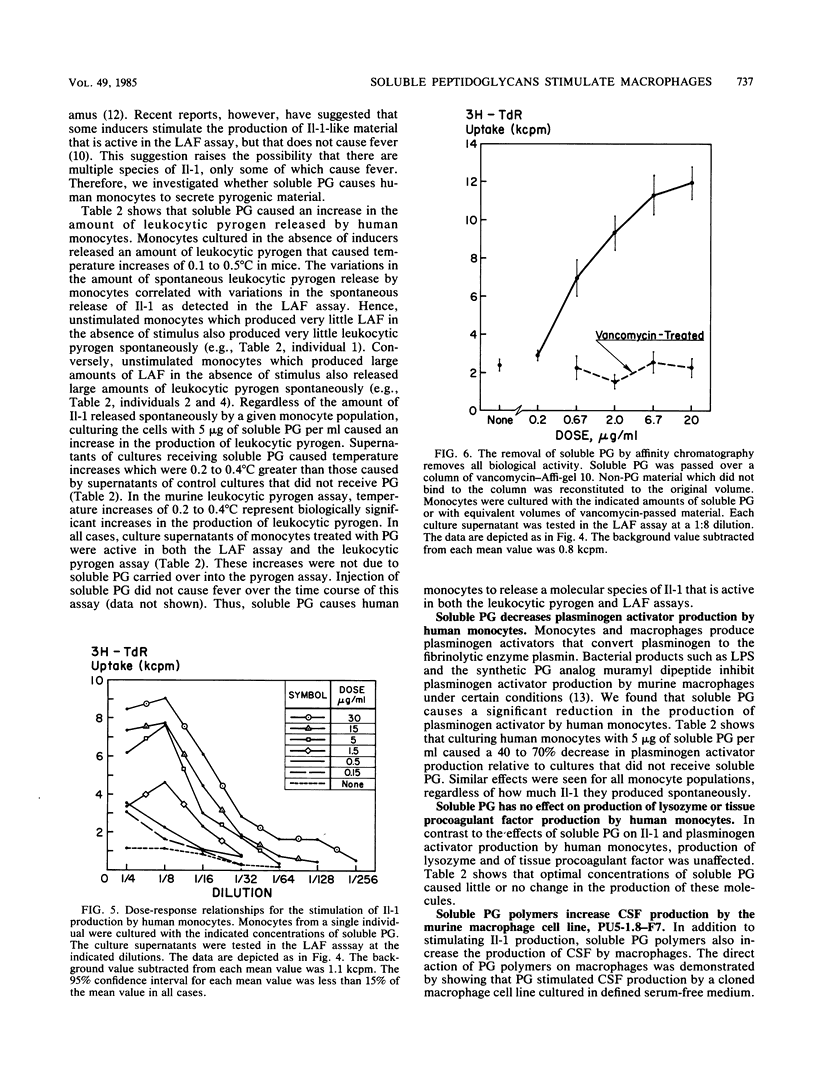
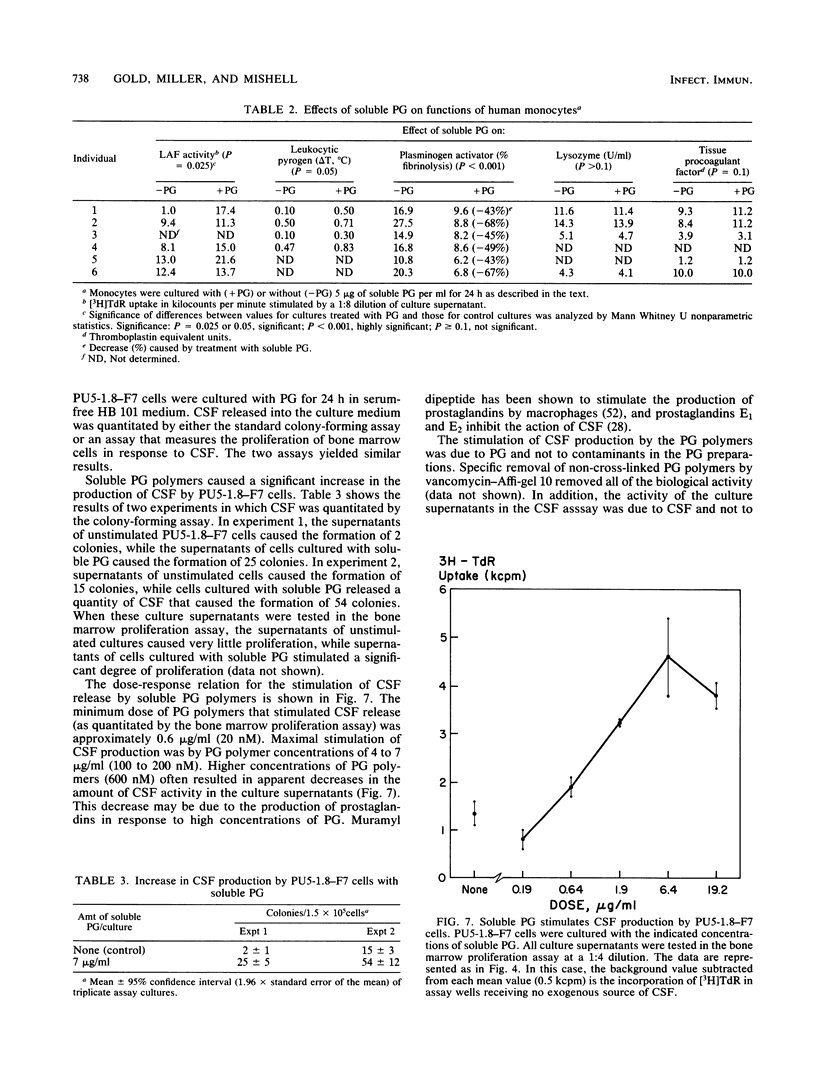
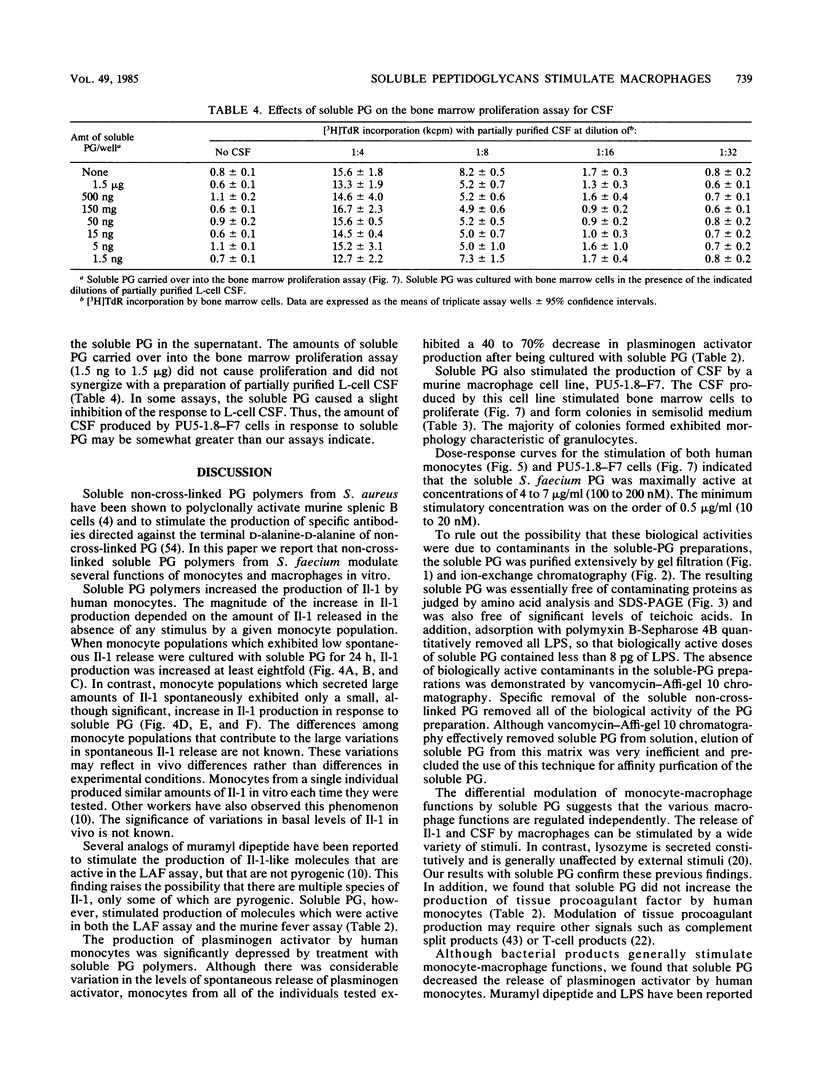
Abstract
Soluble non-cross-linked peptidoglycan polymers are released by gram-positive bacteria when beta-lactam antibiotics are administered to humans. In this report, we show that this type of peptidoglycan can stimulate monocyte-macrophage functions that cause inflammation. Non-cross-linked peptidoglycan polymers from penicillin-treated Streptococcus faecium were purified and shown to stimulate the production of interleukin 1 by human monocytes and of colony-stimulating factors by a murine macrophage cell line. In addition, the release of plasminogen activator by human monocytes was inhibited by the soluble peptidoglycan. These in vitro results suggest that prolonged treatment with beta-lactam antibiotics, by causing the production of soluble peptidoglycan, may result in interleukin 1-mediated inflammatory reactions, excessive production of monocytes and granulocytes, and increased fibrin deposition.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- Ackerman S. K., Douglas S. D. Purification of human monocytes on microexudate-coated surfaces. J Immunol. 1978 Apr;120(4):1372–1374. [PubMed] [Google Scholar]
- Adam A., Petit J. F., Lefrancier P., Lederer E. Muramyl peptides. Chemical structure, biological activity and mechanism of action. Mol Cell Biochem. 1981 Dec 4;41:27–47. doi: 10.1007/BF00225295. [DOI] [PubMed] [Google Scholar]
- Babu U. M., Zeiger A. R. Soluble peptidoglycan from Staphylococcus aureus is a murine B-lymphocyte mitogen. Infect Immun. 1983 Dec;42(3):1013–1016. doi: 10.1128/iai.42.3.1013-1016.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baracos V., Rodemann H. P., Dinarello C. A., Goldberg A. L. Stimulation of muscle protein degradation and prostaglandin E2 release by leukocytic pyrogen (interleukin-1). A mechanism for the increased degradation of muscle proteins during fever. N Engl J Med. 1983 Mar 10;308(10):553–558. doi: 10.1056/NEJM198303103081002. [DOI] [PubMed] [Google Scholar]
- Barrett J. F., Shockman G. D. Isolation and characterization of soluble peptidoglycan from several strains of Streptococcus faecium. J Bacteriol. 1984 Aug;159(2):511–519. doi: 10.1128/jb.159.2.511-519.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodel P., Miller H. Pyrogen from mouse macrophages causes fever in mice. Proc Soc Exp Biol Med. 1976 Jan;151(1):93–96. doi: 10.3181/00379727-151-39150. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Cornett J. B., Redman B. E., Shockman G. D. Autolytic defective mutant of Streptococcus faecalis. J Bacteriol. 1978 Feb;133(2):631–640. doi: 10.1128/jb.133.2.631-640.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damais C., Riveau G., Parant M., Gerota J., Chedid L. Production of lymphocyte activating factor in the absence of endogenous pyrogen by rabbit or human leukocytes stimulated by a muramyl dipeptide derivative. Int J Immunopharmacol. 1982;4(5):451–462. doi: 10.1016/0192-0561(82)90020-0. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A., Bernheim H. A. Ability of human leukocytic pyrogen to stimulate brain prostaglandin synthesis in vitro. J Neurochem. 1981 Sep;37(3):702–708. doi: 10.1111/j.1471-4159.1982.tb12544.x. [DOI] [PubMed] [Google Scholar]
- Drapier J. C., Lemaire G., Petit J. F. Regulation of plasminogen activator secretion in mouse peritoneal macrophages. II. Inhibition by immunomodulators of bacterial origin. Int J Immunopharmacol. 1982;4(1):21–34. doi: 10.1016/0192-0561(82)90005-4. [DOI] [PubMed] [Google Scholar]
- Durum S. K., Schmidt J. A., Oppenheim J. J. Interleukin 1: an immunological perspective. Annu Rev Immunol. 1985;3:263–287. doi: 10.1146/annurev.iy.03.040185.001403. [DOI] [PubMed] [Google Scholar]
- Emori K., Tanaka A. Granuloma formation by synthetic bacterial cell wall fragment: muramyl dipeptide. Infect Immun. 1978 Feb;19(2):613–620. doi: 10.1128/iai.19.2.613-620.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freundlich B., Avdalovic N. Use of gelatin/plasma coated flasks for isolating human peripheral blood monocytes. J Immunol Methods. 1983 Aug 12;62(1):31–37. doi: 10.1016/0022-1759(83)90107-2. [DOI] [PubMed] [Google Scholar]
- Galelli A., Chedid L. Modulation of myelopoiesis in vivo by synthetic adjuvant-active muramyl peptides: induction of colony-stimulating activity and stimulation of stem cell proliferation. Infect Immun. 1983 Dec;42(3):1081–1085. doi: 10.1128/iai.42.3.1081-1085.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian G. G., Moss R. L., Greaser M. Improved methodology for analysis and quantitation of proteins on one-dimensional silver-stained slab gels. Anal Biochem. 1983 Mar;129(2):277–287. doi: 10.1016/0003-2697(83)90551-1. [DOI] [PubMed] [Google Scholar]
- Gordon S., Todd J., Cohn Z. A. In vitro synthesis and secretion of lysozyme by mononuclear phagocytes. J Exp Med. 1974 May 1;139(5):1228–1248. doi: 10.1084/jem.139.5.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowen M., Wood D. D., Ihrie E. J., Meats J. E., Russell R. G. Stimulation by human interleukin 1 of cartilage breakdown and production of collagenase and proteoglycanase by human chondrocytes but not by human osteoblasts in vitro. Biochim Biophys Acta. 1984 Feb 14;797(2):186–193. doi: 10.1016/0304-4165(84)90121-1. [DOI] [PubMed] [Google Scholar]
- Helin H. J., Fox R. I., Edgington T. S. The instructor cell for the human procoagulant monocyte response to bacterial lipopolysaccharide is a Leu-3a+ T cell by fluorescence-activated cell sorting. J Immunol. 1983 Aug;131(2):749–752. [PubMed] [Google Scholar]
- Heymer B. Biological properties of the peptidoglycan. Z Immunitatsforsch Exp Klin Immunol. 1975 Jul;149(2-4):245–257. [PubMed] [Google Scholar]
- Issekutz A. C. Removal of gram-negative endotoxin from solutions by affinity chromatography. J Immunol Methods. 1983 Jul 29;61(3):275–281. doi: 10.1016/0022-1759(83)90221-1. [DOI] [PubMed] [Google Scholar]
- Keglević D., Ladesić B., Hadzija O., Tomasić J., Valinger Z., Pokorny M., Naumski R. Isolation and study of the composition of a peptidoglycan complex excreted by the biotin-requiring mutant of Brevibacterium divaricatum NRRL-2311 in the presence of penicillin. Eur J Biochem. 1974 Mar 1;42(2):389–400. doi: 10.1111/j.1432-1033.1974.tb03351.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Allred C., Cohen S., Yoshida T. Role of interleukin 1 in experimental pulmonary granuloma in mice. J Immunol. 1985 Jan;134(1):358–364. [PubMed] [Google Scholar]
- Kohashi O., Pearson C. M., Shimono T., Kotani S. Preparation of various fractions from Mycobacterium smegmatis, their arthritogenicity and their preventive effect on adjuvant disease. Int Arch Allergy Appl Immunol. 1976;51(4):451–461. doi: 10.1159/000231619. [DOI] [PubMed] [Google Scholar]
- Kurland J. I., Broxmeyer H. E., Pelus L. M., Bockman R. S., Moore M. A. Role for monocyte-macrophage-derived colony-stimulating factor and prostaglandin E in the positive and negative feedback control of myeloid stem cell proliferation. Blood. 1978 Aug;52(2):388–407. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Miller C. L., Graziano C. J., Lim R. C. Human monocyte plasminogen activator production: correlation to altered M phi-T lymphocyte interaction. J Immunol. 1982 May;128(5):2194–2200. [PubMed] [Google Scholar]
- Miller C. L., Graziano C., Lim R. C., Chin M. Generation of tissue factor by patient monocytes: correlation to thromboembolic complications. Thromb Haemost. 1981 Aug 28;46(2):489–495. [PubMed] [Google Scholar]
- Mirelman D., Bracha R., Sharon N. Penicillin-induced secretion of soluble, uncross-linked peptidoglycan by Micrococcus luteus cells. Biochemistry. 1974 Nov 19;13(24):5045–5053. doi: 10.1021/bi00721a028. [DOI] [PubMed] [Google Scholar]
- Mizel S. B., Dayer J. M., Krane S. M., Mergenhagen S. E. Stimulation of rheumatoid synovial cell collagenase and prostaglandin production by partially purified lymphocyte-activating factor (interleukin 1). Proc Natl Acad Sci U S A. 1981 Apr;78(4):2474–2477. doi: 10.1073/pnas.78.4.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizel S. B. Interleukin 1 and T cell activation. Immunol Rev. 1982;63:51–72. doi: 10.1111/j.1600-065x.1982.tb00411.x. [DOI] [PubMed] [Google Scholar]
- Mizel S. B., Oppenheim J. J., Rosenstreich D. L. Characterization of lymphocyte-activating factor (LAF) produced by the macrophage cell line, P388D1. I. Enhancement of LAF production by activated T lymphocytes. J Immunol. 1978 May;120(5):1497–1503. [PubMed] [Google Scholar]
- Moore R. N., Rouse B. T. Enhanced responsiveness of committed macrophage precursors to macrophage-type colony-stimulating factor (CSF-1) induced in vitro by interferons alpha + beta 1. J Immunol. 1983 Nov;131(5):2374–2378. [PubMed] [Google Scholar]
- Mortensen R. F., Sarlo K., Le P. T. Monokine-induced hepatocyte synthesis of acute-phase reactants in mice. Lymphokine Res. 1984;3(1):17–22. [PubMed] [Google Scholar]
- Nagao S., Tanaka A. Muramyl dipeptide-induced adjuvant arthritis. Infect Immun. 1980 May;28(2):624–626. doi: 10.1128/iai.28.2.624-626.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H., Zeiger A. R., Schumacher H. R. Detection of soluble peptidoglycan in urine after penicillin administration. Infect Immun. 1984 Jan;43(1):139–142. doi: 10.1128/iai.43.1.139-142.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins H. R. Specificity of combination between mucopeptide precursors and vancomycin or ristocetin. Biochem J. 1969 Jan;111(2):195–205. doi: 10.1042/bj1110195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prydz H., Allison A. C., Schorlemmer H. U. Further link between complement activation and blood coagulation. Nature. 1977 Nov 10;270(5633):173–174. doi: 10.1038/270173a0. [DOI] [PubMed] [Google Scholar]
- Rickles F. R., Hardin J. A., Pitlick F. A., Hoyer L. W., Conrad M. E. Tissue factor activity in lymphocyte cultures from normal individuals and patients with hemophilia A. J Clin Invest. 1973 Jun;52(6):1427–1434. doi: 10.1172/JCI107316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schill W. B., Schumacher G. F. Radial diffusion in gel for micro determination of enzymes. I. Muramidase, alpha-amylase, DNase 1, RNase A, acid phosphatase, and alkaline phosphatase. Anal Biochem. 1972 Apr;46(2):502–533. doi: 10.1016/0003-2697(72)90324-7. [DOI] [PubMed] [Google Scholar]
- Sinha R. K., Rosenthal R. S. Effect of penicillin G on release of peptidoglycan fragments by Neisseria gonorrhoeae: characterization of extracellular products. Antimicrob Agents Chemother. 1981 Jul;20(1):98–103. doi: 10.1128/aac.20.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart-Tull D. E. The immunological activities of bacterial peptidoglycans. Annu Rev Microbiol. 1980;34:311–340. doi: 10.1146/annurev.mi.34.100180.001523. [DOI] [PubMed] [Google Scholar]
- Tipper D. J., Strominger J. L. Mechanism of action of penicillins: a proposal based on their structural similarity to acyl-D-alanyl-D-alanine. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1133–1141. doi: 10.1073/pnas.54.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tynecka Z., Ward J. B. Peptidoglycan synthesis in Bacillus licheniformis. The inhibition of cross-linking by benzylpenicillin and cephaloridine in vivo accompanied by the formation of soluble peptidoglycan. Biochem J. 1975 Jan;146(1):253–267. doi: 10.1042/bj1460253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassalli J. D., Dayer J. M., Wohlwend A., Belin D. Concomitant secretion of prourokinase and of a plasminogen activator-specific inhibitor by cultured human monocytes-macrophages. J Exp Med. 1984 Jun 1;159(6):1653–1668. doi: 10.1084/jem.159.6.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl S. M., Wahl L. M., McCarthy J. B., Chedid L., Mergenhagen S. E. Macrophage activation by mycobacterial water soluble compounds and synthetic muramyl dipeptide. J Immunol. 1979 Jun;122(6):2226–2231. [PubMed] [Google Scholar]
- Waxman D. J., Yu W., Strominger J. L. Linear, uncross-linked peptidoglycan secreted by penicillin-treated Bacillus subtilis. Isolation and characterization as a substrate for penicillin-sensitive D-alanine carboxypeptidases. J Biol Chem. 1980 Dec 10;255(23):11577–11587. [PubMed] [Google Scholar]
- Worton R. G., McCulloch E. A., Till J. E. Physical separation of hemopoietic stem cells from cells forming colonies in culture. J Cell Physiol. 1969 Oct;74(2):171–182. doi: 10.1002/jcp.1040740209. [DOI] [PubMed] [Google Scholar]
- Zeiger A. R., Tuazon C. U., Sheagren J. N. Antibody levels to bacterial peptidoglycan in human sera during the time course of endocarditis and bacteremic infections caused by Staphylococcus aureus. Infect Immun. 1981 Sep;33(3):795–800. doi: 10.1128/iai.33.3.795-800.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiger A. R., Wong W., Chatterjee A. N., Young F. E., Tuazon C. U. Evidence for the secretion of soluble peptidoglycans by clinical isolates of Staphylococcus aureus. Infect Immun. 1982 Sep;37(3):1112–1118. doi: 10.1128/iai.37.3.1112-1118.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]