Abstract
The inducible prostaglandin synthase isoform cyclooxygenase-2 (COX-2) is overexpressed in ~40% of human breast carcinomas, and in precancerous breast lesions, particularly in association with overexpression of human epidermal growth factor receptor 2 (HER2/neu). Experimental breast cancer can be suppressed by pharmacological inhibition or genetic ablation of Cox-2, suggesting potential clinical utility of COX-2 inhibitors with respect to breast cancer. Importantly, several clinical trials have found reduced colorectal adenoma formation in individuals administered selective COX-2 inhibitors. However, such trials also identified increased cardiovascular risk associated with COX-2 inhibitor use. The goal of this research was to test whether improved chemopreventive efficacy could be achieved by combining submaximal doses of a selective COX-2 inhibitor and a retinoid X receptor-selective retinoid (rexinoid). The rate of HER2/neu-induced mammary tumor formation was substantially delayed by coadministration of the COX-2 inhibitor celecoxib (500 ppm in diet) and the rexinoid LGD1069 (10 mg/kg body weight; oral gavage) to MMTV/neu mice. Median time to tumor formation was increased from 304 days to >600 days (P<0.0001). The combination was substantially more effective than either drug individually. Similarly, potent suppression of aromatase activity was observed in mammary tissues from the combination cohort (44% of control; P<0.001). Regulation of aromatase expression and activity by COX-derived prostaglandins is well established. Interestingly however, single agent LGD1069 significantly reduced mammary aromatase activity (71% of control; P<0.001) without modulating eicosanoid levels. Our data demonstrate that simultaneous blockade of COX/prostaglandin signaling and retinoid X receptor-dependent transcription confers potent anticancer efficacy, suggesting a novel avenue for clinical evaluation.
Keywords: HER2/neu, Cox-2, breast cancer, celecoxib, RXR-selective retinoid
Introduction
The inducible prostaglandin (PG) synthase cyclooxygenase-2 (COX-2) is strongly implicated in breast neoplasia (1, 2). COX-2 is overexpressed in approximately 40% of human breast carcinomas, and in 60–80% of preinvasive ductal carcinoma in situ lesions (1, 2). COX-2 overexpression in breast carcinomas correlates with poor prognosis, and with several associated clinical variables, including overexpression of HER2 (human epidermal growth factor receptor 2; also called neu and c-ERBB2) (3–8). Epidemiologic data are broadly consistent with a protumorigenic role for COX enzymes. Several studies have identified reduced breast cancer incidence associated with the use of COX-inhibiting drugs, including aspirin, non-steroidal antiinflammatory drugs (NSAIDs), and selective COX-2 inhibitors (9–17).
Consistent with the human expression data, Cox-2 is also upregulated in rodent mammary tumors (18–22), and thus rodent breast cancer models have proven useful for analyzing the contribution of Cox-2 to mammary neoplasia. Numerous studies have shown that experimental breast cancer can be suppressed by inhibiting Cox activity using either conventional NSAIDs or selective Cox-2 inhibitors (COXibs) (1, 2). Given the observed correlations between COX-2 and HER2/neu overexpression in human breast tumors (3–6), HER2/neu-driven models are of particular interest. Both we and others have shown that HER2/neu-induced tumor formation is significantly delayed by administration of the selective COX-2 inhibitor celecoxib (20, 23). Furthermore, knocking out Cox-2 significantly decreases HER2/neu-induced mammary tumor formation in mice (24). Conversely, transgenic overexpression of COX-2 is sufficient to induce mammary neoplasia in multiparous mice, providing direct evidence of the in vivo oncogenicity of COX-2 (25). Mechanistically, the antitumor action of Cox-2 inhibition has been ascribed to induction of apoptosis, suppression of proliferation, and modulation of tumor cell invasiveness and immune surveillance (2). Additionally, genetic manipulations of Cox-2 gene dosage suggest an important role for Cox-2 in regulating both angiogenesis and the activity of the estrogen synthase aromatase in breast tissues (24, 26–28).
Together, the above datasets identify COX-2 as a potential target for inhibiting breast carcinogenesis, stimulating the development of several clinical trials designed to evaluate COX-2 inhibitors in breast cancer patients (http://www.cancer.gov/clinicaltrials). In support of this approach, randomized clinical trials have shown that COX-2 inhibitors can reduce the incidence of colorectal adenomas (29, 30). Less propitiously, some studies reported an increased risk of cardiovascular events associated with COXib use, reducing enthusiasm for COX-2 inhibitors as cancer chemopreventive agents (29, 30). Nevertheless, the demonstrated role of COX/PG signaling in neoplasia identifies this pathway as an important target. Thus, it behooves us to identify anticancer strategies capable of suppressing this signaling axis with minimal associated toxicity. We hypothesized that enhanced anti-breast cancer activity and diminished side effects could be achieved by using a COXib in combination with a drug from a distinct class. Such combination approaches offer the potential to achieve substantial cancer suppression while minimizing collateral toxicity by combining submaximal doses of two or more agents (31–33). To test this concept, here we have assessed the chemopreventive efficacy with respect to HER2/neu-induced breast cancer of celecoxib in combination with the retinoid LGD1069.
Retinoids have been extensively evaluated as cancer chemopreventive agents (34, 35). Early clinical trials established the utility of retinoids for preventing cancer, using Vitamin A derivatives such as 13-cis-retinoid acid, all-trans retinoic acid and 9-cis-retinoic acid. However, these trials also identified unacceptable toxicity associated with high dose regimens of these naturally-occurring retinoids. Limiting toxicities include teratogenicity, hepatotoxicity, severe headaches, and mucocutaneous toxicity. More recently, compounds with greater selectivity, and hence decreased toxicity, have been developed through increased understanding of retinoid receptor biology (31, 36). Two distinct classes of retinoid receptor have been identified, retinoic acid receptors (RARs) and retinoid X receptors (RXRs). While both RARs and RXRs are ligand-regulated transcription factors of the steroid/thyroid hormone receptor superfamily, the two classes are distinguished by their differential ligand affinities, and by the spectrum of proteins with which they interact. Importantly, RXR-selective retinoids, or rexinoids, have markedly diminished toxicities relative to panretinoids and RAR ligands (37), and thus offer considerable promise as anticancer agents. Of particular interest, both LGD1069 (bexarotene, Targretin) and LG100268 have robust chemopreventive efficacy with respect to experimental breast cancer, including HER2/neu-induced tumorigenesis (31, 36, 38–40).
Here we have evaluated the combination of the selective COX-2 inhibitor celecoxib and the RXR-selective retinoid LGD1069 for preventing HER2/neu-induced mammary tumorigenesis. We show that celecoxib and LGD1069 in combination mediate greater-than-additive suppression of mammary tumor formation in HER2/neu transgenic mice. Furthermore, parallel decreases in mammary aromatase activity were observed, suggesting that aromatase modulation may contribute to tumor suppression mediated by the celecoxib/LGD1069 combination.
Materials and Methods
Reagents and Chemicals
Celecoxib (Celebrex; 4-[5-(4-methylphenyl)-3-trifluoromethyl)-1H-pyrazol-1-yl]benzene-sulfonamide) was purchased from LKT Laboratories, Inc. (St. Paul, MN). LGD1069 (Targretin; bexarotene) was provided by Ligand Pharmaceuticals (San Diego, CA).
Mouse Experimental Procedures
FVB/N-Tg(MMTVneu)202Mul/J mice (MMTV/neu) were obtained from The Jackson Laboratory and bred to produce multiple litters. Virgin female offspring were used in three separate studies.
Study I
Study I has been previously reported (20), and tumor latency data from that study are provided in Table 4 for comparative purposes only. To briefly recapitulate the study design, females were randomly assigned to one of two groups at three weeks of age (Vehicle or Celecoxib), both of which were fed Laboratory Autoclavable Rodent Diet 5010 ad libitum. The diet of mice in the Celecoxib group was supplemented with 500 ppm celecoxib.
Study II
In Study II, females were randomly assigned to one of four groups at eight weeks of age as shown in Table 1 (Group 1, Vehicle; Group 2, Celecoxib; Group 3, LGD1069; Group 4, Celecoxib and LGD1069). All mice were fed AIN-76A diet (Research Diets Inc., New Brunswick, NJ) ad libitum. The diet of groups 2 and 4 was supplemented with 500 ppm celecoxib. Animals in groups 1 and 2 were gavaged five days per week with 100μl sesame oil (Croda, Inc., Edison, NJ). Animals in groups 3 and 4 were gavaged five days per week with LGD1069 (10 mg/kg body weight) in 100μl sesame oil. Mild dermatitis was observed in a subset of animals in each group, predominantly manifesting as auricular irritation and alopecia on dorsal surfaces in the neck and scapular region. Onset occurred between 7 and 15 months of age (Group 1, n=1; Group 2, n=2; Group 3, n=5; Group 4, n=6). Mouse weights were measured weekly. No significant difference in animal weight was observed in any treatment group relative to the control group, indicative of the absence of drug-induced cachexia. Thus, all drug regimens were well-tolerated with minimal side-effects. Expression of the neu transgene was unaltered by any of the treatment regimens (data not shown).
Table 1.
Experimental Groups
| Group | Diet, ad libitum | Gavage, 5 days per week |
|---|---|---|
| 1. Vehicle | AIN-76A | Sesame oil |
| 2. Celecoxib | AIN-76A containing Celecoxib (500 ppm) | Sesame oil |
| 3. LGD1069 | AIN-76A | LGD1069 in Sesame oil (10 mg/kg body weight) |
| 4. Both (Celecoxib and LGD1069) | AIN-76A containing Celecoxib (500 ppm) | LGD1069 in Sesame oil (10 mg/kg body weight) |
Study III
In Study III, females were randomly assigned to one of two groups at three weeks of age (Vehicle or Celecoxib) and fed control AIN-76A diet or AIN-76A diet supplemented with 500 ppm celecoxib, respectively.
In all three studies, mice were palpated twice weekly for mammary gland tumors, and the age at appearance of the first tumor was recorded (tumor latency). In Studies II and III, animals were sacrificed two months after initial tumor detection, and the number of tumors of at least 0.5 cm diameter was scored (tumor multiplicity). Non-tumor-bearing animals were maintained for their natural lifespan, or up to 98 weeks, whichever was shorter. All animals were euthanized by CO2 asphyxiation, in accordance with the recommendations of the Panel on Euthanasia of the American Veterinary Medical Association. Mammary tissues were harvested from sacrificed animals, snap-frozen in liquid nitrogen and stored at −80oC. Additionally, a portion of mammary tissues and tumors were formalin-fixed and embedded in paraffin. Tumors were confirmed by histological evaluation. Non-tumorous mammary glands were used for subsequent analyses.
Determination of Eicosanoid Levels
Resected mammary glands were homogenized, and eicosanoids were extracted and assayed using LC-MS-MS as previously described (41).
Assay of Celecoxib Levels
Blood was collected from 5.5 month old animals by retroorbital bleeding, and plasma levels of celecoxib were assayed by LC-MS as previously described (20).
Aromatase Activity Assays
Resected mammary glands were homogenized, and mammary aromatase activity was assayed by measuring the release of 3H2O from [1-3H]androstenedione as described previously (28).
Statistical Analysis
Kaplan-Meier curves of tumor-free survival in control and drug-treated cohorts were compared using a log-rank test (two-tailed) (Prism 4.0 for Macintosh, GraphPad Software Inc., San Diego, CA). Poisson regression analysis was used to compare tumor multiplicity in each group, based on the assumption that the number of tumors per animal would follow a Poisson distribution. Mean tumor multiplicity was compared in a pairwise manner, and P-values were corrected for multiple comparisons using Tukey’s method. Repeated measure ANOVA was used to compare the mean mouse weights in each experimental group over time. Mammary eicosanoid data, shown as mean±SD, were compared by one-way ANOVA, and further analyzed by Dunnett’s test to effect comparisons between each treatment group and the control group adjusting for multiple comparisons. Aromatase activity data, shown as mean+SD, were evaluated for statistical significance using one-way ANOVA, followed by pairwise testing using Tukey’s method to adjust for multiple comparisons.
Results and Discussion
Celecoxib and LGD1069 mediate greater-than-additive suppression of mammary tumorigenesis
The primary goal of this research was to compare the chemopreventive efficacy of the COX-2 inhibitor celecoxib and the RXR-selective retinoid LGD1069 alone and in combination, in a breast cancer model. Our ultimate aim was to determine whether greater protection could be achieved by combining drugs from two distinct classes than could be achieved using either drug individually. This combination chemoprevention approach offers the possibility of increased preventive efficacy with minimal associated toxicity through utilization of submaximal doses of individual agents. Thus, drug doses for our study were selected based on the following information. Celecoxib delays tumor formation in MMTV/neu mice when administered in food at either 500 or 900 ppm, while 1500 ppm celecoxib induces cachexia and weight loss in this mouse strain (20, 23). LGD1069 has dose-dependent activity in the MMTV/neu model over the range 10–100mg/kg body weight (40). Therefore, we selected 500 ppm celecoxib and 10 mg/kg LGD1069 as submaximal doses for use in our study.
Virgin female MMTV/neu mice were randomly assigned to one of four experimental groups at 8 weeks of age, as shown in Table 1. Tumor incidence was measured as a function of time in all four groups. The rate of formation of mammary tumors was significantly delayed in the LGD1069 cohort relative to the Vehicle group (Fig. 1, Table 2), consistent with the previously reported chemopreventive action of LGD1069 with respect to HER2/neu-induced breast cancer (40). Mammary tumors were detected in 50% of control animals by 304 days of age vs. 420 days of age in LGD1069-treated mice (T50 = 304 days vs. T50 = 420 days; P=0.0085). Unexpectedly, celecoxib alone failed to delay tumor onset in this study (Fig. 1, Table 2), in contrast to previous reports (20, 23). Failure to achieve comparable circulating celecoxib levels in this study relative to our earlier study would provide a trivial explanation for the lack of effect of celecoxib in the present study. However, serum celecoxib levels in this experiment were 2.8±0.7μM (mean±SD; range 2.2–3.6μM), and thus extremely similar to those achieved in our previous study (mean, 2.4μM) (20). We hypothesize that the variable protective efficacy of celecoxib between studies may be attributable to the use of different diets in each experiment (discussed below).
Fig. 1. Suppression of mammary tumorigenesis by Celecoxib and LGD1069.
Female MMTV/neu transgenic mice were subjected to the following drug regimens, starting at 8 weeks of age. Group 1 were fed control diet and gavaged 5 days per week with 100μl sesame oil (Vehicle; black squares). Group 2 were fed diet containing 500 ppm celecoxib and and gavaged 5 days per week with 100μl sesame oil (Celecoxib; yellow diamonds). Group 3 received control diet and were gavaged 5 days per week with 10 mg/kg body weight LGD1069 in 100μl sesame oil (LGD1069; red triangles). Group 4 were fed celecoxib-containing diet and gavaged with LGD1069 (Both; blue circles). Statistical comparisons of tumor latency were effected using the log-rank test, and data are shown in Table 2. The median time to tumor formation was significantly delayed by LGD1069 administration. Celecoxib alone was ineffective, but significantly increased the delay in tumor formation mediated by LGD1069.
Table 2.
Effect of celecoxib and LGD1069 on tumor latency and multiplicity in MMTV/neu mice
| Drug Regimen | Median time to tumor formation, days | P | Tumor Multiplicity (mean ± SD) | P |
|---|---|---|---|---|
| Vehicle | 304 days (n=32) | - | 1.31 ± 1.00 (n=32) | - |
| Celecoxib (500 PPM) | 285 days (n=29) | 0.26 * | 2.03 ± 1.38 (n=29) | 0.13 ‡ |
| LGD1069 (10 mg/kg) | 420 days (n=26) | 0.0085 * | 1.27 ± 0.87 (n=26) | 1.0 ‡ |
| Celecoxib and LGD1069 | > 600 days (n=26) | <0.0001 * | 0.58 ± 0.78 (n=24) | 0.041 ‡ |
| 0.0125 $ | 0.068 # |
P value obtained by comparison with vehicle-treated cohort (log-rank test)
P value obtained by comparison with LGD1069-treated cohort (log-rank test)
P value obtained by comparison with vehicle-treated cohort (Poisson regression, corrected for multiple comparisons using Tukey’s method)
P value obtained by comparison with LGD1069-treated cohort (Poisson regression, corrected for multiple comparisons using Tukey’s method)
The combination of celecoxib and LGD1069 was markedly more effective at suppressing tumor formation than either drug alone (Fig. 1, Table 2). Tumor latency was more than doubled in the Celecoxib/LGD1069 cohort relative to the untreated group (Group 1, T50 = 304 days vs. Group 4, T50 > 600 days; P=0.0001). Tumor multiplicity was also significantly reduced in the combination group relative to the control cohort (Table 2, P=0.041). Celecoxib, although ineffective as a single agent in this study, substantially enhanced the delay in tumor onset elicited by LGD1069 (LGD1069 alone, T50 = 420 days vs. LGD1069/Celecoxib, T50 > 600 days; P=0.0125). Importantly, serum celecoxib levels were not elevated in the combination group relative to the Celecoxib cohort, excluding the possibility that increased efficacy reflected elevated circulating celecoxib levels (Group 2, 2.8±0.7μM; Group 4, 1.6±0.8μM; P=0.12). A similar greater-than-additive antitumor effect of celecoxib and LGD1069 was also observed in the C3(1)-SV40 T antigen breast cancer model (data not shown), in which we have previously demonstrated single agent activity of LGD1069 (42). Thus, enhanced suppression of mammary tumorigenesis can be achieved by combining the selective COX-2 inhibitor celecoxib and the RXR-selective retinoid LGD1069.
Drug-mediated peturbations of mammary eicosanoid levels
We previously reported a reduction of approximately 50% in mammary PGE2 levels in celecoxib-treated mice (20). In the present study, celecoxib administration resulted in a modest 34% decrease in mean mammary PGE2 levels and a 49% decrease in mean mammary PGD2 levels (Table 3), although neither of these changes achieved statistical significance. Interestingly, substantially increased levels of leukotriene B4 (LTB4) and 12-hydroxyeicosatetraenoic acid (12-HETE) were observed in mammary tissues from celecoxib-treated animals (Table 3). Mean levels of mammary LTB4 and 12-HETE were increased to 337% and 183%, respectively, of those in mammary tissues from vehicle-treated mice (P<0.001 and P=0.11, respectively). These data suggest that a metabolic shunt is occurring in mammary tissues from celecoxib-treated animals, with arachidonic acid being metabolized by 5-lipoxygenase to LTA4 and subsequent byproducts, and by 12-lipoxygenase to 12-HETE, rather than by Cox-2.
Table 3.
Effect of celecoxib and LGD1069 on mammary eicosanoid levels in MMTV/neu mice
| Drug Regimen | PGE2, ng/mg protein (P=0.09) * | PGD2, ng/mg protein (P=0.15) * | LTB4, ng/mg protein (P<0.001) * | 12-HETE, ng/mg protein (P=0.19) * |
|---|---|---|---|---|
| Vehicle | 1.29±0.50 | 2.86±1.55 | 0.070±0.029 | 7.85±2.99 |
| Celecoxib | 0.85±0.49 (P=0.46) ‡ | 1.45±0.65 (P=0.17) ‡ | 0.236±0.082 (P<0.001) ‡ | 14.36±7.20 (P=0.11) ‡ |
| LGD1069 | 1.50±0.93 (P=0.91) ‡ | 2.63±1.82 (P=0.98) ‡ | 0.097±0.049 (P=0.78) ‡ | 9.04±5.55 (P=0.96) ‡ |
| Celecoxib and LGD1069 | 0.62±0.37 (P=0.18) ‡ | 1.53±0.57 (P=0.20) ‡ | 0.102±0.064 (P=0.69) ‡ | 9.79±4.31 (P=0.86) ‡ |
P value obtained by one-way ANOVA analysis across the different treatment groups
P value obtained by comparison with vehicle-treated cohort (using Dunnett’s test and correcting for multiple comparisons)
Although LGD1069 has been shown to downregulate Cox-2 expression both in vitro in human breast cancer cell lines and in vivo in mammary glands from MMTV/neu mice (43, 44), LGD1069 alone was ineffective at modulating mammary PG levels in vivo (Table 3). Unexpectedly, LGD1069 coadministration appeared to suppress the celecoxib-mediated arachidonic acid shunt to 5-lipoxygenase and 12-lipoxygenase. These data demonstrate that LGD1069 has complex effects on eicosanoid metabolism, elucidation of which will require further studies.
Mouse diet modulates susceptibility to celecoxib-mediated tumor suppression
The failure of celecoxib to delay tumor formation in MMTV/neu mice in the present study contrasts with previous data from our group and from other investigators (20, 23). Three alterations in experimental design relative to our earlier study were implemented in the present study, any of which could provide a basis for the discrepant observations. Firstly, the age at initiation of drug administration was changed from three weeks in our first study (Study I) to eight weeks in the present study (Study II). Secondly, the diet was switched from 5010 mouse chow to AIN-76A, a purified diet. Thirdly, all animals received 100μl sesame oil by gavage five days per week. To evaluate the influence of timing of drug initiation on celecoxib efficacy, a third study was performed (Study III) in which animals were fed AIN-76A, as in Study II, and celecoxib administration was initiated at three weeks, as in Study I. Tumor latency in the Vehicle cohort was similar to that observed in Study II, and the rate of tumor formation was not delayed by celecoxib administration (Table 4). Comparison of tumor latencies in control cohorts in all three experiments suggests that the alteration in diet could account for the lack of concordance between these studies. Specifically, tumors formed substantially more rapidly in the Vehicle cohort in Study I than in Study II or III (for example, T50 = 32 weeks in Study I vs. T50 = 43 weeks in Study II; Table 4), suggesting that tumor formation is accelerated in MMTV/neu mice fed 5010 mouse chow compared with those fed AIN-76A diet, presumably due to differences in nutritional constituents. Since tumor latency was similar in the Vehicle cohort in Studies II and III, but only animals in Study II received sesame oil, we infer that sesame oil administration in Study II is unlikely to account for the increased tumor latency observed in Studies II/III relative to Study I.
Table 4.
Diet affects tumor latency and the protective efficacy of celecoxib in MMTV/neu mice
| Experiment | Mouse Diet (gavage) | Age at drug initiation | Tumor Latency | |
|---|---|---|---|---|
| Vehicle | Celecoxib | |||
| Study I $ | 5010 (no gavage) | 3 weeks | 32 weeks | 40 weeks * |
| Study II | AIN-76A (sesame oil) | 8 weeks | 43 weeks | 41 weeks‡ |
| Study III | AIN-76A (no gavage) | 3 weeks | 41 weeks | 39 weeks ¶ |
Data for Study I were previously reported in Howe et al. (2002) Cancer Research, 62: 405–7 (20)
P=0.003, compared to vehicle-treated cohort
P=0.26, compared to vehicle-treated cohort
P=0.17, compared to vehicle-treated cohort
Comparisons of tumor latencies in Vehicle-treated animals in the three studies, and of the relative chemopreventive efficacy of celecoxib, thus support the following conclusions. Firstly, the rate of mammary tumor formation is accelerated in MMTV/neu mice fed 5010 mouse chow relative to those consuming AIN-76A purified diet (Study I vs. Studies II/III). Secondly, consumption of 5010 mouse chow is associated with susceptibility to celecoxib-mediated chemoprotection against breast cancer (Study I vs. Studies II/III). Thirdly, initiating drug treatment at three weeks of age in AIN-76A-fed mice is insufficient to confer susceptibility to celecoxib-mediated protection (Study II vs. III).
Since HER2/neu-induced breast neoplasia is suppressed by either pharmacological inhibition or genetic ablation of the PG synthase Cox-2 (20, 23, 24), increasing mammary PG levels would be predicted to accelerate HER2/neu-driven mammary tumor formation. We speculate that 5010 chow may contain components that increase mammary eicosanoid levels. Consistent with this notion, dietary administration of celecoxib reduced mammary PGE2 by only 34% in animals consuming AIN-76A (Table 3), compared with the 47% reduction we previously reported for 5010-fed animals (20), suggesting that animals consuming 5010 chow may have elevated basal mammary eicosanoid levels.
Celecoxib and LGD1069 suppress mammary aromatase activity
Our next goal was to investigate potential mechanisms by which celecoxib and LGD1069 might induce greater-than-additive suppression of HER2/neu-induced mammary tumor formation. Substantial evidence supports a functional relationship between COX/PG signaling and the estrogen synthase aromatase. Correlations have been identified between expression of cyclooxygenase enzymes and the aromatase cytochrome P450 in human breast cancers (45, 46). These correlations are believed to reflect a causal relationship since PG signaling can stimulate transcription of the CYP19 gene encoding aromatase (28, 47–51). Furthermore, aromatase activity is reduced in mammary tissues from Cox-2 knockout mice and, conversely, is increased by transgenic COX-2 overexpression (27, 28). Therefore, we focused on aromatase modulation as a potential effector of celecoxib/LGD1069-mediated chemoprotection.
Aromatase activity was compared in mammary tissues from all four experimental groups of the celecoxib/LGD1069 combination chemoprevention experiment (Study II). Celecoxib alone caused a 17% decrease in mammary aromatase activity relative to that in the Vehicle cohort (P<0.001; Fig. 2). The magnitude of the celecoxib effect was substantially less than the approximately 60% reduction in mammary aromatase activity we had previously observed in Cox-2 knockout mice (28), consistent with the comparatively modest celecoxib-mediated reductions in mammary PGE2 levels in the current experiment (Table 3).
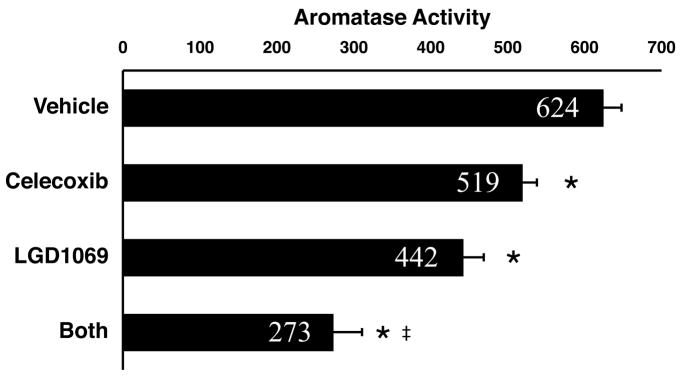
Fig. 2. Celecoxib and LGD1069 suppress mammary aromatase activity.
Aromatase activity was measured in mammary glands harvested from mice from each of the four experimental groups in the experiment shown in Fig. 1 (Study II). Aromatase activity in all three drug-treated groups was significantly lower than that in the control group (*; P<0.001). Celecoxib caused a modest reduction in mammary aromatase activity (83% of control). Aromatase activity was decreased to 71% in tissues from mice treated with LGD1069 alone, and to 44% in tissues from mice treated with both celecoxib and LGD1069. Aromatase activity in tissues from the celecoxib/LGD1069 combination group was significantly less than that in samples from animals treated with LGD1069 alone (‡, P<0.001). Enzyme activity is expressed as fmol/μg protein/h. Data shown are mean + SD, n=6 per group.
Mammary aromatase activity in LGD1069-treated animals was reduced by 29% (P<0.001; Fig. 2). The rexinoid LG101305 has previously been shown to reduce transcription from the CYP19 promoter in cultured human breast adipose cells (52). Our data provide the first in vivo correlate for these tissue culture data. The mechanism by which rexinoids suppress aromatase transcription has not yet been elucidated, but may be independent of prostanoid metabolism since LGD1069 alone did not significantly affect in vivo mammary eicosanoid levels (Table 3).
Strikingly, the combination of celecoxib and LGD1069 potently suppressed mammary aromatase activity, causing a greater-than-additive reduction (56%, P<0.001 compared with Vehicle, Fig. 2). The relative potency of the three drug regimens for depressing mammary aromatase activity was: celecoxib/LGD1069 > LGD1069 > celecoxib. Since the drug regimens ranked in the same order of efficacy with respect to tumor suppression, we speculate that inhibition of aromatase expression may contribute to the observed chemopreventive activity. While frank tumors in MMTV/neu mice lack estrogen receptor (ER) expression, ER is expressed in normal-appearing and hyperplastic MMTV/neu mammary tissues (40, 53). Importantly, tumor formation in the MMTV/neu strain is antagonized by the selective ER modulators (SERMs) arzoxifene and acolbifene, indicating that estrogen signaling contributes to tumorigenesis in this model (39). Thus, we conclude that drug-mediated alterations in mammary aromatase activity may contribute to the observed chemopreventive activity of the celecoxib/LGD1069 combination.
Conclusions
The major goal of this study was to compare the chemopreventive efficacy of celecoxib and LGD1069 in combination with that of each of the two drugs individually, in a breast cancer model. The MMTV/neu strain was used for this experiment, since we had previously demonstrated chemopreventive efficacy of both celecoxib and LGD1069 as single agents in this model (20, 40). We observed a potent, greater-than-additive action of celecoxib and LGD1069 in suppressing mammary tumor formation (Fig. 1). Interestingly, we detected corresponding decreases in mammary aromatase activity (Fig. 2), leading us to conclude that aromatase suppression may be a component of the anticancer action of the celecoxib/LGD1069 combination.
Our data have potential implications for the clinical management of breast cancer. Combination approaches are thought likely to afford greater benefit than can be achieved using single agents, with the additional benefit of minimizing collateral toxicity (31–33). For example, simultaneous targeting of COX-2 and HER-2/neu, using celecoxib and anti-HER-2 antibodies, has been shown to inhibit experimental colorectal carcinoma growth more effectively than either agent alone (54). This combination approach has been extended to experimental breast cancer. Rexinoid/SERM combinations, for example, have striking synergistic efficacy in rodent breast cancer models (31, 39). Furthermore, clinical trials are ongoing to evaluate celecoxib in combination with aromatase inhibitors as neoadjuvant therapy. LGD1069 is FDA-approved for treating refractory advanced stage cutaneous T-cell lymphoma, and is currently being evaluated in numerous clinical studies, including breast cancer-related trials. Additionally, several rexinoids with even greater RXR selectivity and hence diminished side effects have been developed, including LG100268 and UAB30 (31, 36, 55). These agents have demonstrated cancer preventive activity and may be useful candidates for future clinical development. The results of our present study suggest that drug combinations targeting COX/PG signaling and RXR-dependent transcription may have clinical utility for breast cancer prevention.
Acknowledgments
Preliminary statistical support for this study was provided by Paul Christos.
Grant Support: This work was supported by NIH Grants R03 CA105458 (L.R.H.), R03 CA119273 (L.R.H.), R01 CA078480 (P.H.B.), a Breast Cancer Alliance Young Investigator Grant (L.R.H.), the Breast Cancer Research Foundation (P.H.B., A.J.D.), and the Irving Weinstein Foundation, Inc. (L.R.H.).
References
- 1.Howe LR. Inflammation and breast cancer. Cyclooxygenase/prostaglandin signaling and breast cancer. Breast Cancer Res. 2007;9:210. doi: 10.1186/bcr1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howe LR. Cyclooxygenase-2 and Breast Cancer. In: Yao AP, editor. Trends in Breast Cancer Research. New York: Nova; 2005. pp. 1–38. [Google Scholar]
- 3.Boland GP, Butt IS, Prasad R, Knox WF, Bundred NJ. COX-2 expression is associated with an aggressive phenotype in ductal carcinoma in situ. Br J Cancer. 2004;90:423–9. doi: 10.1038/sj.bjc.6601534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ristimaki A, Sivula A, Lundin J, et al. Prognostic significance of elevated cyclooxygenase-2 expression in breast cancer. Cancer Res. 2002;62:632–5. [PubMed] [Google Scholar]
- 5.Subbaramaiah K, Norton L, Gerald W, Dannenberg AJ. Cyclooxygenase-2 is Overexpressed in HER-2/neu-Positive Breast Cancer. Evidence for Involvement of AP-1 and PEA3. J Biol Chem. 2002;277:18649–57. doi: 10.1074/jbc.M111415200. [DOI] [PubMed] [Google Scholar]
- 6.Wulfing P, Diallo R, Muller C, et al. Analysis of cyclooxygenase-2 expression in human breast cancer: high throughput tissue microarray analysis. J Cancer Res Clin Oncol. 2003;129:375–82. doi: 10.1007/s00432-003-0459-1. [DOI] [PubMed] [Google Scholar]
- 7.Denkert C, Winzer KJ, Muller BM, et al. Elevated expression of cyclooxygenase-2 is a negative prognostic factor for disease free survival and overall survival in patients with breast carcinoma. Cancer. 2003;97:2978–87. doi: 10.1002/cncr.11437. [DOI] [PubMed] [Google Scholar]
- 8.Spizzo G, Gastl G, Wolf D, et al. Correlation of COX-2 and Ep-CAM overexpression in human invasive breast cancer and its impact on survival. Br J Cancer. 2003;88:574–8. doi: 10.1038/sj.bjc.6600741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedman GD, Ury HK. Initial screening for carcinogenicity of commonly used drugs. J Natl Cancer Inst. 1980;65:723–33. doi: 10.1093/jnci/65.4.723. [DOI] [PubMed] [Google Scholar]
- 10.Harris RE, Beebe-Donk J, Alshafie GA. Reduction in the risk of human breast cancer by selective cyclooxygenase-2 (COX-2) inhibitors. BMC Cancer. 2006;6:27. doi: 10.1186/1471-2407-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris RE, Chlebowski RT, Jackson RD, et al. Breast cancer and nonsteroidal anti-inflammatory drugs: prospective results from the Women's Health Initiative. Cancer Res. 2003;63:6096–101. [PubMed] [Google Scholar]
- 12.Harris RE, Namboodiri KK, Farrar WB. Nonsteroidal antiinflammatory drugs and breast cancer. Epidemiology. 1996;7:203–5. doi: 10.1097/00001648-199603000-00017. [DOI] [PubMed] [Google Scholar]
- 13.Khuder SA, Mutgi AB. Breast cancer and NSAID use: a meta-analysis. Br J Cancer. 2001;84:1188–92. doi: 10.1054/bjoc.2000.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rahme E, Ghosn J, Dasgupta K, Rajan R, Hudson M. Association between frequent use of nonsteroidal anti-inflammatory drugs and breast cancer. BMC Cancer. 2005;5:159. doi: 10.1186/1471-2407-5-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schreinemachers DM, Everson RB. Aspirin use and lung, colon, and breast cancer incidence in a prospective study. Epidemiology. 1994;5:138–46. doi: 10.1097/00001648-199403000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Sharpe CR, Collet JP, McNutt M, Belzile E, Boivin JF, Hanley JA. Nested case-control study of the effects of non-steroidal anti-inflammatory drugs on breast cancer risk and stage. Br J Cancer. 2000;83:112–20. doi: 10.1054/bjoc.2000.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Terry MB, Gammon MD, Zhang FF, et al. Association of frequency and duration of aspirin use and hormone receptor status with breast cancer risk. Jama. 2004;291:2433–40. doi: 10.1001/jama.291.20.2433. [DOI] [PubMed] [Google Scholar]
- 18.Hamid R, Singh J, Reddy BS, Cohen LA. Inhibition by dietary menhaden oil of cyclooxygenase-1 and -2 in N- nitrosomethylurea-induced rat mammary tumors. Int J Oncol. 1999;14:523–8. doi: 10.3892/ijo.14.3.523. [DOI] [PubMed] [Google Scholar]
- 19.Howe LR, Crawford HC, Subbaramaiah K, Hassell JA, Dannenberg AJ, Brown AMC. PEA3 is upregulated in response to Wnt1 and activates the expression of cyclooxygenase-2. J Biol Chem. 2001;276:20108–15. doi: 10.1074/jbc.M010692200. [DOI] [PubMed] [Google Scholar]
- 20.Howe LR, Subbaramaiah K, Patel J, et al. Celecoxib, a selective cyclooxygenase-2 inhibitor, protects against human epidermal growth factor receptor 2 (HER-2)/neu-induced breast cancer. Cancer Res. 2002;62:5405–7. [PubMed] [Google Scholar]
- 21.Nakatsugi S, Ohta T, Kawamori T, et al. Chemoprevention by nimesulide, a selective cyclooxygenase-2 inhibitor, of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP)-induced mammary gland carcinogenesis in rats. Jpn J Cancer Res. 2000;91:886–92. doi: 10.1111/j.1349-7006.2000.tb01030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robertson FM, Parrett ML, Joarder FS, et al. Ibuprofen-induced inhibition of cyclooxygenase isoform gene expression and regression of rat mammary carcinomas. Cancer Lett. 1998;122:165–75. doi: 10.1016/s0304-3835(97)00387-x. [DOI] [PubMed] [Google Scholar]
- 23.Lanza-Jacoby S, Miller S, Flynn J, et al. The cyclooxygenase-2 inhibitor, celecoxib, prevents the development of mammary tumors in Her-2/neu mice. Cancer Epidemiol Biomarkers Prev. 2003;12:1486–91. [PubMed] [Google Scholar]
- 24.Howe LR, Chang SH, Tolle KC, et al. HER2/neu-induced mammary tumorigenesis and angiogenesis are reduced in cyclooxygenase-2 knockout mice. Cancer Res. 2005;65:10113–9. doi: 10.1158/0008-5472.CAN-05-1524. [DOI] [PubMed] [Google Scholar]
- 25.Liu CH, Chang SH, Narko K, et al. Overexpression of cyclooxygenase-2 is sufficient to induce tumorigenesis in transgenic mice. J Biol Chem. 2001;276:18563–9. doi: 10.1074/jbc.M010787200. [DOI] [PubMed] [Google Scholar]
- 26.Chang SH, Liu CH, Conway R, et al. Role of prostaglandin E2-dependent angiogenic switch in cyclooxygenase 2-induced breast cancer progression. Proc Natl Acad Sci U S A. 2004;101:591–6. doi: 10.1073/pnas.2535911100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muller-Decker K, Berger I, Ackermann K, et al. Cystic duct dilatations and proliferative epithelial lesions in mouse mammary glands upon keratin 5 promoter-driven overexpression of cyclooxygenase-2. Am J Pathol. 2005;166:575–84. doi: 10.1016/S0002-9440(10)62279-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Subbaramaiah K, Howe LR, Port ER, et al. HER-2/neu status is a determinant of mammary aromatase activity in vivo: evidence for a cyclooxygenase-2-dependent mechanism. Cancer Res. 2006;66:5504–11. doi: 10.1158/0008-5472.CAN-05-4076. [DOI] [PubMed] [Google Scholar]
- 29.Bertagnolli MM. Chemoprevention of colorectal cancer with cyclooxygenase-2 inhibitors: two steps forward, one step back. Lancet Oncol. 2007;8:439–43. doi: 10.1016/S1470-2045(07)70139-0. [DOI] [PubMed] [Google Scholar]
- 30.Bresalier RS. Chemoprevention of colorectal neoplasia: advances and controversies (the COX-2 story) Curr Opin Gastroenterol. 2007;23:44–7. doi: 10.1097/MOG.0b013e328011c482. [DOI] [PubMed] [Google Scholar]
- 31.Liby KT, Yore MM, Sporn MB. Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer. Nat Rev Cancer. 2007;7:357–69. doi: 10.1038/nrc2129. [DOI] [PubMed] [Google Scholar]
- 32.Steele VE, Kelloff GJ. Development of cancer chemopreventive drugs based on mechanistic approaches. Mutat Res. 2005;591:16–23. doi: 10.1016/j.mrfmmm.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 33.Dannenberg AJ, Lippman SM, Mann JR, Subbaramaiah K, DuBois RN. Cyclooxygenase-2 and epidermal growth factor receptor: pharmacologic targets for chemoprevention. J Clin Oncol. 2005;23:254–66. doi: 10.1200/JCO.2005.09.112. [DOI] [PubMed] [Google Scholar]
- 34.Lippman SM, Lotan R. Advances in the development of retinoids as chemopreventive agents. J Nutr. 2000;130:479S–82S. doi: 10.1093/jn/130.2.479S. [DOI] [PubMed] [Google Scholar]
- 35.Moon RC, Mehta RG, Rao KVN. Retinoids and cancer in experimental animals. In: Sporn MB, Roberts AB, Goodman DS, editors. The Retinoids: Biology, Chemistry and Medicine. 2. New York: Raven Press; 1994. pp. 573–95. [Google Scholar]
- 36.Howe LR. Rexinoids and Breast Cancer Prevention. Clin Cancer Res. 2007;13:5983–7. doi: 10.1158/1078-0432.CCR-07-1065. [DOI] [PubMed] [Google Scholar]
- 37.Miller VA, Benedetti FM, Rigas JR, et al. Initial clinical trial of a selective retinoid X receptor ligand, LGD1069. J Clin Oncol. 1997;15:790–5. doi: 10.1200/JCO.1997.15.2.790. [DOI] [PubMed] [Google Scholar]
- 38.Gottardis MM, Bischoff ED, Shirley MA, Wagoner MA, Lamph WW, Heyman RA. Chemoprevention of mammary carcinoma by LGD1069 (Targretin): an RXR-selective ligand. Cancer Res. 1996;56:5566–70. [PubMed] [Google Scholar]
- 39.Liby K, Rendi M, Suh N, et al. The combination of the rexinoid, LG100268, and a selective estrogen receptor modulator, either arzoxifene or acolbifene, synergizes in the prevention and treatment of mammary tumors in an estrogen receptor-negative model of breast cancer. Clin Cancer Res. 2006;12:5902–9. doi: 10.1158/1078-0432.CCR-06-1119. [DOI] [PubMed] [Google Scholar]
- 40.Wu K, Zhang Y, Xu XC, et al. The Retinoid X Receptor-Selective Retinoid, LGD1069, Prevents the Development of Estrogen Receptor-Negative Mammary Tumors in Transgenic Mice. Cancer Res. 2002;62:6376–80. [PubMed] [Google Scholar]
- 41.Yang P, Chan D, Felix E, et al. Determination of endogenous tissue inflammation profiles by LC/MS/MS: COX- and LOX-derived bioactive lipids. Prostaglandins, Leukotrienes, and Essential Fatty Acids. 2006;75:385–95. doi: 10.1016/j.plefa.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 42.Wu K, Kim HT, Rodriquez JL, et al. Suppression of mammary tumorigenesis in transgenic mice by the RXR-selective retinoid, LGD1069. Cancer Epidemiol Biomarkers Prev. 2002;11:467–74. [PubMed] [Google Scholar]
- 43.Kim HT, Kong G, Denardo D, et al. Identification of biomarkers modulated by the rexinoid LGD1069 (bexarotene) in human breast cells using oligonucleotide arrays. Cancer Res. 2006;66:12009–18. doi: 10.1158/0008-5472.CAN-05-2515. [DOI] [PubMed] [Google Scholar]
- 44.Kong G, Kim HT, Wu K, et al. The retinoid X receptor-selective retinoid, LGD1069, down-regulates cyclooxygenase-2 expression in human breast cells through transcription factor crosstalk: implications for molecular-based chemoprevention. Cancer Res. 2005;65:3462–9. doi: 10.1158/0008-5472.CAN-03-2912. [DOI] [PubMed] [Google Scholar]
- 45.Brodie AM, Lu Q, Long BJ, et al. Aromatase and COX-2 expression in human breast cancers. J Steroid Biochem Mol Biol. 2001;79:41–7. doi: 10.1016/s0960-0760(01)00131-5. [DOI] [PubMed] [Google Scholar]
- 46.Brueggemeier RW, Quinn AL, Parrett ML, Joarder FS, Harris RE, Robertson FM. Correlation of aromatase and cyclooxygenase gene expression in human breast cancer specimens. Cancer Lett. 1999;140:27–35. doi: 10.1016/s0304-3835(99)00050-6. [DOI] [PubMed] [Google Scholar]
- 47.Agarwal VR, Bulun SE, Leitch M, Rohrich R, Simpson ER. Use of alternative promoters to express the aromatase cytochrome P450 (CYP19) gene in breast adipose tissues of cancer-free and breast cancer patients. J Clin Endocrinol Metab. 1996;81:3843–9. doi: 10.1210/jcem.81.11.8923826. [DOI] [PubMed] [Google Scholar]
- 48.Chen S, Zhou D, Okubo T, Kao YC, Yang C. Breast tumor aromatase: functional role and transcriptional regulation. Endocr Relat Cancer. 1999;6:149–56. doi: 10.1677/erc.0.0060149. [DOI] [PubMed] [Google Scholar]
- 49.Diaz-Cruz ES, Shapiro CL, Brueggemeier RW. Cyclooxygenase inhibitors suppress aromatase expression and activity in breast cancer cells. J Clin Endocrinol Metab. 2005;90:2563–70. doi: 10.1210/jc.2004-2029. [DOI] [PubMed] [Google Scholar]
- 50.Prosperi JR, Robertson FM. Cyclooxygenase-2 directly regulates gene expression of P450 Cyp19 aromatase promoter regions pII, pI.3 and pI.7 and estradiol production in human breast tumor cells. Prostaglandins Other Lipid Mediat. 2006;81:55–70. doi: 10.1016/j.prostaglandins.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 51.Zhao Y, Agarwal VR, Mendelson CR, Simpson ER. Estrogen biosynthesis proximal to a breast tumor is stimulated by PGE2 via cyclic AMP, leading to activation of promoter II of the CYP19 (aromatase) gene. Endocrinology. 1996;137:5739–42. doi: 10.1210/endo.137.12.8940410. [DOI] [PubMed] [Google Scholar]
- 52.Rubin GL, Duong JH, Clyne CD, et al. Ligands for the peroxisomal proliferator-activated receptor gamma and the retinoid X receptor inhibit aromatase cytochrome P450 (CYP19) expression mediated by promoter II in human breast adipose. Endocrinology. 2002;143:2863–71. doi: 10.1210/endo.143.8.8932. [DOI] [PubMed] [Google Scholar]
- 53.Zhang X, Podsypanina K, Huang S, et al. Estrogen receptor positivity in mammary tumors of Wnt-1 transgenic mice is influenced by collaborating oncogenic mutations. Oncogene. 2005;24:4220–31. doi: 10.1038/sj.onc.1208597. [DOI] [PubMed] [Google Scholar]
- 54.Mann M, Sheng H, Shao J, et al. Targeting cyclooxygenase 2 and HER-2/neu pathways inhibits colorectal carcinoma growth. Gastroenterology. 2001;120:1713–9. doi: 10.1053/gast.2001.24844. [DOI] [PubMed] [Google Scholar]
- 55.Grubbs CJ, Lubet RA, Atigadda VR, et al. Efficacy of new retinoids in the prevention of mammary cancers and correlations with short-term biomarkers. Carcinogenesis. 2006;27:1232–9. doi: 10.1093/carcin/bgi308. [DOI] [PubMed] [Google Scholar]