Abstract
The epithelial Na+ channel (ENaC) is an essential channel responsible for Na+ reabsorption. Co-expression of Rab11a and Rab3a small G proteins with ENaC results in a significant increase in channel activity. In contrast, co-expression of Rab5, Rab27a and Arf-1 had no effect or slightly decreased ENaC activity. Inhibition of MEK with PD98059, Rho-kinase with Y27632 or PI3-kinase with LY294002 had no effect on ENaC activity in Rab11a-transfected CHO cells. Fluorescence imaging methods demonstrate that Rab11a colocalized with ENaC. Rab11a increases ENaC activity in an additive manner with dominant–negative dynamin, which is a GTPase responsible for endocytosis. Brefeldin A, an inhibitor of intracellular protein translocation, blocked the stimulatory action of Rab11a on ENaC activity. We conclude that ENaC channels, present on the apical plasma membrane, are being exchanged with channels from the intracellular pool in a Rab11-dependent manner.
Keywords: ENaC, Epithelial sodium channels, small G proteins, small GTPases, Rab, Rab11, Rab3, trafficking
The epithelial Na+ channel (ENaC) plays a central role in control of epithelial surface hydration and vascular volume. ENaC is localized to the apical plasma membrane of epithelia particularly that lining the distal renal nephron and colon and alveolar space [1; 2]. ENaC activity in this epithelia is limiting for sodium reabsorption. Abnormalities in ENaC function have been linked to disorders of total body Na+ homeostasis, blood volume, blood pressure, and lung fluid balance. In contrast to voltage- and ligand-gated ion channels, which are regulated trough rapid changes in channel gating, ENaC is regulated in large part through mechanisms that control its expression at the cell surface [3; 4].
Small G proteins act as GTP-dependent switches to control the activity of effector proteins and thus initiate cellular signaling cascades [5]. Evidence that ion channels are final effectors of small G proteins has accumulated for the last decade [6]. Signaling mediated by small G proteins can impinge upon the activity of a wide variety of membrane-resident ion channels. In some cases, small GTPases interact directly with ion channels to elicit regulation and in others, regulation is mediated by intermediary signaling proteins. A large body of evidence has been accumulated in support of a role for Rab proteins in vesicle trafficking. Rab proteins use the guanine nucleotide-dependent switch mechanism to regulate each of the four major steps in intracellular vesicle transport: vesicle budding from the donor membrane, targeting of the vesicle to the acceptor membrane, docking of the vesicle, and fusion of the vesicle with the acceptor membrane. Substantial evidence has been gathered that most Rab proteins regulate the targeting/docking/fusion processes and that only some of them regulate the budding process, which is primarily regulated by Sar1/Arf small G proteins [5].
The rationale for these studies comes from our preliminary studies showing that several small G proteins, including K-Ras, RhoA and Rac1 alter ENaC activity [7-11]. Moreover, recent studies reveal that multiple Rab isoforms physically interact with and/or modulate the activity of several epithelial ion channels [6; 12]. Van de Graaf et al. recently identified Rab11a as a novel TRPV5- and TRPV6-associated protein. Rab11a colocalizes with TRPV5 and TRPV6 in Ca2+-transporting epithelial cells of the kidney [13]. Trafficking of the chloride channel, CLC-2 via the early endosomal compartment occurs in part through Rab5-associated vesicles and recycling of CLC-2 to the cell surface occurs through a Rab11-dependent pathway [14]. Rab11 maintains the proper distribution of the subapical AQP2 water channels and regulates the trafficking of AQP2 [15; 16]. Rab proteins have also been implicated in the regulation of ENaC and CFTR trafficking [12; 17]. Thus, we focused on the regulation of ENaC by Rab GTPases.
The present study was designed to investigate the trafficking of ENaC and mechanisms regulating this process. Specifically, we tested the hypothesis that Rab GTPases are involved in regulation of ENaC activity via affecting trafficking of the channel. We find that Rab3a and Rab11a increase ENaC activity with Rab11a affecting ENaC activity by influencing channel insertion into the plasma membrane.
Materials and Methods
cDNA constructs and cell culture
All chemicals and materials were purchased from either Fisher Scientific, Sigma or CalBiochem unless noted otherwise. CHO or Cos-7 cells were maintained with standard culture conditions and transfected using Polyfect reagent (Qiagen, Valencia, CA) as described previously [10]. For imaging experiments immortalized mouse cortical collecting duct (mpkCCDc14) cells were grown in defined medium as described previously [18]. For expression of ENaC, subunit cDNA transfection ratios of 1: 1: 1 were used with 0.1 - 0.3 μg of total cDNA per 35 mm2 dish transfected. The expression vectors encoding Rab11a, Rab3a, Rab27a and Arf-1 were from the UMR cDNA Resource Center (http://www.cdna.org). The expression vectors encoding wild type and constitutively active (Q79L) Rab5 and dominant negative (S25N) Rab11 were a kind gift of Dr. G. Seebohm [19]. The expression vector encoding dominant-negative (K44A) dynamin was from Dr. M. McNiven [20].
Electrophysiology
Whole-cell macroscopic current recordings of mENaC expressed in CHO cells were made under voltage-clamp conditions using standard methods. In brief, current through ENaC was the inward, amiloride-sensitive Na+-current with a bath solution of (in mM) 160 NaCl, 1 CaCl2, 2 MgCl2 and 10 HEPES (pH 7.4) and a pipette solution of (in mM) 120 CsCl, 5 NaCl, 2 MgCl2, 5 EGTA, 10 HEPES, 2.0 ATP and 0.1 GTP (pH 7.4). Current recordings were acquired with an Axopatch 200B (Axon Instr.) patch clamp amplifier interfaced via a Digidata 1440A (Mol Dev) to a PC running the pClamp 10.2 suite of software (Mol Dev). Cells were clamped to a 40 mV holding potential with voltage ramps (500 ms) from 60 mV down to –100 mV used to elicit current. ENaC activity is the amiloride-sensitive current density at −80 mV. Whole-cell capacitance, on average 8-10 pF, was compensated. Series resistances, on average 2-5 MΩ, were also compensated.
Confocal Microscopy
To investigate the colocalization of ENaC with wild type and dominant negative Rab11, mpkCCDc14 cells were grown on glass bottom dishes (MalTek, Ashland, MA), and transfected 2-3 days before experiments with CFP-tagged ENaC subunits and Ds-Red-tagged Rab small GTPases. The plasmids encoding NH2-terminal eCFP-tagged α-, β- and γ-mouse ENaC have been described previously [10; 21]. As noted in these earlier publications, channels comprised of eCFP-tagged subunits exhibit functional behavior indistinguishable from native channels. The expression vectors encoding wild type and dominant negative (S25N) Ds-Red-tagged Rab11 were described previously [19]. Micrographs were collected using a Leica TCS SP5 confocal microscope equipped with the air cooled photomultiplier tubes (PMTs) for detecting five channels simultaneously and Acousto-Optical Beam Splitter (AOBS) for dynamic beam splitting. Images were collected with 0.3-0.5 μm intervals in z-sectioning using 63× oil-immersion high-resolution (1.4 numerical aperture) objective. Quantitative analysis of colocalization rate of ENaC and Rab11a was performed using Leica LAS-AF software based on 30% background subtractions in both channels and 30% threshold for determining co-localization. Moreover, the theory of image correlation spectroscopy (ICS) and image cross-correlation spectroscopy (ICCS) developed by Petersen and Wiseman [22; 23] was used to confirm degrees of cross-correlation between ENaC and Rab11 a. The normalized intensity fluctuation spatial auto correlation and cross correlation functions were computed and were fitted to 2-d aussians of the form: . The mean number of colocalized particles per confocal beam area are given by: , following Petersen and Wiseman. In addition to N12 we computed, the correlation coefficient, which is given by the covariance of both channels divided by the product of square roots of autocovariances of each channel. The covariance is the cross correlation at zero lag and the autocovariances are the autocorrelations at zero lag: . Strong colocalization is indicated by a value of ρ12 greater than 0.5 and a value of 1 indicates perfect co-localization. All imaging experiments were performed in the microscopy core facility housed within the B1iotechnology and Bioengineering Center at the Medical College of Wisconsin.
Statistics
All summarized data reported as mean ± SEM. Data compared with either the Student's (two-tailed) t-test or a one way ANOVA. P ≤ 0.05 considered significant.
Results
Rab11a and Rab3a increase ENaC activity
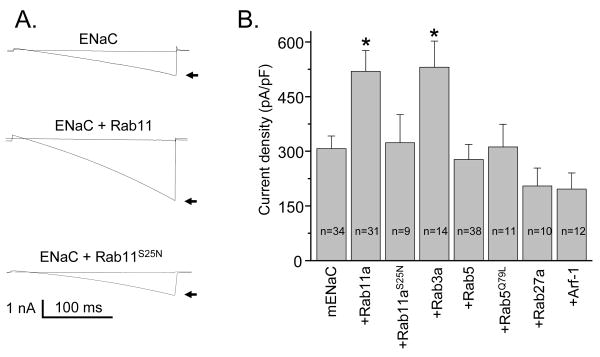
To investigate the actions of small G proteins on ENaC, we reconstituted the channel in CHO cells in the absence and presence of co-expressed wild type and mutant various small G proteins. mENaC was reconstituted by co-expressing α-, β- and γ-channel subunits together. Fig. 1A shows ENaC currents before (arrows) and after treatment with amiloride (10 μM) in a cell expressing the channel alone and in a cell expressing both the channel and either wild type or dominant negative (S25N) Rab11a. Currents were elicited by voltage ramping from 60 mV down to -100 mV (holding potential = 40 mV). As summarized in Fig. 1B, co-expression of wild type Rab11a and Rab3a markedly increased ENaC activity. The average amiloride-sensitive current density at -80 mV, as shown in Fig. 1B, in control experiments (mENaC alone) was 307.2 ± 34.9 pA/pF (n=34). Co-expression of Rab11a and Rab3a with mENaC significantly increased current density to 519.7 ± 57.1 pA/pF (n=31) and 530.9 ± 71.8 pA/pF (n=14), respectively. In contrast, dominant negative Rab11a (S25N) had no effect on basal ENaC activity (323.3 ± 77.5 pA/pF; n=9). Surprisingly, coexpression of ENaC with either wild type or constitutively active (Q79L) Rab5 also had no effect on ENaC activity. Co-expression of Rab27a and Arf-1 with mENaC slightly, but not significantly, decreased ENaC activity (Fig. 1B). These results are consistent with Rab11a and Rab3a enhance ENaC activity.
Fig. 1.
Rab11a and Rab3a enhance ENaC activity. (A) Overlays of typical macroscopic current traces before (arrow) and after 10 μM amiloride from voltage-clamped CHO cells transfected with mENaC alone and wild type or dominant negative (S25N) Rab11a. Currents evoked with a voltage ramp (60 to -100 mV from a holding potential of 40 mV). Inward Na+ currents are downward. (B) Summary graph of amiloride-sensitive current density at -80 mV for CHO cells expressing mENaC in the absence and presence of various small G proteins. The numbers of observations for each group are shown. *, versus mENaC alone.
Rho-kinase, MAPK and PI3-Kinase signaling cascades are not involved in Rab11 dependent increase of ENaC activity
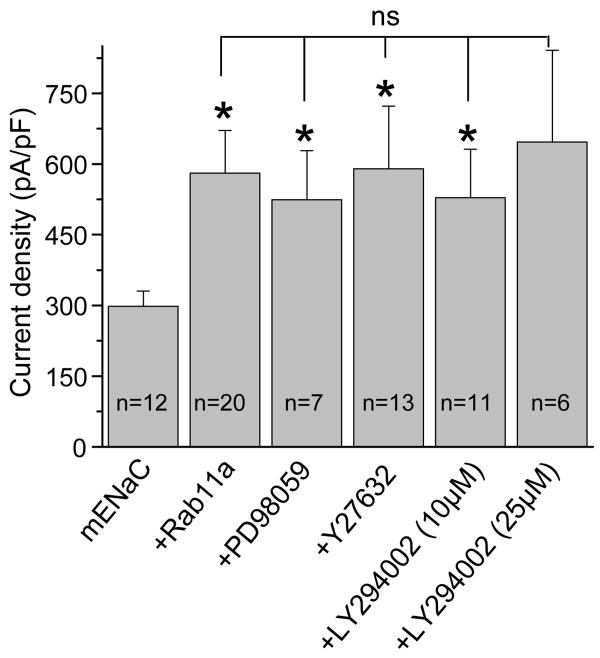
We next asked whether Rab11a activated ENaC via one of its downstream effector proteins/cascades: Rho-kinase, MAPK and PI3-Kinase signaling cascades. Our rationale was that Rab11a most likely communicates with ENaC through a signaling cascade like small G proteins K-Ras and RhoA that increase ENaC activity via PI3-kinase signaling, and via Rho-kinase and subsequent activation of PI(4)P5-kinase, respectively [7; 9-11]. Fig. 2 summarizes the effects of inhibitors of MAPK (10 μM PD98059, 2-4 hrs.), Rho-kinase (1 μM Y27632, 0.5-1 hrs.) and PI3-kinase (10 and 25 μM LY294002, 0.5-1 hrs.) signaling on Rab11a-dependent activation of ENaC. Co-expression of Rab11a with ENaC significantly increased channel current density from 298.2 ± 32.2 to 580.5 ± 90.3 pA/pF. As shown in Fig. 2, pretreating cells overexpressing mENaC and Rab11a with PD98059, Y27632 and LY294002 had no effect on the increase of amiloride sensitive current density mediated by Rab11a. We conclude from these results that these signaling cascades are not involved in Rab11-dependent increases of ENaC activity.
Fig. 2.
Rab11a does not affect ENaC activity via MAPK, Rho-kinase and PI3-kinase signaling cascades. Summary graph of current density for mENaC in the absence and presence of Rab11a with and without pretreatment with inhibitors of MAPK (Mek1/2 inhibitors PD98059), Ral/Rac/Rho (Rho kinase inhibitor Y27632), and PI3-kinase (LY294002) signaling. *, versus mENaC alone.
Rab11a colocalizes with ENaC
We next tested whether ENaC colocalizes with wild type and dominant negative Rab11. Shown in Fig. 3 are fluorescence images of mpkCCDc14 cells expressing CFP-tagged ENaC and wild type (Fig. 3A) and mutant (Fig. 3B) dsRed-Rab11a. The subcellular localization of ENaC with Rab11a was visualized by two-photon laser scanning microscopy. We determined that Rab11a localizes to vesicular structures as well as at the cell surface and colocalizes with ENaC as recently reported by Lu et al. [24]. ENaC was clearly present in many vesicles distributed through the cytoplasm, where it showed prominent colocalization with wild type Rab11a. Moreover, z-stacks analysis of these cells showed the colocalization of ENaC and Rab11a in a plasma membrane region (upper images). For subcellular localization studies, serial sections were acquired by line averaging the frames and descending z levels. Dominant negative Rab11aS25N is expressed in a more focal, perinuclear distribution pattern (Fig. 3B). Interestingly, we have observed a vesicular colocalization of ENaC with GDP-locked Rab11aS25N (Figs. 3B & D). These findings are akin to previously published about colocalization of TRPV5 with Rab11aS25N [13]. The ENaC colocalization with wild type and dominant negative (S25N) Rab11 was quantified by two independent methods: by the cross-correlation between two channels using the inverse Fourier transform (Fig. 3C) and by using digital quantification with Leica LAS-AF software as summarized in Fig. 3D. Fig. 3C shows the cross-correlation spectroscopy analysis for ENaC and wild type Rab11a shown in 3A. The correlation coefficient (ρ12) was 0.83 showing strong colocalization between ENaC and Rab11a. Fig. 3D shows the summary graph of quantification analysis of ENaC colocalization with wild type and dominant negative (S25N) Rab11a using Leica software. Thus, these results show that ENaC colocalizes with Rab11a at both cytosol and submembrane fractions. Fluorescent images of ENaC and Rab11a colocalization in Cos-7 cells were consistent with current results in mpkCCDc14 cells (data not shown).
Fig. 3.
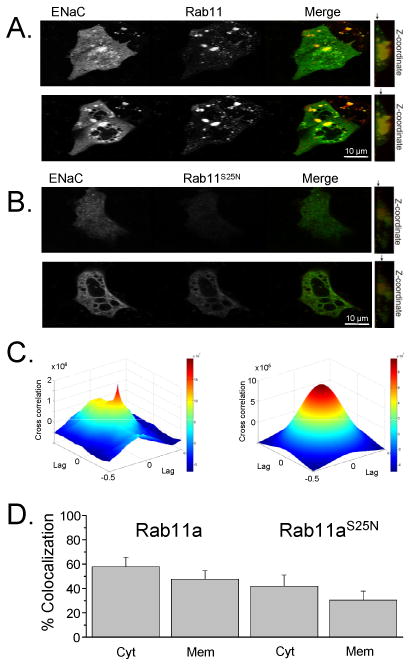
Rab11a colocalizes with ENaC. (A, B) mpkCCDc14 cells were transiently transfected with eCFP-tagged α-, β- and γ-ENaC subunits and wild type (A) or dominant negative (B) DsRed-Rab11. In the merged picture, the colocalization of the 2 proteins is depicted in yellow. Cytoplasm (bottom) and membrane (up) fractions are shown. The arrows on the z-cross-section (right) point to a slice location from where the representative images where taken. (C) Image cross-correlation analysis. Fitting the raw data (left) with an analytical correlation function allowed to calculate the correlation coefficient (ρ12) and the mean number of colocalized particles within a confocal beam area (right). (D) Summary graph of the mean ± SE of the rate of ENaC colocalization with wild type (left columns) and dominant negative (S25N) Rab11 (right columns) from cytosol (Cyt) and membrane (Mem) fractions.
Rab11a promotes ENaC insertion into the plasma membrane
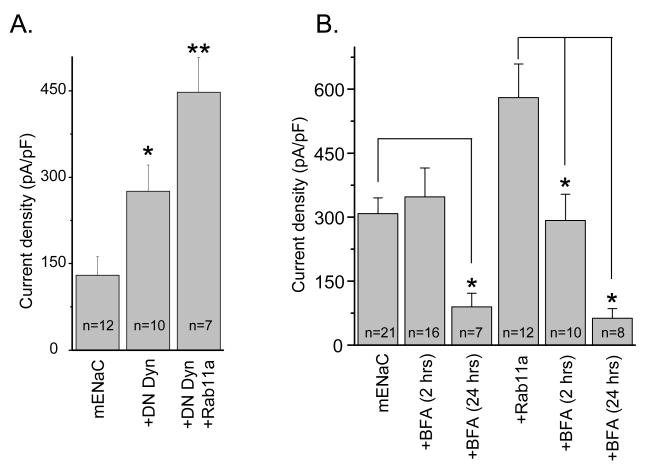
Retrieval of ENaC from the plasma membrane, in part, involves the action of dynamin [25-27]. As shown in Fig. 4A co-expression of dominant negative dynamin (DN-Dyn) with ENaC significantly increases channel activity. We next asked if Rab11a and DN-Dyn have additive effects on ENaC. Co-expression of Rab11a and DN-Dyn together with ENaC significantly increased channel activity above DN-Dyn alone. This suggests that Rab11a influences channel activity through a mechanism independent of dynamin possibly involving increased channel insertion.
Fig. 4.
(A) Rab11a increases ENaC activity in a manner additive with decreases in channel retrieval. Summary graph of ENaC activity from cells expressing channel alone and channel plus dominant-negative dynamin (DN-Dyn), and both DN-Dyn and Rab11a together. *, versus mENaC alone; **, versus mENaC and DN-Dyn. (B) Effects of BFA on ENaC alone and coexpressed with Rab11a. Summary graph of ENaC activity from cells expressing ENaC alone or coexpressed with wild type Rab11a in the absence and presence (2 and 24 hrs.) of pretreatment with BFA. *, versus untreated cells.
To study whether the stimulatory action of Rab11a on ENaC activity is mediated through a pathway dependent on translocation of proteins, we pretreated cells expressing either channel alone or coexpressed with Rab11a with brefeldin A (BFA), which is an inhibitor of intracellular protein trafficking. We and other laboratories have used BFA previously to study trafficking of proteins, including ENaC, to the plasma membrane [8; 28; 29]. As shown in Fig. 4B, BFA (5 μg/ml) abolished ENaC activity after 24 hrs. in both cells expressing channel alone and cells coexpressed with Rab11a. In contrast, disrupting the endomembrane system with BFA within 2 hrs., significantly decreased ENaC activity only in cells coexpressing Rab11a. Thus, BFA, which blocks trafficking, decreased Rab11-dependent movement of ENaC toward the membrane.
Discussion
A family of Rab GTPases has been implicated in the regulation of intracellular vesicular traffic with specialized transport functions of the different Rab proteins in the secretory and endocytic pathways [5]. Rab11a is well-established as a participant in the regulation of recycling endosomal trafficking. Rab11a is associated with vesicles in the apical portion of epithelial cells near the centrosome and beneath the apical plasma membrane. Rab3 is involved in Ca2+-dependent exocytosis [30]. Rab5 are present on early endosomes and regulate early steps of the endocytic process [14; 19; 31]. Arf-1 belongs to the Sar1/Arf family of small G proteins and is important in the regulation of exocytosis [32]. Our results demonstrate an important role for both Rab11a and Rab3a in upregulation of ENaC activity. In contrast, neither Rab5 nor Rab27a or Arf-1, were found to regulate ENaC activity. The current results demonstrate for the first time that Rab11a small GTPases are important in the trafficking of ENaC. Together, these results identify an important new pathway regulating ENaC channel density necessary for the maintenance of proper cell homeostasis.
Saxena et al. recently reported that Rab 3, Rab 4 and Rab27a inhibit ENaC activity in the colon cancer cell line via protein–protein interactions. In contrast, Rab 5 and Rab 6 have no effect on this channel [33; 34]. In these two works authors studied short-circuit current (Isc) in HT-29 cells transiently expressed with wild type and mutant Rab small G proteins. Our experiments are partially consistent with those findings. In our studies we confirmed that Rab27a slightly decreases and Rab5 has no effect on ENaC activity. However, we have shown that Rab3a significantly increases ENaC activity. This discrepancy, though, calls for further study for it may reflect true differences between the response of various Rab3 isoform on ENaC activity or rather be attributable to differences in expression systems. It is unclear which model system better offers a true reflection of the prevalent pathways for trafficking in native epithelial cells. Thus, translation of the current findings to regulation of ENaC in native epithelia remains to be fully explored.
Rab11 small G proteins have been implicated in the regulation of intracellular vesicular traffic. Rab11a associated recycling endosomes most likely carry ENaC to the plasma membrane from a perinuclear compartment. Further evidence for a role of Rab11a in ENaC regulation is the predominant colocalization of Rab11a with ENaC. Similarly, it was shown that Rab11 is expressed in apical vesicular populations in collecting duct cells [13; 35]. The effect of disrupting channel trafficking from the trans-Golgi network to the plasma membrane was examined using brefeldin A (BFA). BFA abolished basal ENaC movement towards the membrane after 24 hrs treatment. However, treatment with BFA within 2-3 hrs. did not affect ENaC activity when the channel was expressed alone, but it produced a marked inhibition of the subsequent ENaC activity when it was coexpressed with Rab11a. Thus, BFA, which blocks trafficking, decreased Rab11-dependent movement of ENaC towards the membrane. Therefore, the fast inhibitory action of BFA on the channel is a specific one on the Rab11a effect, but not a nonspecific one on other protein trafficking processes (e.g. the basal ENaC trafficking), indicating that ENaC when coexpressed with Rab11a is recycled through BFA-sensitive compartments.
It currently is not clear whether Rab11a-mediated ENaC trafficking is a constitutive pathway supporting default movement of the channel to the plasma membrane or whether this cascade is capable of responding to signaling input to dynamically increase channel activity and Na+ reabsorption. Rab11a-mediated trafficking of ENaC could represent a general cellular mechanism controlling trafficking of ion channels and other integral membrane proteins to the membrane. Supporting this are the recent findings that Rab11 small GTPases are involved in trafficking of KCNQ1/KCNE1 and Kv1.5 potassium channels [19; 36], TRPV5 and TRPV6 Ca2+ channels [13], CLC-2 and CFTR Cl- channels [14; 17], and AQP2 water channels [15; 16].
Acknowledgments
This research was supported by AHA (0730111N) and ASN Carl W. Gottschalk Research Scholar Grant (to AS). We greatly appreciate Dr. G. Seebohm (Ruhr-Universität Bochum, Germany) for the Rab constructs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alvarez de la Rosa D, Canessa CM, Fyfe GK, Zhang P. Structure and regulation of amiloride-sensitive sodium channels. Annu Rev Physiol. 2000;62:573–594. doi: 10.1146/annurev.physiol.62.1.573. [DOI] [PubMed] [Google Scholar]
- 2.Schild L. The epithelial sodium channel: from molecule to disease. Rev Physiol Biochem Pharmacol. 2004;151:93–107. doi: 10.1007/s10254-004-0023-7. [DOI] [PubMed] [Google Scholar]
- 3.Butterworth MB, Edinger RS, Frizzell RA, Johnson JP. Regulation of the epithelial sodium channel (ENaC) by membrane trafficking. Am J Physiol Renal Physiol. 2008 doi: 10.1152/ajprenal.90248.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Snyder PM. Regulation of epithelial Na+ channel trafficking. Endocrinology. 2005;146:5079–5085. doi: 10.1210/en.2005-0894. [DOI] [PubMed] [Google Scholar]
- 5.Takai Y, Sasaki T, Matozaki T. Small GTP-binding proteins. Physiol Rev. 2001;81:153–208. doi: 10.1152/physrev.2001.81.1.153. [DOI] [PubMed] [Google Scholar]
- 6.Pochynyuk O, Stockand JD, Staruschenko A. Ion channel regulation by Ras, Rho, and Rab small GTPases. Exp Biol Med (Maywood) 2007;232:1258–1265. doi: 10.3181/0703-MR-76. [DOI] [PubMed] [Google Scholar]
- 7.Pochynyuk O, Medina J, Gamper N, Genth H, Stockand JD, Staruschenko A. Rapid translocation and insertion of the epithelial Na+ channel in response to RhoA signaling. J Biol Chem. 2006;281:26520–26527. doi: 10.1074/jbc.M603716200. [DOI] [PubMed] [Google Scholar]
- 8.Pochynyuk O, Staruschenko A, Bugaj V, Lagrange L, Stockand JD. Quantifying RhoA Facilitated Trafficking of the Epithelial Na+ Channel toward the Plasma Membrane with Total Internal Reflection Fluorescence-Fluorescence Recovery after Photobleaching. J Biol Chem. 2007;282:14576–14585. doi: 10.1074/jbc.M701348200. [DOI] [PubMed] [Google Scholar]
- 9.Staruschenko A, Nichols A, Medina JL, Camacho P, Zheleznova NN, Stockand JD. Rho small GTPases activate the epithelial Na(+) channel. J Biol Chem. 2004;279:49989–49994. doi: 10.1074/jbc.M409812200. [DOI] [PubMed] [Google Scholar]
- 10.Staruschenko A, Patel P, Tong Q, Medina JL, Stockand JD. Ras activates the epithelial Na(+) channel through phosphoinositide 3-OH kinase signaling. J Biol Chem. 2004;279:37771–37778. doi: 10.1074/jbc.M402176200. [DOI] [PubMed] [Google Scholar]
- 11.Staruschenko A, Pochynyuk OM, Tong Q, Stockand JD. Ras couples phosphoinositide 3-OH kinase to the epithelial Na+ channel. Biochim Biophys Acta. 2005;1669:108–115. doi: 10.1016/j.bbamem.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Saxena SK, Kaur S. Regulation of epithelial ion channels by Rab GTPases. Biochem Biophys Res Commun. 2006;351:582–587. doi: 10.1016/j.bbrc.2006.10.087. [DOI] [PubMed] [Google Scholar]
- 13.van de Graaf SF, Chang Q, Mensenkamp AR, Hoenderop JG, Bindels RJ. Direct interaction with Rab11a targets the epithelial Ca2+ channels TRPV5 and TRPV6 to the plasma membrane. Mol Cell Biol. 2006;26:303–312. doi: 10.1128/MCB.26.1.303-312.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dhani SU, Kim CP, Huan LJ, Bear CE. ATP depletion inhibits the endocytosis of ClC-2. J Cell Physiol. 2008;214:273–280. doi: 10.1002/jcp.21192. [DOI] [PubMed] [Google Scholar]
- 15.Takata K, Matsuzaki T, Tajika Y, Ablimit A, Hasegawa T. Localization and trafficking of aquaporin 2 in the kidney. Histochem Cell Biol. 2008;130:197–209. doi: 10.1007/s00418-008-0457-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tajika Y, Matsuzaki T, Suzuki T, Ablimit A, Aoki T, Hagiwara H, Kuwahara M, Sasaki S, Takata K. Differential regulation of AQP2 trafficking in endosomes by microtubules and actin filaments. Histochem Cell Biol. 2005;124:1–12. doi: 10.1007/s00418-005-0010-3. [DOI] [PubMed] [Google Scholar]
- 17.Guggino WB, Stanton BA. New insights into cystic fibrosis: molecular switches that regulate CFTR. Nat Rev Mol Cell Biol. 2006;7:426–436. doi: 10.1038/nrm1949. [DOI] [PubMed] [Google Scholar]
- 18.Bens M, Vallet V, Cluzeaud F, Pascual-Letallec L, Kahn A, Rafestin-Oblin ME, Rossier BC, Vandewalle A. Corticosteroid-dependent sodium transport in a novel immortalized mouse collecting duct principal cell line. J Am Soc Nephrol. 1999;10:923–934. doi: 10.1681/ASN.V105923. [DOI] [PubMed] [Google Scholar]
- 19.Seebohm G, Strutz-Seebohm N, Birkin R, Dell G, Bucci C, Spinosa MR, Baltaev R, Mack AF, Korniychuk G, Choudhury A, Marks D, Pagano RE, Attali B, Pfeufer A, Kass RS, Sanguinetti MC, Tavare JM, Lang F. Regulation of endocytic recycling of KCNQ1/KCNE1 potassium channels. Circ Res. 2007;100:686–692. doi: 10.1161/01.RES.0000260250.83824.8f. [DOI] [PubMed] [Google Scholar]
- 20.Cao H, Thompson HM, Krueger EW, McNiven MA. Disruption of Golgi structure and function in mammalian cells expressing a mutant dynamin. J Cell Sci. 2000;113:1993–2002. doi: 10.1242/jcs.113.11.1993. [DOI] [PubMed] [Google Scholar]
- 21.Staruschenko A, Adams E, Booth RE, Stockand JD. Epithelial Na+ channel subunit stoichiometry. Biophys J. 2005;88:3966–3975. doi: 10.1529/biophysj.104.056804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Costantino S, Comeau JW, Kolin DL, Wiseman PW. Accuracy and dynamic range of spatial image correlation and cross-correlation spectroscopy. Biophys J. 2005;89:1251–1260. doi: 10.1529/biophysj.104.057364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petersen NO, Hoddelius PL, Wiseman PW, Seger O, Magnusson KE. Quantitation of membrane receptor distributions by image correlation spectroscopy: concept and application. Biophys J. 1993;65:1135–1146. doi: 10.1016/S0006-3495(93)81173-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu C, Pribanic S, Debonneville A, Jiang C, Rotin D. The PY motif of ENaC, mutated in Liddle syndrome, regulates channel internalization, sorting and mobilization from subapical pool. Traffic. 2007;8:1246–1264. doi: 10.1111/j.1600-0854.2007.00602.x. [DOI] [PubMed] [Google Scholar]
- 25.Shimkets RA, Lifton RP, Canessa CM. The activity of the epithelial sodium channel is regulated by clathrin-mediated endocytosis. J Biol Chem. 1997;272:25537–25541. doi: 10.1074/jbc.272.41.25537. [DOI] [PubMed] [Google Scholar]
- 26.Staruschenko A, Pochynyuk O, Stockand JD. Regulation of epithelial Na+ channel activity by conserved serine/threonine switches within sorting signals. J Biol Chem. 2005;280:39161–39167. doi: 10.1074/jbc.M509608200. [DOI] [PubMed] [Google Scholar]
- 27.Wang H, Traub LM, Weixel KM, Hawryluk MJ, Shah N, Edinger RS, Perry CJ, Kester L, Butterworth MB, Peters KW, Kleyman TR, Frizzell RA, Johnson JP. Clathrin-mediated endocytosis of the epithelial sodium channel. Role of epsin. J Biol Chem. 2006;281:14129–14135. doi: 10.1074/jbc.M512511200. [DOI] [PubMed] [Google Scholar]
- 28.Butterworth MB, Edinger RS, Johnson JP, Frizzell RA. Acute ENaC stimulation by cAMP in a kidney cell line is mediated by exocytic insertion from a recycling channel pool. J Gen Physiol. 2005;125:81–101. doi: 10.1085/jgp.200409124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Staub O, Gautschi I, Ishikawa T, Breitschopf K, Ciechanover A, Schild L, Rotin D. Regulation of stability and function of the epithelial Na+ channel (ENaC) by ubiquitination. EMBO J. 1997;16:6325–6336. doi: 10.1093/emboj/16.21.6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin-no Y, Shirataki H, Senbonmatsu T, Yamamoto T, Fujita Y, Nakanishi H, Takai Y. A novel function of the C-terminal lipid moieties of Rab3A small G protein implicated in Ca2+-dependent exocytosis--inhibition of interaction with GTP and reduction of this inhibition by phospholipid. Genes Cells. 1997;2:273–288. doi: 10.1111/j.1365-2443.1997.120gc0318.x. [DOI] [PubMed] [Google Scholar]
- 31.Christensen EI, Devuyst O, Dom G, Nielsen R, Van der SP, Verroust P, Leruth M, Guggino WB, Courtoy PJ. Loss of chloride channel ClC-5 impairs endocytosis by defective trafficking of megalin and cubilin in kidney proximal tubules. Proc Natl Acad Sci U S A. 2003;100:8472–8477. doi: 10.1073/pnas.1432873100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumari S, Mayor S. ARF1 is directly involved in dynamin-independent endocytosis. Nat Cell Biol. 2008;10:30–41. doi: 10.1038/ncb1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saxena SK, Singh M, Shibata H, Kaur S, George C. Rab4 GTP/GDP modulates amiloride-sensitive sodium channel (ENaC) function in colonic epithelia. Biochem Biophys Res Commun. 2006;340:726–733. doi: 10.1016/j.bbrc.2005.12.036. [DOI] [PubMed] [Google Scholar]
- 34.Saxena S, Singh M, Engisch K, Fukuda M, Kaur S. Rab proteins regulate epithelial sodium channel activity in colonic epithelial HT-29 cells. Biochem Biophys Res Commun. 2005;337:1219–1223. doi: 10.1016/j.bbrc.2005.09.186. [DOI] [PubMed] [Google Scholar]
- 35.Goldenring JR, Smith J, Vaughan HD, Cameron P, Hawkins W, Navarre J. Rab11 is an apically located small GTP-binding protein in epithelial tissues. Am J Physiol. 1996;270:G515–G525. doi: 10.1152/ajpgi.1996.270.3.G515. [DOI] [PubMed] [Google Scholar]
- 36.McEwen DP, Schumacher SM, Li Q, Benson MD, Iniguez-Lluhi JA, Van Genderen KM, Martens JR. Rab-GTPase-dependent endocytic recycling of Kv1.5 in atrial myocytes. J Biol Chem. 2007;282:29612–29620. doi: 10.1074/jbc.M704402200. [DOI] [PubMed] [Google Scholar]