Abstract
The mitochondrial pathway of cell death, in which apoptosis proceeds following mitochondrial outer membrane permeablization (MOMP), release of cytochrome c, and APAF-1 apoptosome-mediated caspase activation, represents the major pathway of physiological apoptosis in vertebrates. However, the well-characterized apoptotic pathways of the invertebrates C. elegans and D. melanogaster indicate that this apoptotic pathway is not universally conserved among animals. This review will compare the role of the mitochondria in the apoptotic programs of mammals, nematodes, and flies, and will survey our knowledge of the apoptotic pathways of other, less familiar model organisms in an effort to explore the evolutionary origins of the mitochondrial pathway of apoptosis.
The events that define apoptosis in all animal cells are mediated by the actions of the caspase proteases. The mechanisms, however, by which apoptotic stimuli are integrated and translated into caspase activation differ markedly among the species in which the apoptotic pathways have been elucidated. In most mammalian cells, caspase activation and apoptosis proceed after mitochondrial outer membrane permeabilization. A wide range of signals converge on the mitochondria, and MOMP is observed in response to apoptotic stimuli as diverse as DNA damage, nutrient deficiency, ER stress, growth factor withdrawal, heat shock and developmental cues. While a central role for the mitochondria is well recognized in mammalian apoptosis, it is worthwhile to take an unclouded look at what remains a fundamentally surprising and intriguing finding: that our cells have evolved a coordinated program to rupture an indispensable organelle, and that once this occurs an apparently innocuous piece of the respiratory machinery—cytochrome c--assembles a group of proteins lurking in the cytosol to bring about the rapid and total destruction of the cell. When examining the minutiae of this process, we often fail to step back and contemplate why these events take place as they do. When did the centrality of the mitochondria arise? How conserved is the role of the mitochondria in general, and phenomenon of MOMP in particular, among metazoans? The well-characterized death programs of C. elegans and D. melanogaster provide fundamental insight, but the experienced researcher will regard trends based on two data points with a jaundiced eye. Much-needed additional information is beginning to become available from non-canonical invertebrate model organisms; herein we shall present a comparison between the role of the mitochondria in the apoptotic pathways of mammals, nematodes and flies, and begin working to fill in the sizable gaps between these phylogenically disparate animals with a summary of the information gleaned thus far from more obscure creatures (at least from the point of view of apoptosis research). We will take a self-centered approach and use the pathways and processes of mammalian apoptosis as a referential baseline, so let us begin by reviewing these pathways with an eye to molecules and mechanisms that bear comparison to those of other species.
In mammals, the intrinsic pathway of apoptosis is initiated by MOMP, which leads to diffusion of the contents of the mitochondrial intermembrane space into the cytosol. MOMP is controlled by the Bcl-2 family of proteins, which is divided between pro-apoptotic (MOMP-promoting) and anti-apoptotic (MOMP-inhibiting) members. Members of the Bcl-2 family share up to four Bcl-2 homology (BH) domains; chief among the pro-apoptotic Bcl-2 proteins are the multidomain proteins Bax and Bak. These contain BH-1, -2, and -3 domains, and knockout studies have identified them as being indispensable for MOMP; their activation is believed to lead to MOMP via a conformational change, inducing their oligomerization and insertion into the outer mitochondrial membrane (OMM), where they form large pores through which proteins from the intermembrane space can access the cytosol. Bax and Bak activation is controlled, in turn, but the complex interplay between the other members of the Bcl-2 family, including the anti-apoptotic multidomain proteins Bcl-2, Bcl-xL, and MCL-1, and the numerous BH-3-only proteins. Though the specifics of this process remain somewhat controversial, it is thought that the anti-apoptotic multidomain proteins inhibit Bax and Bak by binding to and sequestering the BH-3 only proteins capable of inducing the activating conformational change in Bax and Bak, as well as active Bax and Bak themselves, while another subset of BH-3 only proteins act by releasing this inhibition1.
Once MOMP has taken place, proteins heretofore sequestered within the mitochondria gain access to the cytosol. Among these is cytochrome c, which binds to the WD domain repeats of the cytosolic protein APAF-1. This binding, along with that of dATP, causes a conformational change in APAF-1, which in turn leads to its oligomerization into a septameric structure called the apoptosome2. The conformational change induced in APAF-1 upon binding to cytochrome c also exposes the former’s caspase activation and recruitment domain (CARD), which then recruits and dimerizes caspase-9 via interaction with the CARD in that molecule’s prodomain. Caspase-9 is an apical caspase, and as such exists as an inactive monomer in the cytosol of non-apoptotic cells. This caspase, like the apical caspases-2, -8 and -10, has a long N-terminal prodomain, which contains a protein interaction motif. Apical caspases are actived by dimerization3; when two caspase-9 monomers are pulled into proximity by the oligomerized CARD domains of the apoptosome, they dimerize and undergo interchain autocatlytic cleavage, yielding a mature, active caspase. This protease then activates the executioner caspases, caspase-3 and -7. Executioner caspases are present as inactive dimers in the cytosol, and lack a long prodomain; they are activated by interchain processing by an apical caspase, and once active lead to the destruction of the cell that we observe as apoptosis. (Figure 1)
Figure 1.
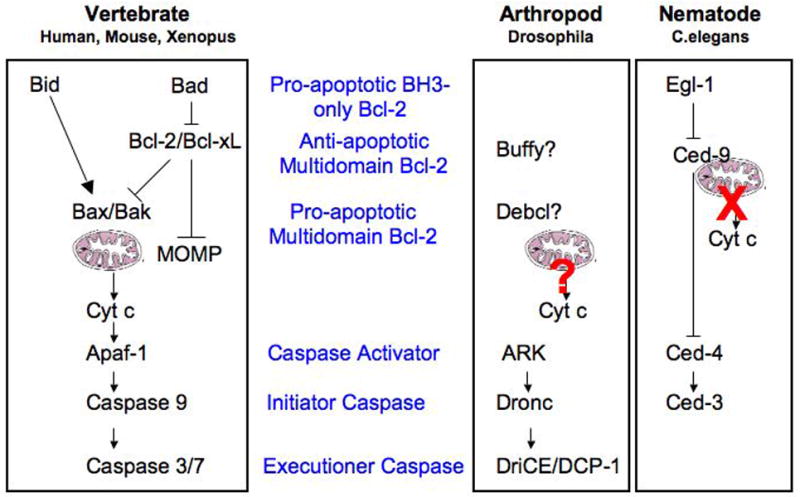
Mammalian cytochrome-c dependent apoptotic pathways and their homologs in flies and worms. Leftmost panel depicts the proteins upstream and downstream of cytochrome c release in mammals. Panels on the right show homologs of these proteins found in Drosophila and C.elegans; the degree to which homologs conserve the function of their mammalian counterparts varies, and is summarized in the text. Cytochrome c release is controversial in the fly, and is not observed in the worm. Note that this figure depicts only cytochrome c dependent caspase activation; effects of IAPs and their inhibitors, as well as other released mitochondrial factors, are omitted.
In addition to cytochrome c, several additional factors released upon MOMP have been implicated in apoptosis. Notably, Smac/DIABLO and Omi/HtrA2 bind to and inhibit the inhibitor of apoptosis (IAP) proteins. The IAPs were first identified in baculoviruses 4, and feature 1–3 copies of a zinc binding domain, the BIR domain. These domains allow some IAPs to bind to and inhibit caspases; XIAP is a strong in vitro inhibitor of caspases-3, -7 and-9, while cIAP1 and 2 are weaker caspase inhibitors 5. XIAP overexpression prevents apoptosis in cultured cells 6,7 and IAPs are often upregulated in human cancers, which initially led researchers to posit a model in which IAPs block basal caspase activity in healthy cells, thereby preventing “accidental” cell death until MOMP occurs, at which point Smac and Omi bind to the IAPs, releasing the caspases and allowing apoptosis to proceed. However, this model was not supported by in vivo mouse studies, which found that genetic ablation of smac, omi, or XIAP failed to produce a clear apoptotic phenotype8–10. Nonetheless, IAP inhibition via small-molecule Smac mimetics has shown remarkable promise as a selective anti-cancer therapy. Recent reports 11–13 have indicated that apoptosis in response to IAP inhibition occurs not due to release of caspases-9 and -3 by XIAP, but rather in a caspase-8 and TNF dependent fashion following autoubiquitination and degradation of cIAP1 and -2. Despite these encouraging findings, the relevance of the Smac/Omi-IAP pathway in the apoptosis of normal mammalian cells remains unclear.
Nematodes: like us, only different
The study of apoptosis in the nematode worm, C.elegans, has an illustrious past; it was the observation that precisely 131 of the 1090 cells generated in the development of an adult hermaphrodite worm die in a predictable, repeatable way that helped to inaugurate the field of apoptosis research at the molecular level. A landmark finding was that programmed cell death in C.elegans is under genetic control14, and subsequent genetic analyses identified several genes responsible for cell death defective (ced) phenotypes. Chief among these were ced-3, ced-4, and egg laying defective (egl)-1, in which loss-of-function mutations led to survival of nearly all the cells programmed to die, and ced-9, in which loss of function leads to excessive apoptosis. Sequence comparison revealed that the protein products of these genes bear striking resemblances to the major players in mammalian apoptosis, and indeed the mechanisms of nematode apoptosis are in many respects similar to those described above for mammals.
The CED-9 protein is an anti-apoptotic protein with four BH domains, homologous to Bcl-2. It is anchored to the mitochondria, and in healthy cells is associated with a dimer of CED-4. CED-4 is homologous to mammalian APAF-1, and like APAF-1 it contains a CARD, though importantly it lacks the WD domain repeats that mediate APAF-1’s interaction with cytochrome c. Developmental cues in the worm lead to upregulation of EGL-1, which is a BH-3 only protein; this protein binds to CED-9, disrupting its interaction with CED-4. CED-4 is then free to form a tetrameric apoptosome-like structure, which recruits CED-3. CED-3 is a CARD-containing caspase, most closely homologous to mammalian caspase-2; C.elegans lacks the initiatior-executioner caspase hierarchy observed in mammalian cells, so the catalytic activity of CED-3 is directly responsible for cellular disruption (for review see 15). (Figure 1)
While many of the players in nematode apoptosis have mammalian homologs, the way in which they interact differs in several important ways. Most obviously, neither MOMP nor cytochrome c plays a role in initiation of apoptosis in the nematode, though the mitochondrially localized proteins CPS-6 and WAH-1 (nematode homologs of mammalian endonuclease-G and AIF, respectively) have been suggested to contribute to DNA degradation and phosphatydilserine externalization later in the apoptotic program16–19. As noted, CED-4 lacks the WD domain repeats present in APAF-1 that are necessary for cytochrome c binding, and the release of CED-4 from sequestration by CED-9 appears to be sufficient to cause formation of the nematode apoptosome. Furthermore, despite early reports to the contrary20,21 it is now accepted that mammalian Bcl-2 homologs do not functionally interact with APAF-122–24.
Given these significant differences, it is intriguing that the anti-apoptotic functions of both Bcl-2 and CED-9 involve the mitochondria. Indeed, the functions of the mammalian Bcl-2 family members and those of CED-9/EGL-1 overlap in a number of interesting ways: Despite sharing only around 22% homology with CED-9, expression of Bcl-2 inhibits apoptosis in C.elegans 25, and Bcl-2 expression was found to rescue the excess of apoptosis found in CED-9 loss-of-function worms 26. This is particularly surprising in light of the inability of Bcl-2 to interact with APAF-1. How, then, is Bcl-2, which acts by preventing MOMP, able to substitute for CED-9, whose anti-apoptotic effect is exerted through sequestration of the nematode APAF-1 homolog CED-4? A more recent study 27 found that Bcl-2 does not interact with CED-4 in a yeast two hybrid screen; it does, however, interact with EGL-1. Furthermore, when the basic nematode apoptotic program is recapitulated in yeast, CED-9, but not Bcl-2, was able to prevent lethality caused by CED-3/CED-4 co-expression. The authors proposed that Bcl-2 prevents cell death in wild-type nematodes by binding EGL-1 via that protein’s BH-3 domain, and that the ability of Bcl-2 to rescue the apoptotic phenotype of CED-9-deficient worms could be due to Bcl-2 preventing EGL-1 from neutralizing the small amount of maternally derived CED-9 present at the developmental stage at which the original experiments were performed 26–28.
Despite their frequent depiction as isolated, globular organelles, in healthy cells the mitochondria form a complex and interconnected network, subject to frequent fusion, fission, and remodeling events (reviewed in 29). Imaging of mammalian cells undergoing apoptosis has revealed that mitochondria fragment during MOMP; subsequent studies have lent credence to the idea that mitochondrial dynamics and MOMP are intimately linked, with increased mitochondrial fission leading to sensitivity to MOMP and increased fusion blocking or delaying it 30–32. While both pro- and anti-apoptotic proteins of the Bcl-2 family have been implicated in these processes, causal links have been difficult to draw, due in part to the challenge of distinguishing between their roles in mitochondrial dynamics and their roles in apoptosis. Intriguingly, given the lack of MOMP in the apoptotic program of C.elegans, it appears that mitochondrial dynamics do play a role in nematode cell death. Jagasia et al. 33 found that EGL-1 causes mitochondrial fragmentation in apoptotic nematode cells, and that this function is antagonized by CED-9; furthermore, upregulation of the mitochondrial fission protein Drp-1 was necessary and partially sufficient to induce apoptosis. Surprisingly, Delvani and colleagues 34 found that the roles of these C.elegans proteins in mitochondrial dynamics extend to mammalian cells. Expression of CED-9 in mammalian cells causes mitochondrial fusion, while EGL-1 co-expression antagonized this function, leading to mitochondrial fragmentation. Perhaps most interestingly, neither CED-9-mediated fusion nor EGL-1-mediated fission affected the dynamics or timing of cytochrome c release. These findings indicate that CED-9 and EGL-1 play a role in mitochondrial dynamics independent of their apoptotic function in regulating the release of CED-4, as well as implying that changes in mitochondrial morphology per se do not affect sensitivity to MOMP in mammalian cells. They also raise questions about the idea that mitochondrial remodeling is required for efficient release of intermembrane space proteins, as nematode cells remodel their mitochondria in similar ways in the absence of MOMP. Finally, it is unclear what role mitochondrial fragmentation plays in C.elegans apoptosis, since the only known role for mitochondria in the process is as localization platforms for sequestration of CED-4 by CED-9.
Conservation of a mitochondrial role in cell death: a fly in the ointment?
Let us now turn our attention to Drosophila melanogaster, the third model organism in which the particulars of the apoptotic pathway have been worked out in some detail. The Drosophila caspase family comprises seven members, and like mammalian caspases (but unlike the nematode), these can be divided into initiator and executioner caspases based upon the presence or absence of long N-terminal pro-domains. DRONC has emerged as the essential apoptotic initiator caspase in Drosophila; like mammalian caspases 2 and 9, as well as CED-3, DRONC contains an N-terminal CARD, through which it interacts with ARK, the fly homolog of APAF-1 and CED-4. Once activated, DRONC cleaves DRICE and DCP-1, Drosophila executioner caspases homologous to mammalian caspase-3.(Figure 1) Unlike APAF-1, however, ARK does not appear to require cytochrome c to activate DRONC. Instead, the Drosophila apoptotic program is controlled by DIAP1, a fly homolog of the mammalian IAP proteins. DIAP1 contains two Zinc-coordinating BIR domains, which allow it to bind to DRONC, keeping that caspase’s activity in check. Apoptosis is initiated by the DIAP1 antagonists HID, Grim and Reaper, which act by binding to DIAP1 and inducing its autoubiquitination and proeasome-mediated degradation (reviewed in 35–37). Cells in which DIAP1 is ablated show spontaneous apoptosis, indicating that removal of the inhibitory function of DIAP1 is sufficient to initiate the apoptotic program in flies. It is worth noting that this stands in contrast to the situation in mammals, where genetic ablation of the IAPs does not produce an overt apoptotic phenotype. Though IAPs and their antagonists are present in mammals, they do not appear to play the central role in the control of apoptosis found in the fly. Smac/DIABLO and Omi/HtrA2, the mammalian IAP antagonists, do not show sequence homology to HID, Grim or Reaper; however, fly and mammalian IAP antagonists do share an unmodified N-terminal alanine, generated by proteolytic cleavage in the case of Smac and Omi, and removal of the initiator methionine in Grim, Reaper and HID38. This functional group is also present in small-molecule Smac mimetics, and is believed to be required for neutralization of BIR domain-mediated inhibition. Smac and Omi also differ from fly IAP antagonists in the mechanism by which they are unleashed. In the fly, HID, Grim and Reaper are transcriptionally upregulated in response to developmental cues in most cell types; transcriptional upregulation of ARK and DRONC may also be involved in evasion of DIAP1-mediated caspase inhibition39. By contrast, in mammalian apoptosis Smac and Omi are released from the mitochondria upon MOMP, along with cytochrome c.
This observation highlights a thorny question: what role, if any, do the mitochondria play in Drosophila apoptosis? ARK is structurally more similar to APAF-1 than to CED-4, and it contains several of the WD domain repeats, absent in CED-4, through which APAF-1 binds cytochrome c during formation of the mammalian apoptosome; however, the role of cytochrome c in Drosophila apoptosis remains controversial. The finding that DIAP1 ablation is sufficient to cause caspase activation indicates that ARK is constitutively able to activate DRONC, independent of mitochondrial factors40. The structure of a putative ARK apoptosome has been solved using electron cryo-microscopy to a resolution of 18.8Å, and this structure does not include cytochrome c, though it does conserve the requirement for dATP found in the mammalian apoptosome41.
Additional biochemical and genetic data has also indicated that apoptosome formation and caspase activation may be cytochrome c independent in Drosophila, though this has been the topic of some debate. In one study, silencing expression of either or both Drosophila cytochrome c proteins was found not to affect apoptosis in the fly, and addition of either recombinant forms of these proteins or whole mitochondrial extracts to cell-free systems failed to significantly activate caspases in Drosophila cell lysates, though addition of recombinant Drosophila cytochrome c does yield caspase activation in mammalian lysates42–44. However, studies on flies lacking d-cyt-c, one of the Drosophila cytochrome c genes, found severely delayed apoptosis in the developing retina45, and a genetic screen found that mutations in d-cyt-c reduced caspase activation during spermatid individualization46, an effect phenocopied by ARK or DRONC loss-of-function mutations. An earlier study found that an otherwise hidden epitope of cytochrome c is presented early in the Drosophila apoptotic program, though this putative conformational change is apparently caspase dependent and proceeds without release of cytochrome c from the mitochondria47. These findings have led to the supposition that alterations in mitochondrial morphology might lead to formation of a cytochrome c dependent apoptosome at the mitochondrial surface as part of a feed-forward signal in caspase activation46,48. While intriguing, such a scenario requires further validation; much of the discord in this area may be due to the differences in cell type and apoptotic stimulus used in the various studies.
In both C.elegans and mammalian cells, proteins of the Bcl-2 family play a pivotal role in the mitochondrial pathway of cell death. Drosophila also expresses two proteins with homology to Bcl-2, termed Buffy and Debcl49–52. These proteins share significant sequence homology, and both contain three BH domains. Sequence comparison and overexpression studies have suggested that Buffy is an anti-apoptotic multidomain Bcl-2 protein similar to mammalian Bcl-2, while Debcl is pro-apoptotic, with greatest observed homology to mammalian Bok. Knockdown of these proteins was consistent with these roles; RNAi against Buffy led to increased apoptosis, while injection of Debcl dsRNA caused an accumulation of posterior glial cells. However, despite these finding, a recent study found that flies with loss-of-function mutations in buffy, debcl, or both display normal developmental cell death and are viable and fertile, though a modest effect on apoptosis following ionizing radiation was observed53. As in the case of cytochrome c involvement in fly apoptosis, these conflicting results may reflect differences in cell type and stimulus.
As noted, significant evidence has been arrayed that mitochondrial factors such as cytochrome c are not required for caspase activation in Drosophila; additionally, Bcl-2 proteins do not appear to play a major role in the core apoptotic pathway of the fly. This stands in stark contrast to the situation observed in the nematode and mammal, and has led to supposition that Drosophila might be an evolutionary outlier, or indeed that the mitochondrial pathway of apoptosis might not be as conserved as once thought. However, a recent study by Abdelwahid and colleagues54 indicates a significant, if somewhat puzzling, role for the mitochondria in programmed cell death in the fly. Abdelwahid et al. found that, upon apoptosis, Reaper and HID proteins cause mitochondrial fragmentation and release of cytochrome c in both cultured S2 cells and in the developing fly embryo. This effect was observed to depend both on HID and Reaper and on caspase activation; death induced by DIAP1 knockdown or genotoxic stress (in which HID and Reaper are not upregulated) did not induce mitochondrial changes, despite significant caspase activation. Furthermore, fragmentation of the mitochondria upon HID or Reaper upregulation was blocked by the pancaspase inhibitor zVAD-fmk. As in mammals and nematodes, Drp1 was found to mediate mitochondrial fragmentation in apoptotic cells, and though the authors confirmed that cytochrome c is not required for apoptosis in the fly, they found that Drp1 knockdown led to a reduction in cell death. It may be that, while the observed mitochondrial disruption does not contribute to caspase activation per se (given that caspase activity is required for the disruption itself), it is nonetheless an important fail-safe mechanism to ensure cell death by rupturing the mitochondria, as well as contributing to efficient packaging of the dying cell for phagocytosis. The role of HID and Reaper in this process is provocative; in nematodes, mammals, and even yeast, the Bcl-2 family proteins are implicated in Drp-1 mediated fragmentation during apoptosis33,55,56. In flies, where the Bcl-2 family evidently plays a less pivotal role in cell death, the essential apoptotic mediators HID and Reaper seem to have assumed this role, and Abdelwahid et al. found that a mutant of Reaper that does not block DIAP-1 function was still able to cause mitochondrial fragmentation, indicating that apoptosis induction and mitochondrial disruption represent two distinct functions of Reaper. This duality of function mirrors the effect found for CED-9/EGL-1 in the nematode33, and may indicate that mitochondrial fragmentation plays an essential, if incompletely understood, role in apoptosis in vivo, leading different organisms to link this process to essential apoptotic factors.
Out of the mainstream, into the ocean
Having surveyed the well-characterized model organisms without emergence of a clear consensus on the role of the mitochondrial pathway in apoptosis, we are forced to turn our attention farther afield. A small group of brave souls has begun working to characterize the pathways of apoptosis in non-canonical invertebrate model organisms; a survey of these findings might prove informative in breaking the stalemate presented by the distinct pathways of C.elegans and Drosophila.
The deuterostome clade consists of three major phyla: (1) the chordates which includes vertebrates (mammals, birds, amphibians, fish, reptiles) and the invertebrate chordates, cephalochordates (amphioxus) and urochordates (sea squirts), (2) the hemichordates (acorn worms) and (3) the echinoderms (sea urchins, starfish)57. Significant, if fragmentary, information is available regarding the features of apoptosis in non-vertebrate deuterostomes. Caspase activation has been observed in organisms of all phyla; however, the mechanisms leading to caspase activation are generally poorly understood. Figure 2 depicts an animal phylogeny showing proposed evolutionary relationships between these phyla, while table 1 summarizes the apoptotic molecules found to date in these animals.
Figure 2.
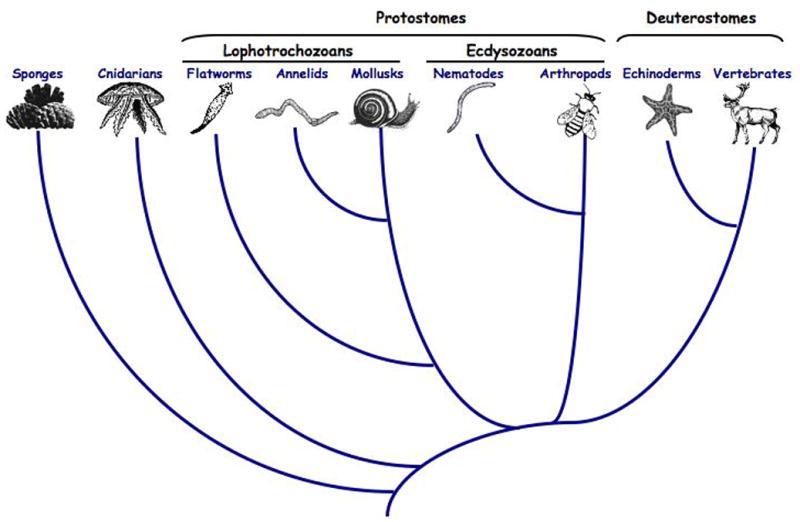
Animal phylogeny. MOMP is well characterized only in vertebrates. The apoptotic programs of the nematode ecdysozoan C.elegans and the arthropod ecdysozoan D.melanogaster have been studied extensively, and show varying degrees of mitochondrial involvement but lack direct evidence of MOMP. This leaves open the question of whether MOMP arose in vertebrates, or arose earlier but was lost in ecdysozoans. Much-need additional information from more basal phyla is emerging (surveyed in the text).
Table 1.
Summary of apoptotic molecules and pathways identified thus far in the invertebrate phyla depicted in figure 2.
| Phylum | Apoptotic molecules identified |
|---|---|
| Echinodermata (Purple sea urchin) | Apical and initiator caspases, three APAF-1 homologs including one with WD repeats, pro- and anti-apopotic Bcl-2 family homologs, seven possible IAPs 61,62 |
| Platyhelminthe (Planaria) | Eleven caspases, one APAF-1 homolog, nine Bcl-2 homologs63,64 |
| Cnidaria (Starlet sea anemone) | p53/p63 homolog, five caspase homologs, APAF-1 homolog containing WD repeats, seven Bcl-2 homologs, four IAP homologs65–69 |
| Porifera (sponges) | Apical and initiator caspase homologs, pro- and anti- apoptotic Bcl-2 family members. Caspase-dependent cell death observed in allograft rejection70–75 |
Cephalochordates are small eel-like animals that live buried in the sand. Apoptosis has been detected in the embryonic and larval stages of amphioxus (Branchiostoma floridae) development58. In addition, AmphiCASP-3/7, a capsase homologous to human caspases 3 and 7, has been cloned. AmphiCASP-3/7 is expressed throughout amphioxus development, from the gastrula to larval stage. When transfected into 293T cells, wild type AmphiCASP-3/7 promotes apoptosis, and a mutant with the prodomain deleted\had an enhanced ability to promote apoptosis, indicating that AmphiCASP-3/7 is activated by cleavage. Recombinant AmphiCASP-3/7 preferentially cleaves synthetic caspase substrates containing a DEVD sequence, but not substrates containing either VEID or IETD sequences. However, transfection of AmphiCASP-3/7 into MCF7 cells (deficient in caspase-3) did not rescue the apoptotic phenotype58.
The urochordate ascidian Ciona intestinalis (sea squirt) develops through a larval stage, which is characterized by a typical chordate body plan including a notochord. The adult is a sessile filter feeder and metamorphosis requires massive reorganization of the body plan including regression of the tail. The regression of the tail has been demonstrated to be caspase dependent and caspase inhibitors blocked metamorphosis. Fifteen independent caspase-like sequences have been identified in Ciona and while the mechanism of caspase activation is unknown, it appears that ERK activation may be involved in developmental apoptosis in Ciona59. Two Bcl-2 family protein homologs have also been identified in Ciona. ciBAX is a homolog of the proapoptotic multidomain protein BAX, while ciBcl-xL is a homolog of the anti-apoptotic Bcl-xL. Ectopic expression of ciBAX, but not a BH3 deleted mutant, in Ciona embryos resulted in cell dissociation and apoptosis during the gastrula stage. The ciBAX-induced dissociation and apoptosis was inhibited by ciBcl-xL60 suggesting that the mitochondrial pathway may be present in Ciona.
The genome of an echinoderm, the purple sea urchin Strongylocentrotus purpuratus, has recently been sequenced61 and homologs of the components of the vertebrate apoptotic pathways have been identified62. The S. purpuratus genome contains five classes of caspases, including both apical and executioner caspases. Caspase-8 and -9 homologues have been detected, containing tandem DED repeats or a CARD in their prodomains, respectively62. Three APAF-1 gene homologs were also identified in the S. purpuratus genome, one of which contained a CARD and seven WD repeats62. Ten genes coding for putative Bcl-2 family proteins were also identified, including one homolog each of the pro-apoptotic multidomain proteins, BAX and BAK, and one homolog of the anti-apoptotic proteins Bcl-2/BclxL; however, to date no homologs of the BH3-only proteins BAD, BID or BIM have been identified62. In addition, the S. purpuratus genome contains seven sequences that encode at least one BIR (baculoviral IAP repeat) domain characteristic of the IAPs, seven potential TNF receptor genes, four potential TNF ligand genes, and homologs of adaptor proteins involved in the extrinsic pathway62.
The Platyhelminthes includes both parasitic and free living species of flatworms63. Freshwater planarians, a free-living flatworm, have long been studied for their remarkable regenerative abilities. For example, in decapitated planaria both the head and trunk fragments each regenerate the missing body parts and integrate them into the original fragments resulting in two complete, proportional and fully functional animals. Cell loss (and presumably death) has been observed during both morphollaxis (proportional remodeling without cell proliferation) and epimorphosis (regeneration with cell proliferation). While cell death has been observed in planaria for more than a century, the molecular mechanisms regulating death remain unknown. The genome of one species of planaria, Schmidtea mediterranea has been sequenced and has recently been made available on SmedGD64. A number of genes with homology to mammalian genes encoding proteins known to be involved in apoptosis, including eleven caspases, one APAF-1 and nine Bcl-2 proteins have been identified (J. Pelletieri, personal communication).
It has recently been observed that germ cells in the starlet sea anemone Nematostella, a member of the phylum cnidaria, undergo apoptosis in response to ultraviolet radiation in a dose dependent manner. While the molecular mechanism of UV-induced death in the anemone is unknown, it appears to be dependent on nvp6365, a protein with homology to mammalian p53, a pro-apoptotic protein that (1) functions as a transcription factor and (2) binds the anti apoptotic proteins Bcl-2 and BclxL to activate the pro-apoptotic proteins BAX and BAK to initiate MOMP66. Sequencing of the genome of Nematostella vectensis has been completed67, and analysis has revealed a number of apoptotic genes, including five caspase homologs, four IAP homologs, seven Bcl-2 family protein homologs, proteins containing homologs to death domains, death effector domains, CARD domains, and nucleotide binding domains62 and homologs of iCAD and CAD, proteins responsible for the characteristic DNA fragmentation observed in apoptosis68. In addition, an APAF-1 homolog containing two CARDs, a nucleotide binding domain and WD repeats was recently identified69.
Significant study has been given to apoptosis in sponges of the phylum Porifera. Cell death can be readily observed in this organism in transplantation experiments, where an autograft (from the same sponge) or an allograft (from a different sponge) is inserted into a host. While the autografts fuse within five days, approximately half of the cells in the allograft show positive TUNEL staining and characteristic DNA fragmentation during the same period70. Extracts made from allografts, but not autografts, cleave a caspase substrate, indicating caspase-like activity71. Apoptotic genes have been identified in several species of sponges, and two sponge caspases have been cloned (GEOCYCAS3l and GEOCYCAS3s). GEOCYCAS3l has a long prodomain which appears to be somewhat homologous to a CARD domain, while GEOCYCAS3s contains a short prodomain; both are upregulated during allograft rejection71,72. Three genes with Bcl-2 homology have also been cloned in sponges. GCBHP1 and GCBHP2 both show homology to Bcl-2 in the BH1 and BH2 regions and both regions show closer homology to mammalian Bcl-2 family members than to CED-973. GCBHP2 expression is upregulated in sponge cells exposed to low (but not high) levels of tributyltin or heat shock, and GCBHP2 appears to have an anti-apoptotic role. Mammalian cells transfected with GCBHP2 were somewhat resistant to serum starvation and tributylin-induced apoptosis74. In addition, a homolog of BAK (LBBAK2l) with homology in the BH1, BH3 and transmembrane domains and a homolog of Bcl-2 (LBBCL-2a) with homology in all four BH domains have been cloned. Expression of caspases and Bcl-2 family proteins has been analyzed in the developing sponge and it appears that the anti-apoptotic proteins are expressed in the proliferative zone, while the pro-apoptotic proteins are expressed in the non-proliferative zone75.
A fairy tale ending
Having surveyed our current understanding of the subject, the degree to which the mitochondrial pathway of apoptosis is conserved remains inconclusive. Based on the well-characterized apoptotic pathways of the ecdysozoans C.elegans, and Drosophila, as well as those present in mammals, we are left with two possibilities: either the apoptotic pathway requiring MOMP upstream of caspase activation arose in the chordates, or it arose earlier but was lost in some or all of the ecdysozoan groups (Figure 2). Recent work on the role of mitochondria in fly apoptosis notwithstanding, Drosophila does represent something of a fly in the ointment. Mitochondrial localization of the CED-9/CED-4 complex in C.elegans, as well as the conserved role of proteins of the Bcl-2 family in mitochondrial dynamics between mammals and nematodes, could be hypothesized to be vestiges of the mitochondrial pathway of apoptosis. But does Drosophila represent the end of an atypical branch of the evolutionary tree in which the mitochondrial pathway has disappeared all together, or is the variance in apoptotic pathways among the phyla greater than expected?
In pursuing this question, one generality that emerges is the futility of attempting to draw conclusions based on DNA or protein sequence. As mentioned above, numerous invertebrates express homologues to apoptotic proteins described in mammals; however, one need look no further than Drosophila to discern the futility of drawing firm conclusions about apoptotic pathways based on sequence data. Based on sequence, Drosophila might be expected to require MOMP and cytochrome c for caspase activation; after all, ARK contains the WD domains through which APAF-1 binds cytochrome c, and the Drosophila genome also encodes both pro- and anti-apoptotic multidomain proteins of the Bcl-2 family, Debcl and Buffy, respectively. However, a preponderance of data indicates that cytochrome c is not required for Drosophila apoptosis. To turn the argument on its head, Drosophila apoptosis hinges on the action of a BIR-domain containing IAP, DIAP-1; however, despite the presence of mammalian homologues to DIAP-1, in mammals IAPs appear to be dispensable for normal execution of apoptosis. While we are blessed to live in a time in which whole genome sequences seem to appear on a monthly basis, this is a problem that will yield to biochemistry and cell biology, not to molecular sequencing alone.
Supposition as to the reason that mitochondria play an important role in apoptosis in at least some animals is just that: supposition. However, the similarities between many of the molecules involved in apoptosis and those recruited in the cellular response to infection are difficult to ignore (see review by Munoz & Martin in this issue, and 76). APAF-1 shares sequence homology with cytoplasmic mediators of the innate immune response called the NOD-like receptors, and the mitochondria arose as bacterial symbionts living within early eukaryotic cells. The idea that the earliest form of apoptosis may have been in response to pathogen invasion is attractive because it circumvents the problem of altruism in very simple organisms; the purposeful deletion of damaged or developmentally superfluous cells in a complex, multicellular animal confers a clear advantage on the organism as a whole, but the advantageousness of apoptosis is harder to envision in single-celled organisms, where cell death and death of the organism are synonymous. One way apoptosis could arise in unicellular organisms is through infection; apoptosis could be advantageous if a clonally identical group of such organisms acquired a way for group members to commit suicide before invading pathogens could proliferate and infect other members of the group76. What if such suicide were mediated by activation of cysteine proteases by simple, innate-immunity-like pattern-recognizing molecules—not unlike the NOD-like receptors--and what if one of the molecules they recognized was an iron-binding protein unique to metabolism in certain bacteria, a relative of what we now call cytochrome c? Now suppose that, untold eons later, a bacterium expressing that protein established a highly advantageous symbiotic relationship with an early eukaryotic cell, providing the metabolic efficiency needed for an eventual jump to multicellularity. And suppose that, as evolutionary pressures mandated targeted deletion of specific cells during development of increasingly complex organisms, the pathways of pathogen-mediated cell death were dusted off and reconfigured, but remained arranged around the one pathogen-like element present in each eukaryotic cell, the mitochondrion. Interesting though such speculation may be, it will remain nothing more than a fairy tale until further supporting biochemical information is gathered from phylogenically diverse organisms.
References
- 1.Chipuk JE, Green DR. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol. 2008 doi: 10.1016/j.tcb.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Acehan D, Jiang X, Morgan DG, Heuser JE, Wang X, Akey CW. Three-dimensional structure of the apoptosome: implications for assembly, procaspase-9 binding, and activation. Mol Cell. 2002;9:423–32. doi: 10.1016/s1097-2765(02)00442-2. [DOI] [PubMed] [Google Scholar]
- 3.Boatright KM, Renatus M, Scott FL, Sperandio S, Shin H, Pedersen IM, et al. A unified model for apical caspase activation. Mol Cell. 2003;11:529–41. doi: 10.1016/s1097-2765(03)00051-0. [DOI] [PubMed] [Google Scholar]
- 4.Crook NE, Clem RJ, Miller LK. An apoptosis-inhibiting baculovirus gene with a zinc finger-like motif. J Virol. 1993;67:2168–74. doi: 10.1128/jvi.67.4.2168-2174.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deveraux QL, Takahashi R, Salvesen GS, Reed JC. X-linked IAP is a direct inhibitor of cell-death proteases. Nature. 1997;388:300–4. doi: 10.1038/40901. [DOI] [PubMed] [Google Scholar]
- 6.Uren AG, Pakusch M, Hawkins CJ, Puls KL, Vaux DL. Cloning and expression of apoptosis inhibitory protein homologs that function to inhibit apoptosis and/or bind tumor necrosis factor receptor-associated factors. Proc Natl Acad Sci U S A. 1996;93:4974–8. doi: 10.1073/pnas.93.10.4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duckett CS, Nava VE, Gedrich RW, Clem RJ, Van Dongen JL, Gilfillan MC, et al. A conserved family of cellular genes related to the baculovirus iap gene and encoding apoptosis inhibitors. Embo J. 1996;15:2685–94. [PMC free article] [PubMed] [Google Scholar]
- 8.Harlin H, Reffey SB, Duckett CS, Lindsten T, Thompson CB. Characterization of XIAP-deficient mice. Mol Cell Biol. 2001;21:3604–8. doi: 10.1128/MCB.21.10.3604-3608.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okada H, Suh WK, Jin J, Woo M, Du C, Elia A, et al. Generation and characterization of Smac/DIABLO-deficient mice. Mol Cell Biol. 2002;22:3509–17. doi: 10.1128/MCB.22.10.3509-3517.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martins LM, Morrison A, Klupsch K, Fedele V, Moisoi N, Teismann P, et al. Neuroprotective role of the Reaper-related serine protease HtrA2/Omi revealed by targeted deletion in mice. Mol Cell Biol. 2004;24:9848–62. doi: 10.1128/MCB.24.22.9848-9862.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vince JE, Wong WW, Khan N, Feltham R, Chau D, Ahmed AU, et al. IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell. 2007;131:682–93. doi: 10.1016/j.cell.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 12.Varfolomeev E, Blankenship JW, Wayson SM, Fedorova AV, Kayagaki N, Garg P, et al. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell. 2007;131:669–81. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 13.Petersen SL, Wang L, Yalcin-Chin A, Li L, Peyton M, Minna J, et al. Autocrine TNFalpha signaling renders human cancer cells susceptible to Smacmimetic-induced apoptosis. Cancer Cell. 2007;12:445–56. doi: 10.1016/j.ccr.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellis HM, Horvitz HR. Genetic control of programmed cell death in the nematode C. elegans. Cell. 1986;44:817–29. doi: 10.1016/0092-8674(86)90004-8. [DOI] [PubMed] [Google Scholar]
- 15.Lettre G, Hengartner MO. Developmental apoptosis in C. elegans: a complex CEDnario. Nat Rev Mol Cell Biol. 2006;7:97–108. doi: 10.1038/nrm1836. [DOI] [PubMed] [Google Scholar]
- 16.Parrish JZ, Xue D. Functional genomic analysis of apoptotic DNA degradation in C. elegans. Mol Cell. 2003;11:987–96. doi: 10.1016/s1097-2765(03)00095-9. [DOI] [PubMed] [Google Scholar]
- 17.Parrish JZ, Yang C, Shen B, Xue D. CRN-1, a Caenorhabditis elegans FEN-1 homologue, cooperates with CPS-6/EndoG to promote apoptotic DNA degradation. Embo J. 2003;22:3451–60. doi: 10.1093/emboj/cdg320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X, Wang J, Gengyo-Ando K, Gu L, Sun CL, Yang C, et al. C. elegans mitochondrial factor WAH-1 promotes phosphatidylserine externalization in apoptotic cells through phospholipid scramblase SCRM-1. Nat Cell Biol. 2007;9:541–9. doi: 10.1038/ncb1574. [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Yang C, Chai J, Shi Y, Xue D. Mechanisms of AIF-mediated apoptotic DNA degradation in Caenorhabditis elegans. Science. 2002;298:1587–92. doi: 10.1126/science.1076194. [DOI] [PubMed] [Google Scholar]
- 20.Hu Y, Benedict MA, Wu D, Inohara N, Nunez G. Bcl-XL interacts with Apaf-1 and inhibits Apaf-1-dependent caspase-9 activation. Proc Natl Acad Sci U S A. 1998;95:4386–91. doi: 10.1073/pnas.95.8.4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inohara N, Gourley TS, Carrio R, Muniz M, Merino J, Garcia I, et al. Diva, a Bcl-2 homologue that binds directly to Apaf-1 and induces BH3-independent cell death. J Biol Chem. 1998;273:32479–86. doi: 10.1074/jbc.273.49.32479. [DOI] [PubMed] [Google Scholar]
- 22.Conus S, Rosse T, Borner C. Failure of Bcl-2 family members to interact with Apaf-1 in normal and apoptotic cells. Cell Death Differ. 2000;7:947–54. doi: 10.1038/sj.cdd.4400729. [DOI] [PubMed] [Google Scholar]
- 23.Hausmann G, O’Reilly LA, van Driel R, Beaumont JG, Strasser A, Adams JM, et al. Pro-apoptotic apoptosis protease-activating factor 1 (Apaf-1) has a cytoplasmic localization distinct from Bcl-2 or Bcl-x(L) J Cell Biol. 2000;149:623–34. doi: 10.1083/jcb.149.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moriishi K, Huang DC, Cory S, Adams JM. Bcl-2 family members do not inhibit apoptosis by binding the caspase activator Apaf-1. Proc Natl Acad Sci U S A. 1999;96:9683–8. doi: 10.1073/pnas.96.17.9683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vaux DL, Weissman IL, Kim SK. Prevention of programmed cell death in Caenorhabditis elegans by human bcl-2. Science. 1992;258:1955–7. doi: 10.1126/science.1470921. [DOI] [PubMed] [Google Scholar]
- 26.Hengartner MO, Horvitz HR. C. elegans cell survival gene ced-9 encodes a functional homolog of the mammalian proto-oncogene bcl-2. Cell. 1994;76:665–76. doi: 10.1016/0092-8674(94)90506-1. [DOI] [PubMed] [Google Scholar]
- 27.Jabbour AM, Puryer MA, Yu JY, Lithgow T, Riffkin CD, Ashley DM, et al. Human Bcl-2 cannot directly inhibit the Caenorhabditis elegans Apaf-1 homologue CED-4, but can interact with EGL-1. J Cell Sci. 2006;119:2572–82. doi: 10.1242/jcs.02985. [DOI] [PubMed] [Google Scholar]
- 28.Hengartner MO, Ellis RE, Horvitz HR. Caenorhabditis elegans gene ced-9 protects cells from programmed cell death. Nature. 1992;356:494–9. doi: 10.1038/356494a0. [DOI] [PubMed] [Google Scholar]
- 29.Hoppins S, Lackner L, Nunnari J. The machines that divide and fuse mitochondria. Annu Rev Biochem. 2007;76:751–80. doi: 10.1146/annurev.biochem.76.071905.090048. [DOI] [PubMed] [Google Scholar]
- 30.Neuspiel M, Zunino R, Gangaraju S, Rippstein P, McBride H. Activated mitofusin 2 signals mitochondrial fusion, interferes with Bax activation, and reduces susceptibility to radical induced depolarization. J Biol Chem. 2005;280:25060–70. doi: 10.1074/jbc.M501599200. [DOI] [PubMed] [Google Scholar]
- 31.Sugioka R, Shimizu S, Tsujimoto Y. Fzo1, a protein involved in mitochondrial fusion, inhibits apoptosis. J Biol Chem. 2004;279:52726–34. doi: 10.1074/jbc.M408910200. [DOI] [PubMed] [Google Scholar]
- 32.Lee YJ, Jeong SY, Karbowski M, Smith CL, Youle RJ. Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Mol Biol Cell. 2004;15:5001–11. doi: 10.1091/mbc.E04-04-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jagasia R, Grote P, Westermann B, Conradt B. DRP-1-mediated mitochondrial fragmentation during EGL-1-induced cell death in C. elegans. Nature. 2005;433:754–60. doi: 10.1038/nature03316. [DOI] [PubMed] [Google Scholar]
- 34.Delivani P, Adrain C, Taylor RC, Duriez PJ, Martin SJ. Role for CED-9 and Egl-1 as regulators of mitochondrial fission and fusion dynamics. Mol Cell. 2006;21:761–73. doi: 10.1016/j.molcel.2006.01.034. [DOI] [PubMed] [Google Scholar]
- 35.Vaux DL, Silke J. IAPs, RINGs and ubiquitylation. Nat Rev Mol Cell Biol. 2005;6:287–97. doi: 10.1038/nrm1621. [DOI] [PubMed] [Google Scholar]
- 36.Kumar S, Doumanis J. The fly caspases. Cell Death Differ. 2000;7:1039–44. doi: 10.1038/sj.cdd.4400756. [DOI] [PubMed] [Google Scholar]
- 37.Kumar S. Caspase function in programmed cell death. Cell Death Differ. 2007;14:32–43. doi: 10.1038/sj.cdd.4402060. [DOI] [PubMed] [Google Scholar]
- 38.Silke J, Verhagen AM, Ekert PG, Vaux DL. Sequence as well as functional similarity for DIABLO/Smac and Grim, Reaper and Hid? Cell Death Differ. 2000;7:1275. doi: 10.1038/sj.cdd.4400790. [DOI] [PubMed] [Google Scholar]
- 39.Kumar S, Cakouros D. Transcriptional control of the core cell-death machinery. Trends Biochem Sci. 2004;29:193–9. doi: 10.1016/j.tibs.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 40.Wang SL, Hawkins CJ, Yoo SJ, Muller HA, Hay BA. The Drosophila caspase inhibitor DIAP1 is essential for cell survival and is negatively regulated by HID. Cell. 1999;98:453–63. doi: 10.1016/s0092-8674(00)81974-1. [DOI] [PubMed] [Google Scholar]
- 41.Yu X, Wang L, Acehan D, Wang X, Akey CW. Three-dimensional structure of a double apoptosome formed by the Drosophila Apaf-1 related killer. J Mol Biol. 2006;355:577–89. doi: 10.1016/j.jmb.2005.10.040. [DOI] [PubMed] [Google Scholar]
- 42.Dorstyn L, Mills K, Lazebnik Y, Kumar S. The two cytochrome c species, DC3 and DC4, are not required for caspase activation and apoptosis in Drosophila cells. J Cell Biol. 2004;167:405–10. doi: 10.1083/jcb.200408054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Means JC, Muro I, Clem RJ. Lack of involvement of mitochondrial factors in caspase activation in a Drosophila cell-free system. Cell Death Differ. 2006;13:1222–34. doi: 10.1038/sj.cdd.4401821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zimmermann KC, Ricci JE, Droin NM, Green DR. The role of ARK in stress-induced apoptosis in Drosophila cells. J Cell Biol. 2002;156:1077–87. doi: 10.1083/jcb.20112068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mendes CS, Arama E, Brown S, Scherr H, Srivastava M, Bergmann A, et al. Cytochrome c-d regulates developmental apoptosis in the Drosophila retina. EMBO Rep. 2006;7:933–9. doi: 10.1038/sj.embor.7400773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arama E, Bader M, Srivastava M, Bergmann A, Steller H. The two Drosophila cytochrome C proteins can function in both respiration and caspase activation. Embo J. 2006;25:232–43. doi: 10.1038/sj.emboj.7600920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Varkey J, Chen P, Jemmerson R, Abrams JM. Altered cytochrome c display precedes apoptotic cell death in Drosophila. J Cell Biol. 1999;144:701–10. doi: 10.1083/jcb.144.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walker DW, Benzer S. Mitochondrial “swirls” induced by oxygen stress and in the Drosophila mutant hyperswirl. Proc Natl Acad Sci U S A. 2004;101:10290–5. doi: 10.1073/pnas.0403767101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Colussi PA, Quinn LM, Huang DC, Coombe M, Read SH, Richardson H, et al. Debcl, a proapoptotic Bcl-2 homologue, is a component of the Drosophila melanogaster cell death machinery. J Cell Biol. 2000;148:703–14. doi: 10.1083/jcb.148.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Quinn L, Coombe M, Mills K, Daish T, Colussi P, Kumar S, et al. Buffy, a Drosophila Bcl-2 protein, has anti-apoptotic and cell cycle inhibitory functions. Embo J. 2003;22:3568–79. doi: 10.1093/emboj/cdg355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang H, Huang Q, Ke N, Matsuyama S, Hammock B, Godzik A, et al. Drosophila pro-apoptotic Bcl-2/Bax homologue reveals evolutionary conservation of cell death mechanisms. J Biol Chem. 2000;275:27303–6. doi: 10.1074/jbc.M002846200. [DOI] [PubMed] [Google Scholar]
- 52.Igaki T, Kanuka H, Inohara N, Sawamoto K, Nunez G, Okano H, et al. Drob-1, a Drosophila member of the Bcl-2/CED-9 family that promotes cell death. Proc Natl Acad Sci U S A. 2000;97:662–7. doi: 10.1073/pnas.97.2.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sevrioukov EA, Burr J, Huang EW, Assi HH, Monserrate JP, Purves DC, et al. Drosophila Bcl-2 proteins participate in stress-induced apoptosis, but are not required for normal development. Genesis. 2007;45:184–93. doi: 10.1002/dvg.20279. [DOI] [PubMed] [Google Scholar]
- 54.Abdelwahid E, Yokokura T, Krieser RJ, Balasundaram S, Fowle WH, White K. Mitochondrial disruption in Drosophila apoptosis. Dev Cell. 2007;12:793–806. doi: 10.1016/j.devcel.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 55.Fannjiang Y, Cheng WC, Lee SJ, Qi B, Pevsner J, McCaffery JM, et al. Mitochondrial fission proteins regulate programmed cell death in yeast. Genes Dev. 2004;18:2785–97. doi: 10.1101/gad.1247904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, Catez F, et al. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell. 2001;1:515–25. doi: 10.1016/s1534-5807(01)00055-7. [DOI] [PubMed] [Google Scholar]
- 57.Bourlat SJ, Juliusdottir T, Lowe CJ, Freeman R, Aronowicz J, Kirschner M, et al. Deuterostome phylogeny reveals monophyletic chordates and the new phylum Xenoturbellida. Nature. 2006;444:85–8. doi: 10.1038/nature05241. [DOI] [PubMed] [Google Scholar]
- 58.Bayascas JR, Yuste VJ, Benito E, Garcia-Fernandez J, Comella JX. Isolation of AmphiCASP-3/7, an ancestral caspase from amphioxus (Branchiostoma floridae). Evolutionary considerations for vertebrate caspases. Cell Death Differ. 2002;9:1078–89. doi: 10.1038/sj.cdd.4401075. [DOI] [PubMed] [Google Scholar]
- 59.Ciana P, Vegeto E, Beato M, Chambon P, Gustafsson JA, Parker M, et al. Looking at nuclear receptors from the heights of Erice. Workshop on nuclear receptor structure and function. EMBO Rep. 2002;3:125–9. doi: 10.1093/embo-reports/kvf029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takada N, Yamaguchi H, Shida K, Terajima D, Satou Y, Kasuya A, et al. The cell death machinery controlled by Bax and Bcl-XL is evolutionarily conserved in Ciona intestinalis. Apoptosis. 2005;10:1211–20. doi: 10.1007/s10495-005-1391-4. [DOI] [PubMed] [Google Scholar]
- 61.Sodergren E, Weinstock GM, Davidson EH, Cameron RA, Gibbs RA, Angerer RC, et al. The genome of the sea urchin Strongylocentrotus purpuratus. Science. 2006;314:941–52. doi: 10.1126/science.1133609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Robertson AJ, Croce J, Carbonneau S, Voronina E, Miranda E, McClay DR, et al. The genomic underpinnings of apoptosis in Strongylocentrotus purpuratus. Dev Biol. 2006;300:321–34. doi: 10.1016/j.ydbio.2006.08.053. [DOI] [PubMed] [Google Scholar]
- 63.Sanchez Alvarado A. The freshwater planarian Schmidtea mediterranea: embryogenesis, stem cells and regeneration. Curr Opin Genet Dev. 2003;13:438–44. doi: 10.1016/s0959-437x(03)00082-0. [DOI] [PubMed] [Google Scholar]
- 64.Robb SM, Ross E, Alvarado AS. SmedGD: the Schmidtea mediterranea genome database. Nucleic Acids Res. 2008;36:D599–606. doi: 10.1093/nar/gkm684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pankow S, Bamberger C. The p53 tumor suppressor-like protein nvp63 mediates selective germ cell death in the sea anemone Nematostella vectensis. PLoS ONE. 2007;2:e782. doi: 10.1371/journal.pone.0000782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chipuk JE, Green DR. Dissecting p53-dependent apoptosis. Cell Death Differ. 2006;13:994–1002. doi: 10.1038/sj.cdd.4401908. [DOI] [PubMed] [Google Scholar]
- 67.Sullivan JC, Ryan JF, Watson JA, Webb J, Mullikin JC, Rokhsar D, et al. StellaBase: the Nematostella vectensis Genomics Database. Nucleic Acids Res. 2006;34:D495–9. doi: 10.1093/nar/gkj020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Eckhart L, Fischer H, Tschachler E. Phylogenomics of caspase-activated DNA fragmentation factor. Biochem Biophys Res Commun. 2007;356:293–9. doi: 10.1016/j.bbrc.2007.02.122. [DOI] [PubMed] [Google Scholar]
- 69.Zmasek CM, Zhang Q, Ye Y, Godzik A. Surprising complexity of the ancestral apoptosis network. Genome Biol. 2007;8:R226. doi: 10.1186/gb-2007-8-10-r226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wiens M, Krasko A, Blumbach B, Muller IM, Muller WE. Increased expression of the potential proapoptotic molecule DD2 and increased synthesis of leukotriene B4 during allograft rejection in a marine sponge. Cell Death Differ. 2000;7:461–9. doi: 10.1038/sj.cdd.4400671. [DOI] [PubMed] [Google Scholar]
- 71.Wiens M, Krasko A, Perovic S, Muller WE. Caspase-mediated apoptosis in sponges: cloning and function of the phylogenetic oldest apoptotic proteases from Metazoa. Biochim Biophys Acta. 2003;1593:179–89. doi: 10.1016/s0167-4889(02)00388-9. [DOI] [PubMed] [Google Scholar]
- 72.Tepsuporn S, Kaltenbach JC, Kuhns WJ, Burger MM, Fernandez-Busquets X. Apoptosis in Microciona prolifera Allografts. Biol Bull. 2003;205:199–201. doi: 10.2307/1543251. [DOI] [PubMed] [Google Scholar]
- 73.Wiens M, Krasko A, Muller CI, Muller WE. Molecular evolution of apoptotic pathways: cloning of key domains from sponges (Bcl-2 homology domains and death domains) and their phylogenetic relationships. J Mol Evol. 2000;50:520–31. doi: 10.1007/s002390010055. [DOI] [PubMed] [Google Scholar]
- 74.Wiens M, Diehl-Seifert B, Muller WE. Sponge Bcl-2 homologous protein (BHP2-GC) confers distinct stress resistance to human HEK-293 cells. Cell Death Differ. 2001;8:887–98. doi: 10.1038/sj.cdd.4400906. [DOI] [PubMed] [Google Scholar]
- 75.Wiens M, Belikov SI, Kaluzhnaya OV, Schroder HC, Hamer B, Perovic-Ottstadt S, et al. Axial (apical-basal) expression of pro-apoptotic and pro-survival genes in the lake baikal demosponge Lubomirskia baicalensis. DNA Cell Biol. 2006;25:152–64. doi: 10.1089/dna.2006.25.152. [DOI] [PubMed] [Google Scholar]
- 76.James ER, Green DR. Infection and the origins of apoptosis. Cell Death Differ. 2002;9:355–7. doi: 10.1038/sj.cdd.4400986. [DOI] [PubMed] [Google Scholar]


