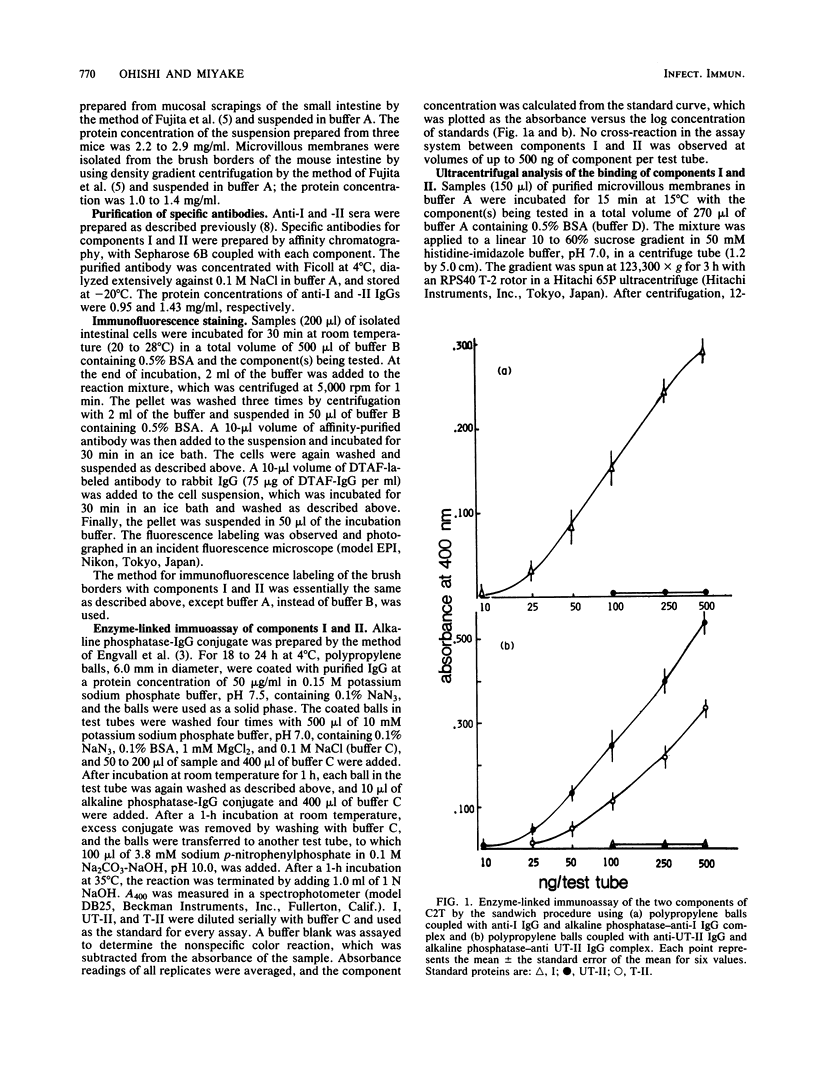
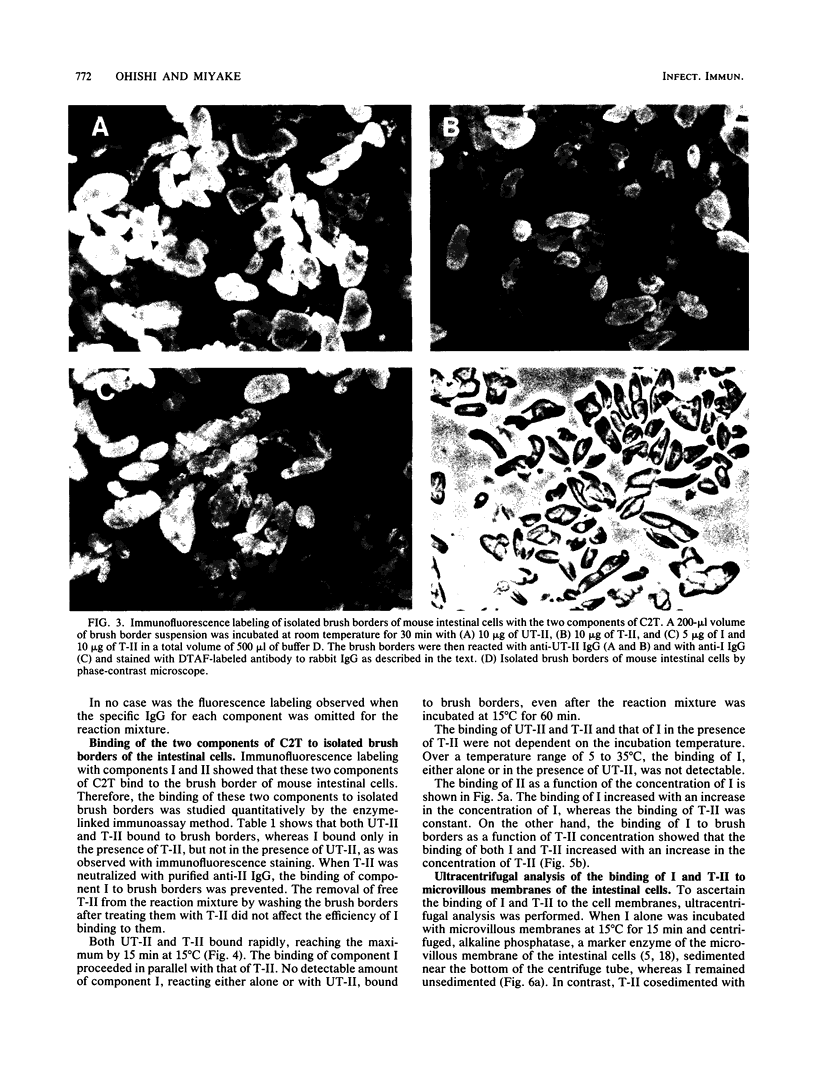
Abstract
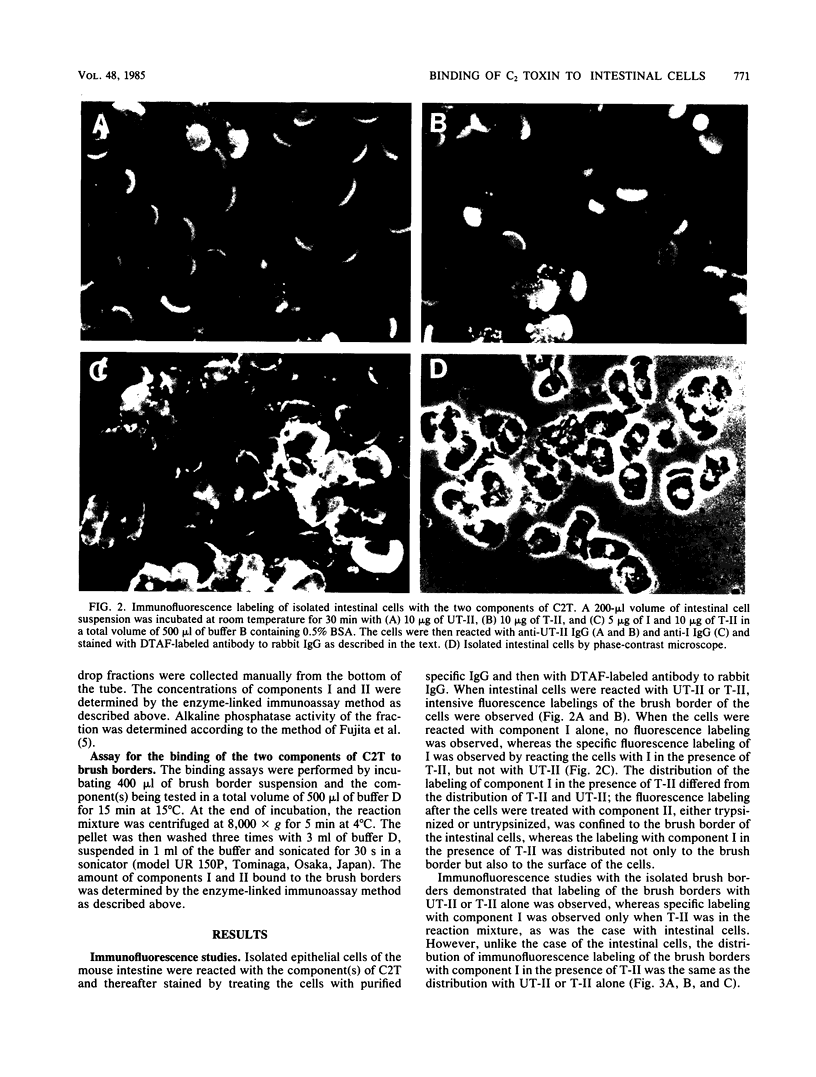
C2 toxin elaborated by Clostridium botulinum types C and D is composed of two nonlinked protein components and has enterotoxic activity, for which the cooperation of these two components is necessary. In the present study, the binding of components I and II, the two components of C2 toxin, to isolated epithelial cells and brush borders of mouse intestine was examined. Immunofluorescence studies showed that component II, either trypsinized (T-II) or untrypsinized (UT-II), bound to the cells and the brush borders of mouse intestine, whereas component I alone did not. The binding of I was observed only when the cells and the brush borders were reacted with T-II, but not when they were reacted with UT-II. These results are consistent with the fact that the biological activities of C2 toxin are elicited by the combination of I and T-II, but not of I and UT-II. The in vitro binding of I and II to isolated brush borders of mouse intestinal cells also showed similar binding characteristics. The binding of I and II to brush borders was rapid and not temperature dependent. Ultracentrifugal analysis revealed that both I and T-II bound to microvillous membranes of the intestinal cells. The data from the present study indicate that the enterotoxic activity of C2 toxin is initiated by the binding of T-II to the microvillous membrane of intestinal cells followed by that of I, for which the site of the cell membrane is induced by the binding of T-II, but not of UT-II.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- DasGupta B. R., Sugiyama H. A common subunit structure in Clostridium botulinum type A, B and E toxins. Biochem Biophys Res Commun. 1972 Jul 11;48(1):108–112. doi: 10.1016/0006-291x(72)90350-6. [DOI] [PubMed] [Google Scholar]
- Engvall E., Jonsson K., Perlmann P. Enzyme-linked immunosorbent assay. II. Quantitative assay of protein antigen, immunoglobulin G, by means of enzyme-labelled antigen and antibody-coated tubes. Biochim Biophys Acta. 1971 Dec 28;251(3):427–434. doi: 10.1016/0005-2795(71)90132-2. [DOI] [PubMed] [Google Scholar]
- Feinstein G. Interaction of insoluble protein inhibitors with proteases. Biochim Biophys Acta. 1970 Jul 27;214(1):224–227. doi: 10.1016/0005-2795(70)90088-7. [DOI] [PubMed] [Google Scholar]
- Fujita M., Ota H., Kawai K., Matsui H., Nakao M. Differential isolation of microvillous and basolateral plasma membranes from intestinal mucosa: mutually exclusive distribution of digestive enzymes and ouabain-sensitive ATPase. Biochim Biophys Acta. 1972 Aug 9;274(2):336–347. doi: 10.1016/0005-2736(72)90181-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Miyazaki S., Iwasaki M., Sakaguchi G. Clostridium botulinum type D toxin: purification, molecular structure, and some immunological properties. Infect Immun. 1977 Aug;17(2):395–401. doi: 10.1128/iai.17.2.395-401.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohishi I., Iwasaki M., Sakaguchi G. Purification and characterization of two components of botulinum C2 toxin. Infect Immun. 1980 Dec;30(3):668–673. doi: 10.1128/iai.30.3.668-673.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohishi I., Iwasaki M., Sakaguchi G. Vascular permeability activity of botulinum C2 toxin elicited by cooperation of two dissimilar protein components. Infect Immun. 1981 Mar;31(3):890–895. doi: 10.1128/iai.31.3.890-895.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohishi I. Lethal and vascular permeability activities of botulinum C2 toxin induced by separate injections of the two toxin components. Infect Immun. 1983 Apr;40(1):336–339. doi: 10.1128/iai.40.1.336-339.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohishi I. Response of mouse intestinal loop to botulinum C2 toxin: enterotoxic activity induced by cooperation of nonlinked protein components. Infect Immun. 1983 May;40(2):691–695. doi: 10.1128/iai.40.2.691-695.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohishi I., Sakaguchi G. Activation of botulinum toxins in the absence of nicking. Infect Immun. 1977 Aug;17(2):402–407. doi: 10.1128/iai.17.2.402-407.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohishi I., Sakaguchi G. Production of C2 toxin by Clostridium botulinum types C and D as determined by its vascular permeability activity. Infect Immun. 1982 Jan;35(1):1–4. doi: 10.1128/iai.35.1.1-4.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson L. L. A comparison of the pharmacological properties of Clostridium botulinum type C1 and C2 toxins. J Pharmacol Exp Ther. 1982 Dec;223(3):695–701. [PubMed] [Google Scholar]
- Syuto B., Kubo S. Isolation and molecular size of Clostridium botulinum type C toxin. Appl Environ Microbiol. 1977 Feb;33(2):400–405. doi: 10.1128/aem.33.2.400-405.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syuto B., Kubo S. Separation and characterization of heavy and light chains from Clostridium botulinum type C toxin and their reconstitution. J Biol Chem. 1981 Apr 25;256(8):3712–3717. [PubMed] [Google Scholar]
- Weiser M. M. Intestinal epithelial cell surface membrane glycoprotein synthesis. I. An indicator of cellular differentiation. J Biol Chem. 1973 Apr 10;248(7):2536–2541. [PubMed] [Google Scholar]




