Abstract
Objective
The purpose of this study is to verify the usefulness of the rabbit model for disc degeneration study.
Materials
The L1-L2, L2-L3, L3-L4, or L4-L5 lumbar intervertebral disc (IVD) of 9 mature male New Zealand White rabbits were injured by inserting a 16-gauge needle to a depth of 5 mm in the left anterolateral annulus fibrosus while leaving L5-L6 IVD uninjured. Three other rabbits also received intradiscal injections of rabbit disc cells transfected with adenovirus and bone morphogenetic protein-2 (ad-BMP-2) at L4-L5 in addition to injury by 16-gauge needle at the L1-L2 level. Using digitized radiographs, measurements of IVD height were made and analyzed by using the disc height index (DHI). Magnetic resonance imaging (MRI) scans of the injured discs, injected discs, and uninjured L5-L6 discs were performed at 15 weeks post surgery and compared with preoperative MRI scans.
Results
All twelve rabbits showed consistent results of disc degeneration within 15 weeks following annular puncture. DHIs of injured discs were significantly lower than that of the uninjured L5-L6 discs (p<0.05). The mean value of disc degeneration grade of injured discs was significantly higher than that of uninjured discs (p<0.05). The injection of disc cell transfected with ad-BMP-2 did not induce disc regeneration at 15 weeks after injection.
Conclusion
This study showed that the injured disc had a significant change in DHI on simple lateral radiograph and disc degeneration grade on MRI scans within 15 weeks in all rabbits. Rabbit annular puncture model can be useful as a disc degeneration model in vivo.
Keywords: Intervertebral disc, Animal model, Rabbit, Disc degeneration, in vivo
INTRODUCTION
Back pain is one of the major causes of disability and has a major socioeconomic impact. The lifetime prevalence of low back pain is 75% to 80% in the population of the United States of America3). Intervertebral disc degeneration is a major contributor to back pain1,3,4,6,8,10,14,19,20,23,24,27), but the precise pathogenesis and pathophysiology of disc degeneration is not known. The direct relationship between disc degeneration and pain generation still needs to be studied.
The current treatment options for back pain range from conservative measures to surgical discectomy with fusion8,14,15,24). However, discectomy due to lumbar disc herniation may cause further degeneration that eventually leads to instability of the motion segment making further operative management necessary to stabilize those segments. The progression of disc degeneration cannot be prevented by these minimally invasive operative procedures3). Arthroplasty with the use of artificial discs is another treatment option for symptoms caused by disc degeneration15). Recently, advancements in molecular biology and tissue engineering have made it possible to contemplate directly treating the intervertebral disc itself to alter the course of disc degeneration1,4,6,15,23).
To study disc degeneration or disc regeneration, establishment of an in vivo animal model is essential6,14,15,18,23,24). Although recent publications have proven the feasibility of the rabbit disc degeneration model, the efficacy and usefulness of this model has not yet been studied. The purpose of this study is to verify the effectiveness of the rabbit model to examine disc degeneration and the effectiveness of various growth factors in promoting disc regeneration.
MATERIALS AND METHODS
Study design with rabbit model
Thirteen 5 month-old adult male New Zealand white rabbits, weighing approximately 3-3.5 kg were used in this study. One rabbit died due to sepsis following surgery and was excluded from this study (Table 1). This study was approved by the Institutional Review Board of University of California, Los Angeles.
Table 1.
The summary of rabbits intervertebral discs used in this study
Disc Cell/ad-BMP-2 indicates disc cells transfected with ad-BMP-2. BMP : bone morphogenetic protein
In each of 9 rabbits, one of the discs at L1-L2, L2-L3, L3-L4, or L4-L5 lumbar IVD was chosen at random to be punctured by a 16-gauge needle to a depth of 5 mm in the left anterolateral annulus fibrosus; the punctured disc in each rabbit is considered part of the injured group. The L5-L6 discs served as an uninjured control group in each of the 9 rabbits, as proposed Masuda et al14).
In another 3 rabbits the L1-L2 IVD was punctured using the same method described above (injured group), L5-L6 IVD was left uninjured (uninjured group), and in addition, the L4-L5 IVD was injected with disc cells transfected by Adenovirus BMP2-gene (ad-BMP-2). The disc cells (40 µL of 1×105 cells) were taken from other New Zealand white rabbits.
Ad-BMP-2 gene transfection procedure
Rabbit disc cells were isolated and cultured from other rabbits in vitro in the presence of adenoviral vector and recombinant human bone morphogenetic protein-2 (rhBMP-2) (INFUSE Bone Graft; Medtronic Sofamor Danek, Memphis, Tennessee) and transfected with BMP-2 gene via transduction.
The first step was disc cell isolation and culture. Tissue specimens were placed in a sterile saline solution and transferred to the laboratory for isolation. Tissue was washed three times with Hank's balanced salt solution (HBSS) (GibcoBRL, Carlsbad, CA) with 1% penicillin/streptomycin (P/S) (Bio-Whittaker, Walkersville, MD) to remove any blood or other bodily contaminants before isolation of nucleus pulposus cells. Any obvious dense annulus tissue was carefully removed. The specimens were minced with scissors and digested for 90 minutes at 37℃ under gentle agitation in 20 mL of sterile Ham's F-12 medium (GibcoBRL, Grand Island, NY) containing 1% P/S, 5% heatinactivated fetal bovine serum (FBS) (GibcoBRL, Grand Island, NY) and 0.2% pronase (Calbiochem, La Jolla, CA). The specimens were then rinsed three times with HBSS with 1% P/S and incubated overnight in a solution of F-12 medium containing 1% P/S, 5% FBS and 0.01% collagenase type II (Sigma, St. Louis, MO). A sterile nylon mesh filter was used to separate the suspended cells from the remaining tissue debris. Isolated cells were then centrifuged at 2,000 RPM for 5 minutes. The cells were re-suspended in a culture medium consisting of F-12 medium with 10% FBS and 1% P/S. The isolated cells were placed in a tissue culture flask (VWR Scientific Products, Bridgeport, NJ) and incubated at 37℃ in a humidified atmosphere of 5% carbon dioxide. The culture medium was changed twice each week and cultures were allowed to grow to full confluence, requiring an average incubation period of 2 weeks.
The second step was transfecting the cells with BMP-2 gene using the method reported by Wallach et al27). Cultured disc cells were plated as a monolayer 48 hours before transduction in tissue culture flasks with 1×106 cells. Solutions of virus particles equal to 50, 100, or 150 multiplicity of infection (MOI) were premixed with HBSS in 1 mL aliquots for the ad-BMP-2 viral vectors9,14). After washing the monolayer cells three times with HBSS to remove any remaining culture medium, the experimental viral solutions were added to the tissue culture flask. After 4 hours of incubation at 37℃, 3 mL of culture medium was added and the cells were allowed to recover over the ensuing 48 hours. The transduced cells were resuspended in culture medium. 1.5×105 cells were placed in individual 1.5 mL microfuge tubes with 1.0 mL culture medium and centrifuged at 2,000 RPM for 5 minutes to form pellet aggregates. This pellet culture system was based on the method proposed by Lee et al11). These pellets were incubated at 37℃ for 48 hours.
Rabbit annular puncture model as disc degeneration model in vivo
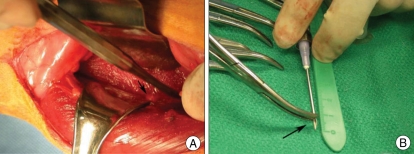
The rabbits were premedicated 30 minutes prior to surgery with subcutaneous injections of (0.3mg/mL) buprenorphine (Buprenex®; Reckitt Benckiser Healthcare, Hull, UK). The rabbits were anesthesized by intramuscular injection of ketamine/xlylocaine followed by isoflurane gas. After anesthesia, the hair over the surgical field was shaved. The rabbits were placed in a lateral decubitus position with a 20 degrees inclination. Aseptic technique was used for all surgical procedures. The surgical field was disinfected and draped. A posterolateral retroperitoneal approach was used to expose the IVD (Fig. 1A). A longitudinal skin incision was made from the inferior margin of the rib cage to the pelvic rim, 2 cm ventral to the paraspinal musculature. The left anterolateral vertebral column from L1-L5 was exposed by sharp and blunt dissection of the overlying subcutaneous tissue, retroperitoneal fat, and musculatures. Disc levels were identified using the pelvic rim as an anatomic landmark for the L5-L6 disc level. A titanium clip was placed on the top of superior endplate of L1 vertebra as a level marker. In 9 of the rabbits, One of the lumbar IVD from L1-L2 to L4-L5 was punctured by a 16-gauge needle to a depth of 5 mm in the left anterolateral annulus fibrosus14,24). The depth of penetration was controlled by a locking forceps clamped 5 mm from the needle tip (Fig. 1B). The L5-L6 discs were left uninjured in all rabbits to serve as a control. In other 3 rabbits, L1-L2 IVDs were injured using the same method described above, L5-L6 was left uninjured to serve as a control, and L4-L5 IVD was injected with disc cells transfected with ad-BMP-2 gene using a 27-gauge needle.
Fig. 1.
Photographs of rabbit during left retroperitoneal approach. The arrow indicates an intervertebral disc of the rabbit (A). Rabbit lumbar intervertebral discs were punctured by a 16-gauge needle to a depth of 5 mm (arrow) in the left anterolateral annulus fibrosus (B).
The surgical wound was irrigated with sterile saline after the procedures. The fascia was closed with absorbable sutures, while a non-absorbable monofilament suture material was used to close the skin incisions. All animals were monitored until they regained consciousness. The rabbits were allowed to eat and drink, while their healing status was monitored on a daily basis. All animals continued to receive pain medication with buprenorphine two times a day for at least two days. After two days, if the animals still showed any signs of pain or distress, buprenorphine was administered on an as-needed basis.
Radiographic analysis of disc height
Lateral plain radiographs obtained pre-operation and at each time point (3, 7, 11, and 15 weeks post-operation) were digitalized. Radiographs were taken after administration of ketamine hydrochloride (2.5 mg/kg). To obtain a similar degrees of muscle relaxation each time, which may affect the disc height, a consistent level of anesthesia was maintained during radiographic imaging of each animal and at each time. IVD heights were measured and expressed as the disc height index (DHI) using the method reported by Lu et al13). The average IVD height was calculated using measurements obtained from the anterior, middle, and posterior portions of the IVD and was divided by the average of adjacent vertebral body heights. Changes in the DHI were expressed as percent DHI and normalized to the measured pre-operative IVD height (percent DHI=post-operation DHI/pre-operation DHI×100).
MRI assessment
To monitor changes in IVD, MRI of the animals were also taken in this study using a 3.0-T Tim Trio scanner (Siemens Medical Solutions, Erlangen, Germany) and 8 channels knee coil at the same time points but on different days from the radiographs. The X-rays and MRIs were taken on different days, allowing the rabbits at least 72 hours to recover from each of the procedures before being sedated for a second time. T2-weighted sections in the sagittal plane were obtained in the following settings : fast spin echo sequence with TR (time to repetition) of 3,500 milliseconds and TE (time to echo) of 104 milliseconds; 320 (h)×320 (v) matrix; field of view of 120; and 3 averages. The section thickness was 2 mm with a 0.2 mm gap. The Pfirrmann's classification21) was used for disc degeneration grading from grade 1 to 5 (1=normal, 2=mild degeneration, 3=moderate degeneration, 4=severe degeneration, and 5=advanced degeneration).
Histological examination
The animals were euthanized at 15 weeks post-operation. After sacrifice, the spine specimens were fixed in 40% ethanol, decalcified using 10% standard decalcifying solution (CAL-Ex, Fischer Scientific, Fairlawn, NJ), washed with running tap water, and then transferred to 75% ethanol. The fixed, decalcified rabbit spines were cut carefully on serial mid-sagittal sections. They were embedded in wax and sectioned. The sections were stained with hematoxylin and eosin.
Statistical Analysis
Statistical analysis was performed using SPSS statistical software version 14.0 (SPSS INC., Chicago, IL). Radiographic changes of disc height index between injured and uninjured groups were compared using student t-test. For the disc degeneration grade, comparison between the injured, uninjured, and injected groups was conducted by ANOVA test. p<0.05 indicated a significant difference.
RESULTS
Disc degeneration compatibility at 15 weeks as animal model
Twelve rabbit discs were injured by needle puncture using 16-gauge needles and disc degeneration was induced within 15 weeks in all 12 discs (Fig. 2). The disc height also decreased significantly in all 12 rabbits (p<0.001).
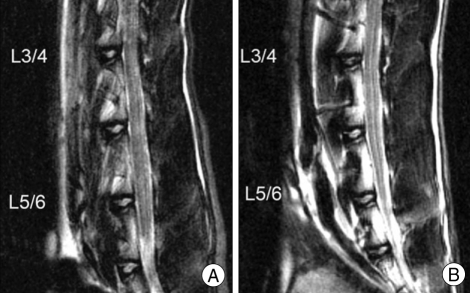
Fig. 2.
Magnetic resonance image (MRI) of rabbit at injured and uninjured discs. MRI examination was performed on all spinal columns from L1 to L6. T2-weighted MRI of the nucleus pulposus at the injured L3-L4 disc shows lower signal intensity at 15 weeks post-operation than both uninjured L5-L6 disc (B) and pre-operative L3-L4 disc (A) in the same rabbit.
Disc height index
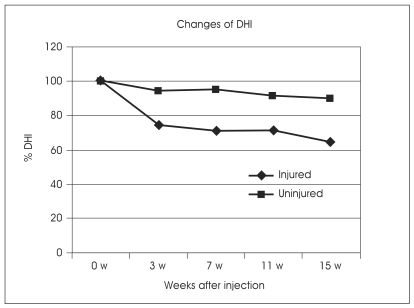
Lateral radiographs taken 15 weeks after initial annulus fibrosus puncture showed a significant narrowing of the injured disc compared to the uninjured control disc of the same animal (Fig. 3). The mean percent DHI in the injured group at 15 weeks post-operation was 72% of that of the uninjured group (p<0.001). In the uninjured discs, there were no significant differences in DHI and %DHI at 15 weeks post-operation. In the injured group, disc height decreased slowly throughout the 15 weeks and significant disc space narrowing was observed after 15 weeks (p<0.05) (Fig. 4).
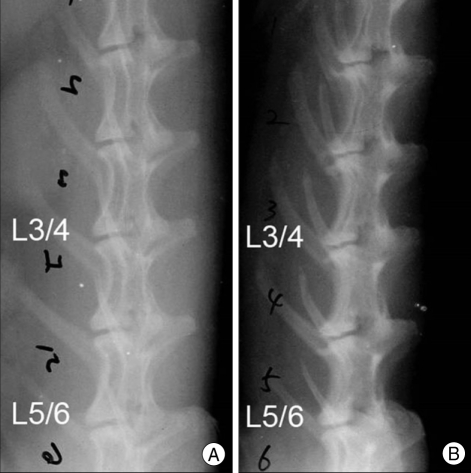
Fig. 3.
Lateral radiographs of a rabbit lumbar spine after annular puncture by a 16-gauge needle. Annulus of the L3-L4 intervertebral disc was punctured to induce disc degeneration. C Comparison of initial radiograph (A) and radiograph at 15 weeks after the initial annular puncture (B) shows a higher degree of disc space narrowing of the punctured L3-L4 disc compared to the uninjured L5-L6 disc.
Fig. 4.
Changes in the intervertebral disc height index (DHI) after the annular puncture. The percent DHI was measured at each time to quantify changes in disc height.
Disc degeneration grade of rabbits with MRI
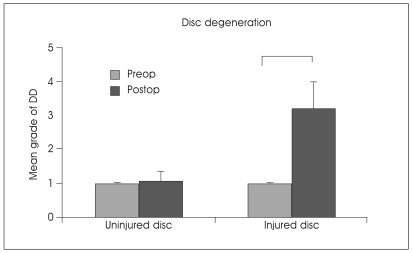
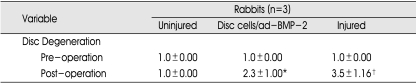
When the mean grade of disc degeneration on MRI was compared between the uninjured and injured groups, grades of disc degeneration in the injured group were significantly higher at 15 weeks post-operation than that of the uninjured group (p<0.001) (Fig. 5). The mean grade of disc degeneration with disc cell/ad-BMP-2 group was significantly higher compared to that of control uninjured group (Table 2). However, it was not significantly different from the injured group. The disc cell/ad-BMP-2 injection did not induce disc regeneration at 15 weeks after injection.
Fig. 5.
Change in disc degeneration grade 15 weeks after annular puncture. Pfirrmann's classification based on disc height and signal intensity from grade 1 to 5 was used to grade the disc degeneration grade of the rabbit discs. *p<0.05. DD : disc degeneration.
Table 2.
The changes of disc degeneration grade 15 weeks after disc cell/ad-BMP-2 injection
The values are mean±SD. *p<0.05, †p<0.01
Histological examination
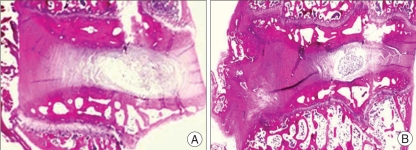
Histological examination (H & E staining) of injured discs showed disc space narrowing by 15 weeks (Fig. 6). Additionally, endochondral ossification of osteophytes was apparent at most of the injured intervertebral disc levels.
Fig. 6.
A histologic section (H & E staining) of healthy, intact rabbit lumbar disc (A) and discs 15 weeks after injury (B) by 16-gauge needle (original magnification X10). Disc space narrowing and endochondral ossification of osteophyte (arrow) was apparent in the disc 15 weeks after injury.
DISCUSSION
Numerous animal models for intervertebral disc degeneration have been proposed in the literature, each with its own advantages and disadvantages for the purposes of studying the pathogenesis and pathophysiology of disc degeneration and testing novel therapies2,6,9,14,15,17,19,23,24,27). Due to the invasiveness of the procedures and concerns involving the care and use of animals, a limited number of in vivo studies have been conducted with animal models such as bovine, murine, feline, or porcine models. Rat IVD is very small in size so measuring disc heights on simple X-ray or MRI is very difficult even though it is possible to measure the proteoglycan production, disc cell proliferation, and mRNA levels. To overcome these shortcomings, the rabbit model for disc degeneration was introduced.
Intervertebral disc degeneration models using rabbits were developed many years ago. Among them, the classic annular stab model was introduced by Lipson and Muir12). Disc degeneration was induced by making a full-thickness, ventral stab of the annulus fibrosus of rabbit lumbar discs with a number 11 scalpel blade. It appeared to produce certain biochemical and histological outcome measures that were similar to changes seen in human disc degeneration. However, it has some disadvantages. First, the classic stab model led to a rapid narrowing of disc space in the first 2 weeks with variable disc height narrowing, indicating acute degeneration14). Second, the immediate herniation of the nucleus pulposus following full-thickness stab limits the usefulness of this model4). More recently, the annulus puncture model was introduced by Masuda K et al14). It involves puncturing the annulus with a 16G to 21G needle with controlled depth (5 mm). Stabbing the anterolateral annulus fibrosus of adult rabbit lumbar discs with a 16-gauge hypothermic needle to a limited (5 mm) depth resulted in a number of slowly progressive and reproducible MRI, radiograph, and histological changes over 24 weeks that show a similarity to changes seen in human intervertebral disc disease. It also results in a slower decrease in disc height than the classic stab model13).
This current study was conducted with the rabbit annulus puncture model. In this study, injured discs had a significant decrease in disc height on simple X-ray within 15 weeks. Disc degeneration grade on MRI was also significantly increased after disc injury. These results demonstrate the usefulness of the rabbit annulus puncture model as a disc degeneration animal model.
During the surgery with this new model, caution is required. Masuda et al.14) reported that excessive irrigation and dissection of the prevertebral structures should be avoided because any damage or irrigation of the periosteum of the vertebral body would result in osteophyte formation. The puncture needle size to provide the most predictable, slow, progressive disc narrowing was 16G or 18G. The use of the 27-gauge needle, as reported in another study, did not cause any significant decrease in disc height, suggesting that a needle as large as 27-gauge should be used to introduce growth factors, plasmids, or other pharmacological reagents or cells while avoiding damage8,14). They also reported that the length of time with which the needle is maintained in position after puncturing is important14).
The authors believe that several other points should also be considered. When puncturing the disc, the trajectory is very important. The disc space in rabbits is small so a great deal of precision is required, and if the trajectory is not towards the center of the disc, the puncture causes less injury to the IVD. Thus, inspection of both superior and inferior margins of the rabbit IVD is essential before puncturing. Confirmation of IVD level is also very important. We used baseline simple X-rays before the procedure and C-arm guidance during the procedure for confirmation of IVD level. Unlike in human, the pelvic rim generally corresponds to the L5-L6 IVD in rabbits. The authors placed a titanium clip at the top of the superior endplate of the L1 vertebra which usually forms a large osteophyte. It was very helpful in that it was visible in simple X-ray but did not disrupt MRI scanning.
Postoperative care of the rabbits also takes a lot of time and effort. The rabbits sometimes scratch the wound due to itching postoperatively. It may lead to skin infection, and rarely sepsis. The authors lost one rabbit due to sepsis caused by scratching and subsequent wound infection, and this rabbit was excluded from this study. A clean environment and wound care is required to avoid infection. Also, stressful conditions such as frequent touch, manipulation, or poor water supply should be avoided.
IVDs are characterized by their abundant extracelluar matrix and low cell density, coupled with an absence of blood vessels, lymphatics, and nerves in all but the most peripheral annulus layers22). In many respects, this absence leaves the disc prone to degeneration because the cells have a large extracellular matrix to maintain without nociceptive feedback to detect and limit damage, and have no source of repair through the vasculature. The lumbar intervertebral disc undergoes very extensive destructive changes with age and degeneration. With aging of the lumbar spine, the elastic nucleus pulposus becomes dehydrated and more fibrotic. Fissures develop in the annular fibers that allow displacement of the nucleus pulposus, causing a decrease in disc height. Many clinicians and researchers believe that IVD degeneration is the predominant source of low back pain.
All spine surgeons dream of biological repair of the damaged tissue by a simple injection of biomaterials into the defective disc. Many techniques using gene transfer7,19,20,27), growth factors2,6,9,15-17,25,26), or other biochemical manipulations on disc integrity1,3) have been introduced to biologically repair intervertebral discs. One of the most commonly used approaches is the injection of inhibitors of proteolytic enzymes or biological growth factors that stimulate cell metabolic activity in order to slow down the degenerative process. Another is implantation of a biomatrix embedded with cells which have the potential to restore functionality and to retard further disc degeneration.
Bone morphogenetic protein (BMP) is one of the most commonly used growth factor for disc degeneration and regeneration studies7,16,25,26). It is purified from bone matrix and is a multifunctional growth factor that belongs to the transforming growth factor β superfamily. About 20 members of the BMP family have been characterized. The activity of BMPs was identified in the 1960s, and the proteins were purified and discovered to be responsible for bone induction in the late 1980s. In 2002, the U.S. Food and Drug Administration approved the use of rhBMP-2, a high-potency osteoinductive material, to promote anterior lumbar interbody fusion in humans16,25,26).
The osteoinductive capacity of rhBMP-2 was demonstrated in preclinical models and evaluated in clinical trials. In addition, rhBMP-2 has a potent anabolic action and a chondroinductive capacity involving articular chondrocytes. RhBMP-2 induces fibroblast-like cells that differentiate into chondrocytes and increases matrix secretion25). RhBMP-2 can increase synthesis of the extracellular matrix and its components in chondrocytes, including proteoglycan, aggrecan, and type-II collagen for use in repairing damaged cartilage. RhBMP-2 also maintains the chondrocyte phenotype and stimulates proteoglycan synthesis in articular cartilage. In 2003, rhBMP-2 was first shown to stimulate the proliferation of rat IVD cells from the annulus fibrosus and transition zone in vitro. However, an in vivo study using 22 rabbits found that degenerative changes were more severe in the groups treated with rhBMP-2 with or without coral in radiographic findings9). Nowadays, a widely used growth factor is osteogenic protein-1 (OP-1), also known as BMP-7. It is also a member of the transforming growth factor β superfamily and was initially found to exert potent effects on osteocyte and chondrocyte differentiation and metabolism. In chondrocytes, OP-1 stimulates the synthesis of proteoglycans and type II collagen. Importantly, a recent study showed that OP-1 strongly stimulates the production and formation of extracellular matrix by rabbit IVD cells as well as by human IVD cells15). An adequate animal model is essential to study the effects of OP-1 in vivo. The rabbit model we describe in this study will have much value for such in vivo studies.
In this current study, injection trials of disc cell/ad-BMP-2 into rabbit IVD were attempted. The authors injected cultured disc cells transfected with ad-BMP-2 into the rabbit IVD. However, we found no restoration of disc height or improvement in disc degeneration grade. Since a very small sample size was used for this preliminary study, further investigation using a larger sample should be conducted in the future.
The present study has several limitations. This study had no biochemical analysis (proteoglycan or other collagen) or histological grading of disc specimens from the rabbits. This study was mainly focused on confirming the usefulness of the rabbit annular puncture model in studying disc degeneration. But, the usefulness of this model for studying the association between disc regeneration and various growth factors should be studied further.
CONCLUSION
This study showed that the injured disc had a significant change in DHI on simple lateral radiograph and disc degeneration grade on MRI scans within 15 weeks in all rabbits. The rabbit annular puncture model can serve as a very useful tool for studying disc degeneration in vivo.
References
- 1.Alini M, Roughley PJ, Antoniou J, Stoll T, Aebi M. A biological approach to treating disc degeneration : not for today, but maybe for tomorrow. Eur Spine J. 2002;11(Suppl 2):S215–S220. doi: 10.1007/s00586-002-0485-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.An HS, Takegami K, Kamada H, Nguyen CM, Thonar EJ, Singh K, et al. Intradiscal administration of osteogenic protein-1 increases intervertebral disc height and proteoglycan content in the nucleus pulposus in normal adolescent rabbits. Spine. 2005;30:25–31. doi: 10.1097/01.brs.0000148002.68656.4d. discussion 31-32. [DOI] [PubMed] [Google Scholar]
- 3.An HS, Thonar EJ, Masuda K. Biological repair of intervertebral disc. Spine. 2003;28(15 Suppl):S86–S92. doi: 10.1097/01.BRS.0000076904.99434.40. [DOI] [PubMed] [Google Scholar]
- 4.Boos N, Weissbach S, Rohrbach H, Weiler C, Spratt KF, Nerlich AG. Classification of age-related changes in lumbar intervertebral discs. Spine. 2002;27:2631–2644. doi: 10.1097/00007632-200212010-00002. [DOI] [PubMed] [Google Scholar]
- 5.Chiba K, Andersson GBJ, Masuda K, Momohara S, Williams JM, Thonar EJ. A new culture system to study the metabolism of the intervertebral disc in vitro. Spine. 1998;23:1821–1827. doi: 10.1097/00007632-199809010-00002. [DOI] [PubMed] [Google Scholar]
- 6.Chujo T, An HS, Akeda K, Mijamoto K, Muehleman C, Attawia M, et al. Effects of Growth differentiation factor-5 on the intervertebral disc-in vitro bovine study and in vivo rabbit disc degeneration model study. Spine. 2006;31:2909–2917. doi: 10.1097/01.brs.0000248428.22823.86. [DOI] [PubMed] [Google Scholar]
- 7.Cowan CM, Jiang X, Hsu T, Soo C, Zhang B, Wang JZ, et al. Synergistic effects of Nell-1 and BMP-2 on the osteogenic differentiation of myoblasts. J Bone Miner Res. 2007;22:918–930. doi: 10.1359/jbmr.070312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haughton V. Imaging intervertebral disc degeneration. J Bone Joint Surg AM. 2006;88:15–20. doi: 10.2106/JBJS.F.00010. [DOI] [PubMed] [Google Scholar]
- 9.Huang KY, Yan JJ, Hsieh CC, Chang MS, Lin RM. The in vivo biological effects of intradiscal recombinant human bone morphogenetic protein-2 on the injured intervertebral disc. Spine. 2007;32:1174–1180. doi: 10.1097/01.brs.0000263369.95182.19. [DOI] [PubMed] [Google Scholar]
- 10.Kroeber MW, Unglaub F, Wang H, Schmid C, Thomsen M, Nerlich A, et al. New in vivo animal model to create intervertebral disc degeneration and to investigate the effects of therapeutic strategies to stimulate disc regeneration. Spine. 2002;27:2684–2690. doi: 10.1097/00007632-200212010-00007. [DOI] [PubMed] [Google Scholar]
- 11.Lee JY, Hall R, Pelinkovic D, Cassinelli E, Usas A, Gilbertson L, et al. New use of a three-dimensional pellet culture system for human intervertebral disc cells. Spine. 2001;26:2316–2322. doi: 10.1097/00007632-200111010-00005. [DOI] [PubMed] [Google Scholar]
- 12.Lipson SJ, Muir H. 1980 Volvo award in basic science. Proteoglycans in experimental intervertebral disc degeneration. Spine. 1981;6:194–210. doi: 10.1097/00007632-198105000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Lu DS, Shono Y, Oda I, Abumi K, Kaneda K. Effects of chondroitinase ABC and chymopapain on spinal motion segment biomechanics. An in vivo biomechanical, radiologic, and histologic canine study. Spine. 1997;22:1828–1834. doi: 10.1097/00007632-199708150-00006. [DOI] [PubMed] [Google Scholar]
- 14.Masuda K, Aota Y, Muehleman C, Imai Y, Okuma M, Thonar EJ, et al. A novel rabbit model of mild, reproducible disc degeneration by an annulus needle puncture : correlation between the degree of disc injury and radiological and histological appearances of disc degeneration. Spine. 2004;30:5–14. doi: 10.1097/01.brs.0000148152.04401.20. [DOI] [PubMed] [Google Scholar]
- 15.Masuda K, Imai Y, Okuma M, Muehleman C, Nakagawa K, Akeda K, et al. Osteogenic protein-1 injection into a degenerated disc induces the restoration of disc height and structural changes in the rabbit anular puncture model. Spine. 2006;31:742–754. doi: 10.1097/01.brs.0000206358.66412.7b. [DOI] [PubMed] [Google Scholar]
- 16.Masuda K, Oegema TR, An HS. Growth factors and treatment of intervertebral disc degeneration. Spine. 2004;29:2757–2769. doi: 10.1097/01.brs.0000146048.14946.af. [DOI] [PubMed] [Google Scholar]
- 17.Miyamoto K, Masuda K, Kim JG, Inoue N, Akeda K, Andersson GB, et al. Intradiscal injections of osteogenic protein-1 restore the viscoelastic properties of degenerated intervertebral discs. Spine J. 2006;6:692–703. doi: 10.1016/j.spinee.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 18.Natarajan RN, Williams JR, Andersson GB. Modeling changes in intervertebral disc mechanics with degeneration. J Bone Joint Surg Am. 2006;88-A:36–40. doi: 10.2106/JBJS.F.00002. [DOI] [PubMed] [Google Scholar]
- 19.Nishida K, Kang JD, Gilbertson LG, Moon SH, Suh JK, Vogt MT, et al. 1999 Volvo award winner in basic science studies. Modulation of the biologic activity of the rabbit intervertebral disc by gene therapy : An in vivo study of adenovirus-mediated transfer of the human transforming growth factor β1 encoding gene. Spine. 1999;24:2419–2425. doi: 10.1097/00007632-199912010-00002. [DOI] [PubMed] [Google Scholar]
- 20.Nishida K, Kang JD, Suh JK, Robbins PD, Evans CH, Gilbertson LG. Adenovirus-mediated gene transfer to nucleus pulposus cells : implications for the treatment of intervertebral disc degeneration. Spine. 1998;23:2437–2442. doi: 10.1097/00007632-199811150-00016. [DOI] [PubMed] [Google Scholar]
- 21.Pfirrmann CWA, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine. 2001;26:1873–1878. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 22.Roughley PJ. Biology of intervertebral disc aging and degeneration. Involvement of the extracellular matrix. Spine. 2004;29:2691–2699. doi: 10.1097/01.brs.0000146101.53784.b1. [DOI] [PubMed] [Google Scholar]
- 23.Sakai D, Mochida J, Iwashina T, Watanabe T, Nakai T, Ando K, et al. Differentiation of mesenchymal stem cells transplanted to a rabbit degenerative disc model. Spine. 2005;30:2379–2387. doi: 10.1097/01.brs.0000184365.28481.e3. [DOI] [PubMed] [Google Scholar]
- 24.Sobajima S, Kompel JF, Kim JS, Wallach CJ, Bobertson DD, Vogt MT, et al. A slowly progressive and reproducible animal model of intervertebral disc degeneration characterized by MRI, x-ray, and histology. Spine. 2004;30:15–24. doi: 10.1097/01.brs.0000148048.15348.9b. [DOI] [PubMed] [Google Scholar]
- 25.Yoon ST, Kim KS, Li J, Park JS, Akamaru T, Elmer WA, et al. The effect of bone morphogenetic protein-2 on rat intervertebral disc cells in vitro. Spine. 2003;28:1773–1780. doi: 10.1097/01.BRS.0000083204.44190.34. [DOI] [PubMed] [Google Scholar]
- 26.Walsh AJL, Bradford DS, Lotz JC. in vitro growth factor treatment of degenerated intervertebral discs. Spine. 2004;29:156–163. doi: 10.1097/01.BRS.0000107231.67854.9F. [DOI] [PubMed] [Google Scholar]
- 27.Wallach CJ, Sobajima S, Watanabe Y, Kim JS, Georgescu HI, Robbins P, et al. Gene transfer of the catabolic inhibitor TIMP-1 increases measured proteoglycans in cells from degenerated human intervertebral discs. Spine. 2003;28:2331–2337. doi: 10.1097/01.BRS.0000085303.67942.94. [DOI] [PubMed] [Google Scholar]