Abstract
Autophagy is a conserved pathway that sequesters cytoplasmic material and delivers it to lysosomes for degradation. Digestion of portions of the cell interiors plays a key role in the recycling of nutrients, remodeling, and dispose of superfluous organelles. Along with its metabolic function, autophagy is an important mechanism for innate immunity against invading bacteria, parasites, and viruses. Multicellular organisms seem to have exploited autophagy to survey the interior of cells to eliminate intracellular pathogens that would otherwise grow in the cytoplasm. Surprisingly, autophagy is involved in the response to extracellular pathogens as well, following their engulfment by conventional phagocytosis. Possible links between these two forms of cellular “eating” represent a new dimension in host defense.
INTRODUCTION
Phagocytosis and macroautophagy (herein, called autophagy) are processes that are likely to have evolved originally to satisfy metabolic requirements of single-celled organisms, the former involved in engulfment of food while the latter is a highly conserved pathway for degradation and utilization of intracellular organelles and proteins for the recycling of nutrients. Intriguingly, both appear to have been subsequently exploited in multicellular organisms as host defense mechanisms. The role of phagocytosis in host defense against extracellular pathogens is well known1–4, and recent studies show that autophagy can target intracellular parasites for destruction (as reviewed herein). Perhaps such a role for autophagy in host defense is not surprising; intracellular parasites cause a drain on metabolic resources in the host, which in turn would trigger autophagy. Any resulting removal of the parasite, however fortuitous, would benefit the host. Therefore mechanisms that promote such autophagy and target it to an invader would subsequently be favored. Indeed, in response to infectious agents, host cells use autophagy to survey the invaders, and eradicate them from the cytoplasm. As with metabolic autophagy, autophagy wraps cytoplasmic microbes within a double-membrane-bound compartment redirecting the free cytoplasmic pathogen to bulk lysosomal degradation.
Interestingly, autophagy has also been associated with immunity against pathogens that do not proliferate in the cytoplasm of host cells. Recent studies have described new roles for autophagy in defense against extracellular pathogens that suggest the existence of collaborations between autophagy and phagocytosis.5–8 The role of the autophagy machinery as an enhancer of phagocytosis reveals that the components of the autophagy pathway may be more important players in defense than had been previously suspected. Here we review the role of autophagy in innate immunity and discuss recent studies that associate the autophagy machinery with the maturation of conventional phagosomes.
AUTOPHAGY IN IMMUNITY
Both autophagy and phagocytosis are cellular catabolic pathways that use lysosomes to digest particles; what makes these two cellular processes different is that autophagy marks material that was originally inside the cell for degradation, while phagocytosis engulfs extracellular particles. The immune system has adapted both cellular responses to the defense against microorganisms. The role of phagocytosis in defense is well known but lately much attention has been directed to those parasites that manage to escape or damage the phagosome to survive intracellularly. In those cases, the cell has a second line of defense, autophagy (which in this case might be regarded as “xenophagy”) that captures parasites that escape to the cytosol. During autophagy, an intracellular pathogen is docked by a nascent phagophore (autophagosomal isolation membrane), which eventually encloses the pathogen entirely within a double membrane. Later, lysosomes fuse with the autophagosome giving rise to a mature organelle, the autolysosome. The formation of such “defense autophagosomes” seems to require similar signals to those of metabolic autophagy. For instance, the PI3-Kinase inhibitors 3-MA and wortmannin blocked the sequestration of Coxiella burnetti9 and BCG6 within LC3 positive compartments. The activation of LC3 depends on a double ubiquitin-like protein conjugation system that produces the conversion of LC3-I to its lipidated form LC3-II. While metabolic autophagy is initiated by starvation or by any treatment that mimics that condition, the triggers of defense autophagy remain obscure. Although key immune cytokines like IFN-gamma6, TRAIL10, or TNF-alpha11 have been shown to increase autophagy and are correlated with increased immunity, it is still unknown how the phagophore targets intracellular pathogens and distinguishes them from the rest of the cytosolic material for degradation.
INTRACELLULAR PATHOGENS SURRENDER TO AUTOPHAGY
Pathogenic organisms can generally be considered to be intracellular or extracellular parasites. Some organisms enter cells via phagocytosis or by other mechanisms, then escape into the cytosol, while others persist in phagosomes or other specialized vesicles. Alternatively, a true extracellular parasite may be phagocytosed as a means to remove it, whereby intracellular processes may facilitate its destruction. Autophagy plays roles in defense under each of these conditions.
The first evidence of the role of autophagy in immunity was found in guinea pig polymorphonuclear cells infected with the intracellular bacterium Rickettsia conorii12. Infected cells were found to accumulate autophagosomes containing dense glycogen granules and Rickettsia cells, which appeared to be rapidly induced by the bacteria. The role of this autophagy-like process in bacterial pathogenesis or defense remains unclear, however. Since then, extensive evidence has accumulated that autophagy participates in defense against intracellular pathogens.
The initial signal that triggers the specific engulfment of the pathogen by autophagy remains unclear. It is hypothesized that the ubiquitination of cytosolic bacteria may be recognized by the phagophore, which could explain how the autophagic machinery discriminates between the parasite and other intracellular organelles that were not extensively ubiquitinized.13 Molecules that are exclusively expressed by the parasite could also be used by the autophagy machinery to find the invader. Listeria monocytogenes, after being phagocytosed manages to escape from the phagosome and uses cytosolic nutrients to grow.14–17 However, defensive autophagy is likely to target this organism, as the onset of intracellular growth is greatly accelerated in cells lacking components of the autophagy pathway, and the cytosolic bacteria are found associated with autophagosomes.7, 18 Bacterial Listeriolysin-O (LLO) appears to be required for the targeting of autophagy to the bacteria, and this molecule is involved in the escape from the phagosome. Thus, it may be that the damage to the phagosome is an initiating signal for autophagy. After escaping from the phagosome, Listeria uses the cellular machinery to move around inside the host cell by forming a comet-like tail that depends on actin cytoskeleton for propelling the bacterial movement within the host. Bacterial phospholipids, and bacterial expression of ActA, which induces actin polymerization, are used by Listeria to evade autophagy.14, 15
Franciscella tularensis, the causative agent of tularemia, also manages to escape from the phagosome, requiring autophagy to clear the infection.19, 20 The mechanism by which Franciscella evades the phagosome is poorly understood. Only the 23 kDa protein IglC, which shows no similarity to other proteins has been related to the phenomena but its function is still elusive.21 However, Franciscella is known to engage the inflammasome signaling pathway via a NOD-like receptor (NLR), cryopyrin/NALP322. It has been proposed that such NLRs may enlist the autophagy pathway23 and it is possible that this represents a way in which intracellular pathogens may be marked for degradation. This intriguing possibility remains to be tested.
Some pathogens transform the phagosome or other intracellular compartments into protective environments within the cell, where they survive and reproduce. In the case of parasites that remain in the lumen of a vesicle, there is growing evidence indicating that modifications to (or damage of) the host vesicle surrounding the parasite could trigger the normal autophagy response to recycle the damage organelle and ultimately eliminating the parasite that resides within.24 For instance, Salmonella Typhimurium proliferates inside host vesicles that are called SCV (Salmonella containing vesicle). To establish an intracellular niche, S. Typhimurium secretes bacterial proteins into the host cell via needle-like appendages at the bacterial surface called type III secretion system (TTSSs). TTSS secretions are known to damage eukaryotic cell membranes that ultimately activate an autophagic response to recycle the damaged organelle, which in turn would also kill the Salmonella cells.19, 25
In defense against the protozoan Toxoplasma gondii, the parasite is found in the lumen of autophagosomes when it escapes from the phagosome24, but it can also be destroyed by autophagy when it is in the lumen of a phagosome.5 Thus, in this case, autophagy appears to target the parasite in multiple ways.
Intriguingly, parasites that survive in phagosomes can be targeted when metabolic autophagy is induced. Mycobacterium Tuberculosis readily persists inside phagosomes but was found in autophagosomes when autophagy was induced by rapamycin or starvation conditions.6 This corresponded to active destruction of the mycobacteria within the phagosomes. The autophagy mediated destruction of Mycobacteria cells depended on Irgm1 (LRG-47), a GTPase that previously was known to protect against mycobacterium infections.6
The importance of autophagy in immunity is evident by the development of pathogen countermeasures to evade destruction in the autophagosome.13 Shigella can escape from phagosomes, once it is cytosolic, it can also evade engulfment by autophagy by secreting the protein IcsB.18 Pathogens like Brucella abortus26, 27,Porphyromonas gingivalis28, 29, Coxiella burnetti30, 31, and Legionella pneumophila32, 33 subvert the autophagosome by blocking the fusion of lysosomes with the organelle.34
AUTOPHAGY IN THE DEGRADATION OF EXTRACELLULAR MICROBES
The role of autophagy in clearance of pathogens is not restricted to intracellular microbes. The fate of group A Streptococci, an extracellular bacteria, after phagocytosis has long been controversial. A recent report has shown that bacterial secretion of streptolysin O induces the clearance of the pathogen by autophagy.7 Streptolysin O is a monomeric water-soluble protein that binds host cholesterol polymerizing and forming ring-like structures that open pores with a inner diameter of 35 nm.35 How streptolysin O promotes autophagy is unclear but it is possible that by damaging the phagosome it recruits the autophagy machinery, removing both the phagosome and the parasite.
We have found that engagement of TLR signaling during phagocytosis of an extracellular organism usurps the autophagy pathway to conjugate LC3 directly to the phagosome, apparently without the formation of an autophagosome structure.8 In contrast to the observations on autophagy of M. tuberculosis,6 discussed above, induction of metabolic autophagy by starvation or rapamycin did not promote the recruitment of LC3 to the phagosome. Instead, this extremely rapid process (about 10 min) requires TLR signaling from within the phagosome. The association of LC3 with the phagosome requires ATG5 and ATG7, and is preceded by association of Beclin-1 and PI3-kinase activity. As a result, enhanced fusion of the phagosome to lysosomes occurs, presumably through the same mechanisms by which autophagosome-lysosome fusion is facilitated, and promotes the destruction of the engulfed organism. A role for TLR signaling in enhancing maturation and acidification of phagosomes was recently demonstrated36, although no mechanism was provided for this effect. The recruitment of the autophagy machinery to the phagosome may be such a mechanism (Figure 1).
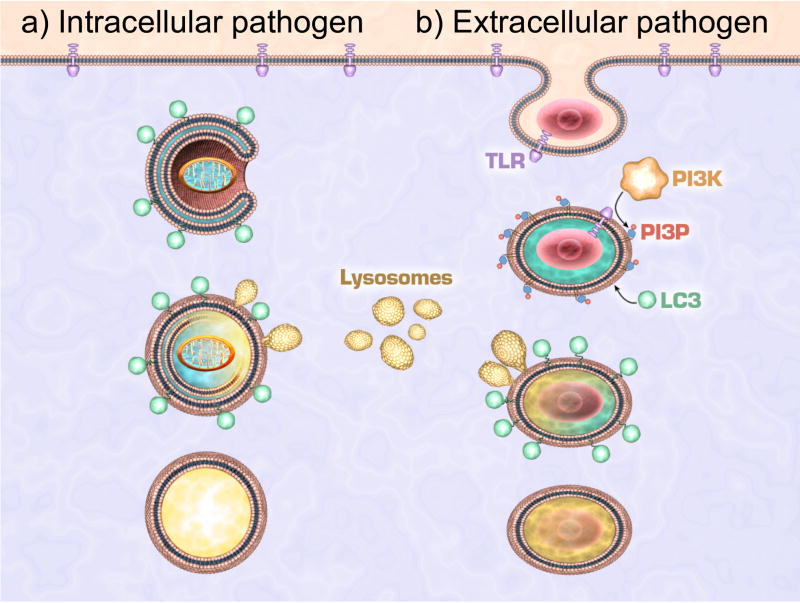
Figure 1. Autophagy machinery in the elimination of intracellular and extracellular pathogens.
(a) Intracellular pathogens that are either free in the cytosol or inside a damaged phagosome are surrounded by an isolation double-membrane. The resulting autophagosome is decorated with LC3 and/or other autophagic molecules that attract lysosomes. Upon fusion these will generate a mature autolysosome. (b) Phagocytosis of pathogens that activate TLR directly recruits LC3 and other autphagic molecules to the phagosome. These promote the fusion of lysosomes that enhance the maturation of phagosomes to phagolysosomes, thus linking the phagocytosis and autophagy pathways.
Signaling via TLR can also induce what appears to be conventional autophagy, although in this case the function is less clear. Cells stimulated for several hours with ligands for TLR437 or other TLRs8 showed appearance of autophagosomes. It is possible that under some conditions (such as described for M. tuberculosis, perhaps), TLR signals may directly or indirectly (e.g., via the autocrine production of TLR-induced cytokines such as TNF) facilitate targeting of intracellular pathogens and/or phagosomes by autophagy.
If association of the autophagy machinery to the phagosome enhances lysosomal clearance of the contents, why need this be coupled to TLR signaling? That is, why aren’t all phagosomes simply targeted by the autophagic pathway as a constitutive process? While answering such teleological questions is always fraught with perils (leading to “Just So” stories), it may be interesting to reflect that both phagocytosis and autophagy almost certainly arose to meet metabolic demands of unicellular organisms, when food is present or limited, respectively. Since autophagy, by reducing intracellular proteins and organelles, necessarily restricts cell division, one might expect that when phagocytosis is occurring (and thus providing metabolic energy) the autophagic pathway would be inhibited (indeed, AKT inhibits autophagy via mTOR38). In linking the autophagic pathway to phagocytosis, as we suggested, it would therefore be necessary to provide a signal that indicates that the engulfed particle is a potential pathogen. The TLRs perform this recognition function.39 Thus, autophagy is not simply augmented (that is, we found no evidence of a general increase in autophagy, per se, in cells in which TLRs were activated) but instead, the autophagy pathway is specifically directed to those membranes where TLR signaling has occurred.
CONCLUSION
Autophagy is a key cellular catabolic pathway that has further been utilized as a defense mechanism by multicellular organisms. Host cells activate autophagy pathways for the elimination of invading microorganisms, but the activation of TLR by pathogens demonstrates that the autophagy machinery could also be directly recruited to phagosomes. We are just scratching the surface on understanding the intricate relationship between host and parasites but it is becoming clearer that cells can respond with creative ways to the increasingly sophisticated attacks of pathogens by using autophagy as the ultimate weapon against the invading activities of infectious microorganisms.
Acknowledgments
The authors thank Joshua R. Stokes at the St. Jude Children’s Research Hospital Biomedical Communications shared resource facility for the artistic design of the figure showed herein.
References
- 1.Blander JM. Signalling and phagocytosis in the orchestration of host defence. Cell Microbiol. 2007;9:290–9. doi: 10.1111/j.1462-5822.2006.00864.x. [DOI] [PubMed] [Google Scholar]
- 2.Desjardins M, Houde M, Gagnon E. Phagocytosis: the convoluted way from nutrition to adaptive immunity. Immunol Rev. 2005;207:158–65. doi: 10.1111/j.0105-2896.2005.00319.x. [DOI] [PubMed] [Google Scholar]
- 3.Russell DG, Yates RM. Toll-like receptors and phagosome maturation. Nat Immunol. 2007;8:217. doi: 10.1038/ni0307-217a. author reply -8. [DOI] [PubMed] [Google Scholar]
- 4.Stuart LM, Ezekowitz RA. Phagocytosis: elegant complexity. Immunity. 2005;22:539–50. doi: 10.1016/j.immuni.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Andrade RM, Portillo JA, Wessendarp M, Subauste CS. CD40 signaling in macrophages induces activity against an intracellular pathogen independently of gamma interferon and reactive nitrogen intermediates. Infect Immun. 2005;73:3115–23. doi: 10.1128/IAI.73.5.3115-3123.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gutierrez MG, Master SS, Singh SB, Taylor GA, Colombo MI, Deretic V. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell. 2004;119:753–66. doi: 10.1016/j.cell.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 7.Nakagawa I, Amano A, Mizushima N, Yamamoto A, Yamaguchi H, Kamimoto T, Nara A, Funao J, Nakata M, Tsuda K, Hamada S, Yoshimori T. Autophagy defends cells against invading group A Streptococcus. Science. 2004;306:1037–40. doi: 10.1126/science.1103966. [DOI] [PubMed] [Google Scholar]
- 8.Sanjuan MA, Dillon CP, Tait SWG, Moshiach S, Dorsey F, Connell S, Komatsu M, Tanaka K, Cleveland JL, Withoff S, Green DR. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature. 2007;450:1253–7. doi: 10.1038/nature06421. [DOI] [PubMed] [Google Scholar]
- 9.Beron W, Gutierrez MG, Rabinovitch M, Colombo MI. Coxiella burnetii localizes in a Rab7-labeled compartment with autophagic characteristics. Infect Immun. 2002;70:5816–21. doi: 10.1128/IAI.70.10.5816-5821.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mills KR, Reginato M, Debnath J, Queenan B, Brugge JS. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is required for induction of autophagy during lumen formation in vitro. Proc Natl Acad Sci U S A. 2004;101:3438–43. doi: 10.1073/pnas.0400443101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Subauste CS, Andrade RM, Wessendarp M. CD40-TRAF6 and autophagy-dependent anti-microbial activity in macrophages. Autophagy. 2007;3:245–8. doi: 10.4161/auto.3717. [DOI] [PubMed] [Google Scholar]
- 12.Rikihisa Y. Glycogen autophagosomes in polymorphonuclear leukocytes induced by rickettsiae. Anat Rec. 1984;208:319–27. doi: 10.1002/ar.1092080302. [DOI] [PubMed] [Google Scholar]
- 13.Ogawa M, Sasakawa C. Bacterial evasion of the autophagic defense system. Curr Opin Microbiol. 2006;9:62–8. doi: 10.1016/j.mib.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Birmingham CL, Canadien V, Gouin E, Troy EB, Yoshimori T, Cossart P, Higgins DE, Brumell JH. Listeria monocytogenes evades killing by autophagy during colonization of host cells. Autophagy. 2007;3:442–51. doi: 10.4161/auto.4450. [DOI] [PubMed] [Google Scholar]
- 15.Py BF, Lipinski MM, Yuan J. Autophagy limits Listeria monocytogenes intracellular growth in the early phase of primary infection. Autophagy. 2007;3:117–25. doi: 10.4161/auto.3618. [DOI] [PubMed] [Google Scholar]
- 16.Rich KA, Burkett C, Webster P. Cytoplasmic bacteria can be targets for autophagy. Cell Microbiol. 2003;5:455–68. doi: 10.1046/j.1462-5822.2003.00292.x. [DOI] [PubMed] [Google Scholar]
- 17.Webster P. Cytoplasmic bacteria and the autophagic pathway. Autophagy. 2006;2:159–61. doi: 10.4161/auto.2826. [DOI] [PubMed] [Google Scholar]
- 18.Ogawa M, Yoshimori T, Suzuki T, Sagara H, Mizushima N, Sasakawa C. Escape of intracellular Shigella from autophagy. Science. 2005;307:727–31. doi: 10.1126/science.1106036. [DOI] [PubMed] [Google Scholar]
- 19.Checroun C, Wehrly TD, Fischer ER, Hayes SF, Celli J. Autophagy-mediated reentry of Francisella tularensis into the endocytic compartment after cytoplasmic replication. Proc Natl Acad Sci U S A. 2006;103:14578–83. doi: 10.1073/pnas.0601838103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clemens DL, Horwitz MA. Uptake and intracellular fate of Francisella tularensis in human macrophages. Ann N Y Acad Sci. 2007;1105:160–86. doi: 10.1196/annals.1409.001. [DOI] [PubMed] [Google Scholar]
- 21.Golovliov I, Sjostedt A, Mokrievich A, Pavlov V. A method for allelic replacement in Francisella tularensis. FEMS Microbiol Lett. 2003;222:273–80. doi: 10.1016/S0378-1097(03)00313-6. [DOI] [PubMed] [Google Scholar]
- 22.Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–32. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 23.Levine B, Deretic V. Unveiling the roles of autophagy in innate and adaptive immunity. Nat Rev Immunol. 2007;7:767–77. doi: 10.1038/nri2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ling YM, Shaw MH, Ayala C, Coppens I, Taylor GA, Ferguson DJ, Yap GS. Vacuolar and plasma membrane stripping and autophagic elimination of Toxoplasma gondii in primed effector macrophages. J Exp Med. 2006;203:2063–71. doi: 10.1084/jem.20061318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Birmingham CL, Brumell JH. Autophagy recognizes intracellular Salmonella enterica serovar Typhimurium in damaged vacuoles. Autophagy. 2006;2:156–8. doi: 10.4161/auto.2825. [DOI] [PubMed] [Google Scholar]
- 26.Pizarro-Cerda J, Meresse S, Parton RG, van der Goot G, Sola-Landa A, Lopez-Goni I, Moreno E, Gorvel JP. Brucella abortus transits through the autophagic pathway and replicates in the endoplasmic reticulum of nonprofessional phagocytes. Infect Immun. 1998;66:5711–24. doi: 10.1128/iai.66.12.5711-5724.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pizarro-Cerda J, Moreno E, Sanguedolce V, Mege JL, Gorvel JP. Virulent Brucella abortus prevents lysosome fusion and is distributed within autophagosome-like compartments. Infect Immun. 1998;66:2387–92. doi: 10.1128/iai.66.5.2387-2392.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dorn BR, Dunn WA, Jr, Progulske-Fox A. Bacterial interactions with the autophagic pathway. Cell Microbiol. 2002;4:1–10. doi: 10.1046/j.1462-5822.2002.00164.x. [DOI] [PubMed] [Google Scholar]
- 29.Rodrigues PH, Belanger M, Dunn W, Jr, Progulske-Fox A. Porphyromonas gingivalis and the autophagic pathway: an innate immune interaction? Front Biosci. 2008;13:178–87. doi: 10.2741/2668. [DOI] [PubMed] [Google Scholar]
- 30.Gutierrez MG, Vazquez CL, Munafo DB, Zoppino FC, Beron W, Rabinovitch M, Colombo MI. Autophagy induction favours the generation and maturation of the Coxiella-replicative vacuoles. Cell Microbiol. 2005;7:981–93. doi: 10.1111/j.1462-5822.2005.00527.x. [DOI] [PubMed] [Google Scholar]
- 31.Romano PS, Gutierrez MG, Beron W, Rabinovitch M, Colombo MI. The autophagic pathway is actively modulated by phase II Coxiella burnetii to efficiently replicate in the host cell. Cell Microbiol. 2007;9:891–909. doi: 10.1111/j.1462-5822.2006.00838.x. [DOI] [PubMed] [Google Scholar]
- 32.Dubuisson JF, Swanson MS. Mouse infection by Legionella, a model to analyze autophagy. Autophagy. 2006;2:179–82. doi: 10.4161/auto.2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Otto GP, Wu MY, Clarke M, Lu H, Anderson OR, Hilbi H, Shuman HA, Kessin RH. Macroautophagy is dispensable for intracellular replication of Legionella pneumophila in Dictyostelium discoideum. Mol Microbiol. 2004;51:63–72. doi: 10.1046/j.1365-2958.2003.03826.x. [DOI] [PubMed] [Google Scholar]
- 34.Kirkegaard K, Taylor MP, Jackson WT. Cellular autophagy: surrender, avoidance and subversion by microorganisms. Nat Rev Microbiol. 2004;2:301–14. doi: 10.1038/nrmicro865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palmer M, Valeva A, Kehoe M, Bhakdi S. Kinetics of streptolysin O self-assembly. Eur J Biochem. 1995;231:388–95. doi: 10.1111/j.1432-1033.1995.tb20711.x. [DOI] [PubMed] [Google Scholar]
- 36.Blander JM, Medzhitov R. Regulation of phagosome maturation by signals from toll-like receptors. Science. 2004;304:1014–8. doi: 10.1126/science.1096158. [DOI] [PubMed] [Google Scholar]
- 37.Xu Y, Jagannath C, Liu XD, Sharafkhaneh A, Kolodziejska KE, Eissa NT. Toll-like receptor 4 is a sensor for autophagy associated with innate immunity. Immunity. 2007;27:135–44. doi: 10.1016/j.immuni.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005;17:596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 39.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]