently activate M phi for fungicidal activity. These data show that IFN can exert an immunoregulatory role on M phi defense against these fungal pathogens.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P., Yip Y. K., Vilcek J. Human interferon-gamma is internalized and degraded by cultured fibroblasts. J Biol Chem. 1983 May 25;258(10):6497–6502. [PubMed] [Google Scholar]
- Anderson S. E., Bautista S., Remington J. S. Induction of resistance to Toxoplasma gondii in human macrophages by soluble lymphocyte products. J Immunol. 1976 Aug;117(2):381–387. [PubMed] [Google Scholar]
- Beaman L., Benjamini E., Pappagianis D. Activation of macrophages by lymphokines: enhancement of phagosome-lysosome fusion and killing of Coccidioides immitis. Infect Immun. 1983 Mar;39(3):1201–1207. doi: 10.1128/iai.39.3.1201-1207.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanden R. V., Mackaness G. B., Collins F. M. Mechanisms of acquired resistance in mouse typhoid. J Exp Med. 1966 Oct 1;124(4):585–600. doi: 10.1084/jem.124.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bout D. T., Joseph M., David J. R., Capron A. R. In vitro killing of S. mansoni schistosomula by lymphokine-activated mouse macrophages. J Immunol. 1981 Jul;127(1):1–5. [PubMed] [Google Scholar]
- Brass C., Volkmann C. M., Klein H. P., Halde C. J., Archibald R. W., Stevens D. A. Pathogen factors and host factors in murine pulmonary blastomycosis. Mycopathologia. 1982 Jun 18;78(3):129–140. doi: 10.1007/BF00466066. [DOI] [PubMed] [Google Scholar]
- Brummer E., Stevens D. A. Activation of murine polymorphonuclear neutrophils for fungicidal activity with supernatants from antigen-stimulated immune spleen cell cultures. Infect Immun. 1984 Aug;45(2):447–452. doi: 10.1128/iai.45.2.447-452.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummer E., Sugar A. M., Stevens D. A. Activation of peritoneal macrophages by concanavalin A or Mycobacterium bovis BCG for fungicidal activity against Blastomyces dermatitidis and effect of specific antibody and complement. Infect Immun. 1983 Feb;39(2):817–822. doi: 10.1128/iai.39.2.817-822.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummer E., Sugar A. M., Stevens D. A. Immunological activation of polymorphonuclear neutrophils for fungal killing: studies with murine cells and blastomyces dermatitidis in vitro. J Leukoc Biol. 1984 Oct;36(4):505–520. doi: 10.1002/jlb.36.4.505. [DOI] [PubMed] [Google Scholar]
- Byrne G. I., Faubion C. L. Lymphokine-mediated microbistatic mechanisms restrict Chlamydia psittaci growth in macrophages. J Immunol. 1982 Jan;128(1):469–474. [PubMed] [Google Scholar]
- Cameron D. J., Churchill W. H. Cytotoxicity of human macrophages for tumor cells: enhancement by bacterial lipopolysaccharides (LPS). J Immunol. 1980 Feb;124(2):708–712. [PubMed] [Google Scholar]
- Campbell P. A., Czuprynski C. J., Cook J. L. Differential expression of macrophage effector functions: bactericidal versus tumoricidal activities. J Leukoc Biol. 1984 Sep;36(3):293–306. doi: 10.1002/jlb.36.3.293. [DOI] [PubMed] [Google Scholar]
- Celada A., Gray P. W., Rinderknecht E., Schreiber R. D. Evidence for a gamma-interferon receptor that regulates macrophage tumoricidal activity. J Exp Med. 1984 Jul 1;160(1):55–74. doi: 10.1084/jem.160.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole P. Activation of mouse peritoneal cells to kill Listeria monocytogenes by T-lymphocyte products. Infect Immun. 1975 Jul;12(1):36–41. doi: 10.1128/iai.12.1.36-41.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doe W. F., Henson P. M. Macrophage stimulation by bacterial lipopolysaccharides. I. Cytolytic effect on tumor target cells. J Exp Med. 1978 Aug 1;148(2):544–556. doi: 10.1084/jem.148.2.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelson P. J., Cohn Z. A. Effects of concanavalin A on mouse peritoneal macrophages. I. Stimulation of endocytic activity and inhibition of phago-lysosome formation. J Exp Med. 1974 Nov 1;140(5):1364–1386. doi: 10.1084/jem.140.5.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson K. L., Cicurel L., Gruys E., Fidler I. J. Murine T-cell hybridomas that produce lymphokine with macrophage-activating factor activity as a constitutive product. Cell Immunol. 1982 Sep 1;72(1):195–201. doi: 10.1016/0008-8749(82)90297-0. [DOI] [PubMed] [Google Scholar]
- Fowles R. E., Fajardo I. M., Leibowitch J. L., David J. R. The enhancement of macrophage bacteriostasis by products of activated lymphocytes. J Exp Med. 1973 Oct 1;138(4):952–964. doi: 10.1084/jem.138.4.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemsa D., Debatin K. M., Kramer W., Kubelka C., Deimann W., Kees U., Krammer P. H. Macrophage-activating factors from different T cell clones induce distinct macrophage functions. J Immunol. 1983 Aug;131(2):833–844. [PubMed] [Google Scholar]
- Haidaris C. G., Bonventre P. F. A role for oxygen-dependent mechanisms in killing of Leishmania donovani tissue forms by activated macrophages. J Immunol. 1982 Aug;129(2):850–855. [PubMed] [Google Scholar]
- Harvey R. P., Schmid E. S., Carrington C. C., Stevens D. A. Mouse model of pulmonary blastomycosis: utility, simplicity, and quantitative parameters. Am Rev Respir Dis. 1978 Apr;117(4):695–703. doi: 10.1164/arrd.1978.117.4.695. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Granger D. L., Cook J. L., Lewis A. M., Jr Activated macrophage mediated cytotoxicity for transformed target cells. Adv Exp Med Biol. 1982;146:315–335. doi: 10.1007/978-1-4684-8959-0_17. [DOI] [PubMed] [Google Scholar]
- Horwitz M. A., Silverstein S. C. Activated human monocytes inhibit the intracellular multiplication of Legionnaires' disease bacteria. J Exp Med. 1981 Nov 1;154(5):1618–1635. doi: 10.1084/jem.154.5.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard D. H., Otto V. Experiments on lymphocyte-mediated cellular immunity in murine histoplasmosis. Infect Immun. 1977 Apr;16(1):226–231. doi: 10.1128/iai.16.1.226-231.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., Mastroianni R. P. Inhibition of phagocytosis by cryptococcal polysaccharide: dissociation of the attachment and ingestion phases of phagocytosis. Infect Immun. 1976 Jul;14(1):62–67. doi: 10.1128/iai.14.1.62-67.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le J., Prensky W., Yip Y. K., Chang Z., Hoffman T., Stevenson H. C., Balazs I., Sadlik J. R., Vilcek J. Activation of human monocyte cytotoxicity by natural and recombinant immune interferon. J Immunol. 1983 Dec;131(6):2821–2826. [PubMed] [Google Scholar]
- Le J., Vilcek J. Lymphokine-mediated activation of human monocytes: neutralization by monoclonal antibody to interferon-gamma. Cell Immunol. 1984 Apr 15;85(1):278–283. doi: 10.1016/0008-8749(84)90299-5. [DOI] [PubMed] [Google Scholar]
- Leonard E. J., Ruco L. P., Meltzer M. S. Characterization of macrophage activation factor, a lymphokine that causes macrophages to become cytotoxic for tumor cells. Cell Immunol. 1978 Dec;41(2):347–357. doi: 10.1016/0008-8749(78)90232-0. [DOI] [PubMed] [Google Scholar]
- MACKANESS G. B. Cellular resistance to infection. J Exp Med. 1962 Sep 1;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer M. S., Benjamin W. R., Farrar J. J. Macrophage activation for tumor cytotoxicity: induction of macrophage tumoricidal activity by lymphokines from EL-4, a continuous T cell line. J Immunol. 1982 Dec;129(6):2802–2807. [PubMed] [Google Scholar]
- Meltzer M. S., Ruco L. P., Boraschi D., Nacy C. A. Macrophage activation for tumor cytotoxicity: analysis of intermediary reactions. J Reticuloendothel Soc. 1979 Oct;26(4):403–415. [PubMed] [Google Scholar]
- Murray H. W., Cartelli D. M. Killing of intracellular Leishmania donovani by human mononuclear phagocytes. Evidence for oxygen-dependent and -independent leishmanicidal activity. J Clin Invest. 1983 Jul;72(1):32–44. doi: 10.1172/JCI110972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Männel D. N., Falk W. Interferon-gamma is required in activation of macrophages for tumor cytotoxicity. Cell Immunol. 1983 Jul 15;79(2):396–402. doi: 10.1016/0008-8749(83)90082-5. [DOI] [PubMed] [Google Scholar]
- Nacy C. A., James S. L., Benjamin W. R., Farrar J. J., Hockmeyer W. T., Meltzer M. S. Activation of macrophages for microbicidal and tumoricidal effector functions by soluble factors from EL-4, a continuous T cell line. Infect Immun. 1983 May;40(2):820–824. doi: 10.1128/iai.40.2.820-824.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacy C. A., Leonard E. J., Meltzer M. S. Macrophages in resistance to rickettsial infections: characterization of lymphokines that induce rickettsiacidal activity in macrophages. J Immunol. 1981 Jan;126(1):204–207. [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983 Sep 1;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Prendergast T. J., Wiebe M. E., Stanley E. R., Platzer E., Remold H. G., Welte K., Rubin B. Y., Murray H. W. Activation of human macrophages. Comparison of other cytokines with interferon-gamma. J Exp Med. 1984 Aug 1;160(2):600–605. doi: 10.1084/jem.160.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira N., Chaplan S., Reesink M., Tydings J., Cohn Z. A. Trypanosoma cruzi: induction of microbicidal activity in human mononuclear phagocytes. J Immunol. 1982 May;128(5):2142–2146. [PubMed] [Google Scholar]
- Pace J. L., Russell S. W., Torres B. A., Johnson H. M., Gray P. W. Recombinant mouse gamma interferon induces the priming step in macrophage activation for tumor cell killing. J Immunol. 1983 May;130(5):2011–2013. [PubMed] [Google Scholar]
- Pedersen J. K., Bennedsen J., Rhodes J. M., Larsen S. O. In vitro effect of mitogen-activated speen cells and their culture supernatants on mouse peritoneal macrophages as assayed by intracellular killing of Listeria monocytogenes and content of beta-galactosidase. J Reticuloendothel Soc. 1979 May;25(5):469–477. [PubMed] [Google Scholar]
- Ratliff T. L., Thomasson D. L., McCool R. E., Catalona W. J. T-cell hybridoma production of macrophage activation factor (MAF) I. Separation of MAF from interferon gamma. J Reticuloendothel Soc. 1982 May;31(5):393–397. [PubMed] [Google Scholar]
- Roberts W. K., Vasil A. Evidence for the identity of murine gamma interferon and macrophage activating factor. J Interferon Res. 1982;2(4):519–532. doi: 10.1089/jir.1982.2.519. [DOI] [PubMed] [Google Scholar]
- Ruskin J., Rengton J. S. Role for the macrophage in acquired immunity to phylogenetically unrelated intracellular organisms. Antimicrob Agents Chemother (Bethesda) 1968;8:474–477. doi: 10.1128/AAC.8.4.474. [DOI] [PubMed] [Google Scholar]
- Salahuddin S. Z., Markham P. D., Lindner S. G., Gootenberg J., Popovic M., Hemmi H., Sarin P. S., Gallo R. C. Lymphokine production by cultured human T cells transformed by human T-cell leukemia-lymphoma virus-I. Science. 1984 Feb 17;223(4637):703–707. doi: 10.1126/science.6320367. [DOI] [PubMed] [Google Scholar]
- Sasada M., Johnston R. B., Jr Macrophage microbicidal activity. Correlation between phagocytosis-associated oxidative metabolism and the killing of Candida by macrophages. J Exp Med. 1980 Jul 1;152(1):85–98. doi: 10.1084/jem.152.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber R. D., Altman A., Katz D. H. Identification of a T cell hybridoma that produces large quantities of macrophage-activating factor. J Exp Med. 1982 Sep 1;156(3):677–689. doi: 10.1084/jem.156.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber R. D., Pace J. L., Russell S. W., Altman A., Katz D. H. Macrophage-activating factor produced by a T cell hybridoma: physiochemical and biosynthetic resemblance to gamma-interferon. J Immunol. 1983 Aug;131(2):826–832. [PubMed] [Google Scholar]
- Sone S., Poste G., Fidler I. J. Rat alveolar macrophages are susceptible to activation by free and liposome-encapsulated lymphokines. J Immunol. 1980 May;124(5):2197–2202. [PubMed] [Google Scholar]
- Svedersky L. P., Benton C. V., Berger W. H., Rinderknecht E., Harkins R. N., Palladino M. A. Biological and antigenic similarities of murine interferon-gamma and macrophage-activating factor. J Exp Med. 1984 Mar 1;159(3):812–827. doi: 10.1084/jem.159.3.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taramelli D., Varesio L. Activation of murine macrophages. I. Different pattern of activation by poly I:C than by lymphokine or LPS. J Immunol. 1981 Jul;127(1):58–63. [PubMed] [Google Scholar]
- Toh K., Sato N., Kikuchi K. Effect of concanavalin A on the cytotoxicity of rat peritoneal macrophages. J Reticuloendothel Soc. 1979 Jan;25(1):17–28. [PubMed] [Google Scholar]
- Toh K., Yamamoto N., Suzuki T., Kikuchi K. Effect of concanavalin A and lipopolysaccharide on the cytotoxicity of macrophages and neutrophils. J Reticuloendothel Soc. 1979 Sep;26(3):273–282. [PubMed] [Google Scholar]
- Wing E. J., Gardner I. D., Ryning F. W., Remington J. S. Dissociation of effector functions in populations of activated macrophages. Nature. 1977 Aug 18;268(5621):642–644. doi: 10.1038/268642a0. [DOI] [PubMed] [Google Scholar]
- Wu-Hsieh B., Zlotnik A., Howard D. H. T-cell hybridoma-produced lymphokine that activates macrophages to suppress intracellular growth of Histoplasma capsulatum. Infect Immun. 1984 Jan;43(1):380–385. doi: 10.1128/iai.43.1.380-385.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S., Leonard E. J., Meltzer M. Molecular weight, isoelectric point, and stability of a murine lymphokine that induces macrophage tumoricidal activity. J Reticuloendothel Soc. 1983 May;33(5):343–351. [PubMed] [Google Scholar]


