Abstract
A neutrophil specific peptide, cinnamoyl-F(D)LF(D)LFK (cFLFLFK), was conjugated consecutively with a polyethylene glycol moiety (3.4 K) and 2,2′,2″,2‴-(1,4,7,10-tetraazacyclododecane-1,4,7,10-tetrayl)tetraacetic acid (DOTA) to form cFLFLFK-PEG-DOTA. After 64Cu labeling, Positron Emission Tomography (PET) imaging was successfully able to detect mouse lung inflammation.
Keywords: Neutrophil antagonist, Peptide tracer, Inflammation imaging, 64Cu
Several peptides targeting receptors on infiltrating leukocytes (e.g. fMLF1, iBoc-MLF1, and fNleLFNleYK2 et al) have been investigated as potential imaging agents for non-invasive detection of acute inflammation. Currently used clinical nuclear imaging probes, e.g. 99mTc or 111In labeled white blood cell3,4 and 67Ga-citrate5, either needs significant preparation time and blood handling or is not specific for inflammation. Peptide probes specifically target neutrophils in vivo, therefore avoiding the disadvantages associated with ex vivo laboratory procedures and non-specificity. While these peptides show promise for in vivo detection of inflammation in terms of early imaging and target-to-background ratio in experimental models, several problems remain. Receptor agonist peptides may cause neutropenia or demargination due to neutrophil activation, while receptor antagonist peptides may show low uptake in infectious foci due to the low receptor affinity. The neutrophil receptor antagonist cFLFLF was reported with high affinity for the neutrophil N-formylpeptide receptor (FPR)6, but we have found this agent to have poor imaging quality due to lipophilicity resulting in high liver uptake. We addressed these issues by modifying the cFLFLF with biocompatible polyethylene glycol (PEG, molecular weight = 3.4k). We reason that the increase in hydrophilicity caused by the addition of PEG should help to attenuate hepatobiliary and intestinal uptake of the peptide. This imaging agent consists of three structural components: neutrophil-specific cFLFLF; biocompatible PEG; and the radioisotope 64Cu. The binding assay of cFLFLF-PEG-64Cu to human neutrophils yielded Kd = 5.7 nM indicating that the PEGylation did not interfere the peptide binding with neutrophil receptor.
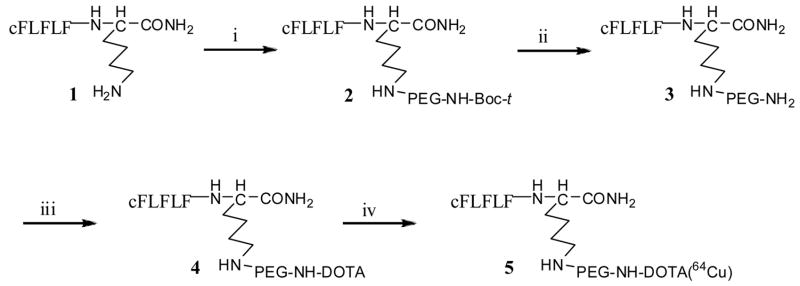
The PEGylation of the peptide was completed according to a modified procedure of Chen et al7. A solution of 10 mg of cFLFLFK 1, synthesized on an Advanced solid-phase peptide synthesizer by Fmoc solid phase chemistry, and 30 mg of bifunctional t-butoxycarbonyl-protected PEG-succinimidyl ester (t-Boc-PEG-NHS) (MW, 3,400. Laysan Bio, Inc., USA) in acetonitrile-sodium borate buffer (0.1N, pH 8.5) (50/50, v/v) was incubated at 4°C overnight to yield 23 mg of cFLFLF-PEG 2 (62% from PEG), purified by HPLC and characterized by mass spectroscopy. After cleavage of the t-Boc by treating with trifluoroacetic acid (TFA) from 2, the mono-activated DOTA-SulfoNHS was coupled to the other end of the PEG moiety to produce cFLFLF-PEG-DOTA 4 which was characterized by MALDI-TOF MS (Matrix assisted laser desorption ionisation time-of-flight mass spectrometry) with a number-average molecular weight of 4776. The DOTA-SulfoNHS was freshly prepared from DOTA (Macrocyclics, Inc., Dallas, TX), N-hydroxysulfosuccinimide (Sulfo-NHS) (PIERCE, Rockford, IL) and 1-ethyl-3-[3-(dimethylamino)-propyl]carbodiimide (EDC) (PIERCE, Rockford, IL) in aqueous (pH 5.5) at 4 °C for 30 minutes. The radiolabeling was completed by addition of 760 μCi of 64CuCl2 (Isotrace, Inc, O’Fallon, MO) to 20μg of cFLFLF-PEG-DOTA 4 in 0.1N ammonium acetate (pH 5.5) buffer and the mixture was incubated at 40°C for 30 minutes. The reaction was terminated by the addition of 5 mL of EDTA solution (10 mmol/L), followed by HPLC purification (HPLC purification with a C18 reversed-phase Apollo column (5μm, 250×10 mm). The mobile phase changed from 40% Solvent A (0.1% TFA in water) and 60% Solvent B (0.1% TFA in 80% aqueous acetonitrile) to 100% Solvent B at 30min at a flow rate 3mL/min. The cFLFLF-PEG-64Cu 5 was collected with retention time at 17.2min with radiochemical yields higher than 90%. The collected fraction was analyzed with HPLC with the same conditions (Figure 2). The radiochemical purity was higher than 90% and the specific activity was ~ 30 mCi/μmol. To characterize cFLFLF-PEG-64Cu 5, the “cold” counterpart cFLFLF-PEG-Cu was synthesized and verified by mass spectroscopy. HPLC retention time of cFLFLF-PEG-64Cu 5 was assigned by coinjection with the “cold” counterpart.
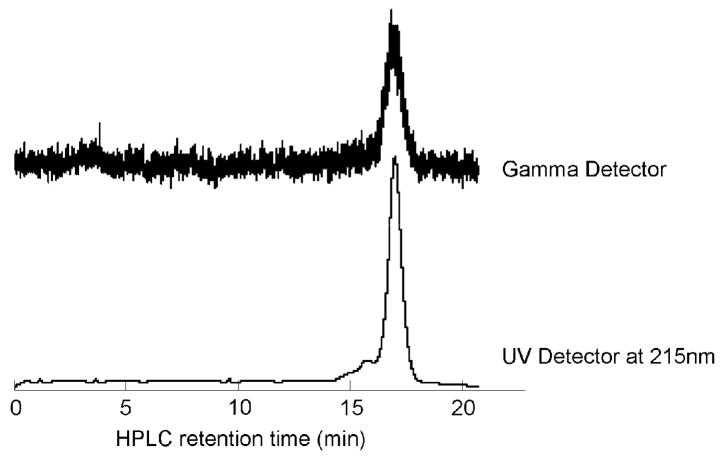
Figure 2.
The HPLC chromatogram shows the collected cFLFLF-PEG-64Cu with radiochemical purity higher than 90%. The retention time of the cFLFLF-PEG-64Cu is identical to its “cold” counterpart, cFLFLF-PEG-Cu, which has been characterized by mass spectroscopy.
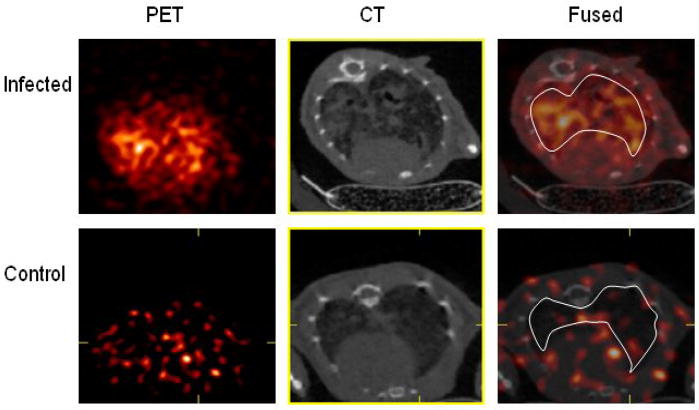
The newly synthesized cFLFLF-PEG-64Cu was evaluated in an animal model of bacterial infection. Pneumonia was induced in male C57Bl/6 mice by oropharyngeal aspiration under light inhalational anesthesia of 106 colony forming units of Klebsiella pneumoniae. A control mouse was sham-treated with aspiration of sterile normal saline. Twenty four hours after bacterial inoculation, 80–100 μCi of cFLFLF-PEG-64Cu was injected through the tail vein. MicroPET (Siemens Focus 120) and respiratory gated microCT (custom-built) were performed 6 hrs after tracer injection. Total image acquisition time was 20–25 minutes. MicroPET and microCT images were fused using a transformation matrix generated by imaging a phantom. CT images were used to guide the placements of the lung regions of interest to obtain lung activity concentration. Tracer Standardized Uptake Values (SUVs) were computed as the ratio of the total ROI activity concentration normalized by the injected dose and the weight of the animal. Figure 3 shows the PET, CT and fused transaxial slices for the infected and the sham mice. The SUV of lung for the infected mouse is higher than the sham by a factor of 7.
Figure 3.
PET, CT and fused transaxial slices for infected and sham mice. The region of interest are depicted in the fused images.
In conclusion, we have shown that cFLFLF-PEG-64Cu is an effective probe for in vivo imaging of acute neutrophilic inflammation. In our mouse model of bacterial pneumonia, using fused microPET and microCT imaging, cFLFLF-PEG-64Cu signal was 7-fold higher in infected than in non-infected lungs. Further biological evaluation of this novel imaging agent is ongoing in our laboratory, with the goal of refining in vivo imaging of lung inflammation to facilitate studies of anti-inflammatory therapies.
Figure 1.
Reagents: (i)t-Boc-NH-PEG-NHS, 0.1N Sodium Borate Buffer, pH 8.5; (ii) TFA (iii) DOTA-SulfoNHS, pH 8.5; (iv) 64CuCl2
Acknowledgments
The research was supported by NIH grants HL-073361 (JL) and HD051609 (KDF).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Babich JW, Tompkins RG, Graham W, Barrow SA, Fischman AJ. Journal of Nuclear Medicine. 1997;38:1316–22. [PubMed] [Google Scholar]
- 2.Pollak A, Goodbody AE, Ballinger JR, Duncan GS, Tran LL, Dunn-Dufault R, Meghji K, Lau F, Andrey TW, Boxen I, Sumner-Smith M. Nuclear Medicine Communications. 1996;17:132–9. doi: 10.1097/00006231-199602000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Thakur ML, Lavender JP, Arnot RN, Silvester DJ, Segal AW. Journal of Nuclear Medicine. 1977;18:1014–21. [PubMed] [Google Scholar]
- 4.McAfee JG, Thakur ML. Journal of Nuclear Medicine. 1976;17:480–7. [PubMed] [Google Scholar]
- 5.Edwards CL, Hayes RL. Journal of Nuclear Medicine. 1969;10:103–5. [PubMed] [Google Scholar]
- 6.Babich JW, Dong Q, Graham W, Barzana M, Ferril K, Pike M, Fischman AJ. J Nucl Med. 1997;38:268P. [Google Scholar]
- 7.Chen X, Hou Y, Tohme M, Park R, Khankaldyyan V, Gonzales-Gomez I, Bading JR, Laug WE, Conti PS. J Nucl Med. 2004;45:1776–1783. [PubMed] [Google Scholar]