Summary
Membrane lipids are structurally diverse in ways that far exceed the role envisioned by Singer and Nicholson of simply providing a fluid bilayer matrix in which proteins reside. Current models of lipid organization in membranes postulate that lipid structural diversity enables nonrandom lipid mixing in each leaflet of the bilayer, resulting in regions with special physical and functional properties, i.e., microdomains. Central to understanding the tendencies of membrane lipids to mix nonrandomly in biomembranes is the identification and evaluation of structural features that control membrane lipid lateral mixing interactions in simple model membranes. The surface balance provides a means to evaluate the lateral interactions among different lipids at a most fundamental level—mixed in binary/ternary combinations that self-assemble at the air–water interface as monomolecular films, i.e., monolayers. Analysis of surface pressure and interfacial potential as a function of average cross-sectional molecular area provide insights into hydrocarbon chain ordering, lateral compressibility/elasticity, and dipole effects under various conditions including those that approximate one leaflet of a bilayer. Although elegantly simple in principle, effective use of the surface balance requires proper attention to various experimental parameters, which are described herein. Adequate attention to these experimental parameters ensures that meaningful insights are obtained into the lipid lateral interactions and enables lipid monolayers to serve as a basic platform for use with other investigative approaches.
Keywords: Ceramide, cholesterol, condensation, dipole moment, film balance, lateral compressibility, phase transition, surface potential, surface pressure, sphingomyelin
1. Introduction
When membrane lipid amphiphiles are dissolved in a water-insoluble solvent and deposited on a water surface with a microsyringe, the solution spreads rapidly to occupy the available area. As the solvent evaporates, the lipid amphiphiles orient to minimize contact of their nonpolar regions with water, although maximizing the water-contact of their polar regions. The resulting one-molecule-thick lipid film, i.e., monolayer, provides a useful model system for studying the lateral packing interactions of lipids in each leaflet of a biomembrane.
Lipid monolayers have a rich history of providing key insights into biomembranes. Perhaps the most famous of such lipid monolayer experiments are those of Gorter and Grendel (1), who used a Langmuir surface balance to deduce the bilayer structure of membranes, by noting that monolayer surface areas produced by the lipid extracts of erythrocytes from several different animals are approximately twice the surface areas of the cells themselves. Since the pioneering work of Gorter and Grendel, it has become clear that many physical phenomena displayed by biomembrane lipids can be observed and modeled in lipid monolayers. These phenomena include phase transitions, lateral diffusion, mixing interactions that bring about changes in hydrocarbon chain ordering and lateral compressibility, and result in critical points and coexisting lateral phases, i.e., domains (2–8). Over the past decade, interest in such phenomena has enjoyed a renaissance, especially among cell biologists, largely because of the “raft” model proposed for biomembrane structure (9). Studies of lateral interactions among “raft” lipids generally involve phosphatidylcholine (PC) (e.g., POPC or DOPC), sphingomyelin (SM), cholesterol, and closely related derivatives in various combinations because of their putative roles in raft microdomain formation (8,9). The focus of this chapter will be on describing the fundamentals of using a surface balance to achieve reliable and reproducible insights into raft lipid lateral interactions.
2. Materials
To obtain meaningful data, serious precautions must be taken to avoid contamination of all materials used in monolayer experiments by surface-active substances. A single human fingerprint contains sufficient surface-active materials to form numerous monolayers in a typical surface balance.
Subphase buffer onto which monolayers are spread is kept stored under purified argon or nitrogen after preparation using purified water (see Notes 1 and 2). If organic-based buffers must be used, for example, HEPES, they should be of the highest purity available and should be tested for the presence of amphiphilic impurities (see Note 3).
Glassware is acid cleaned, rinsed with purified water, and then treated with hexane/ethanol (95:5) before use.
Solvents used to prepare lipid stock solutions and to spread lipids onto the gas–water interface are high-pressure liquid chromatography grade (see Note 3).
Lipid purity is confirmed by thin-layer chromatography using appropriate solvent mixtures (see Note 4).
Lipid stock solutions are kept at −20°C until use in acid-cleaned, borosilicate glass vials equipped with Kimble-Kontes Microflex Mininert® push–pull valves (see Note 5).
3. Methods
The spreading and orienting response of membrane lipid amphiphiles during monolayer compression is a direct consequence of the free energy excess experienced by water molecules at the surface compared with those in the bulk subphase. Water molecules at the surface are more restricted in their hydrogen bonding with other water molecules, i.e., lower entropy, compared with bulk-phase water molecules in the subphase. As a result, water molecules attempt to limit their exposure along the air contact surface by continuously “pulling back” toward the bulk subphase water to maximize hydrogen bonding with neighboring water molecules. The magnitude of the pull by surface water is reflected in its surface tension, which has units of force per unit length. Changes in the surface tension that occur in response to lipid amphiphile addition to the surface provide information about lipid–lipid and lipid–water interactions. When the available area for the lipid monolayer is so large that there is little effect of the lipids on the surface tension of water and the lipid intermolecular lateral interactions are weak, then the monolayer can be regarded as a two-dimensional (2D) gas. When the available surface area of the monolayer is reduced by a movable barrier (see Fig. 1), the lipid amphiphiles start to exert repulsive effects on each other. This 2D analog of a pressure is called surface pressure (π) and is expressed by the following relationship:
where γ0 is the surface tension in absence of a monolayer and γ the surface tension with the monolayer present.
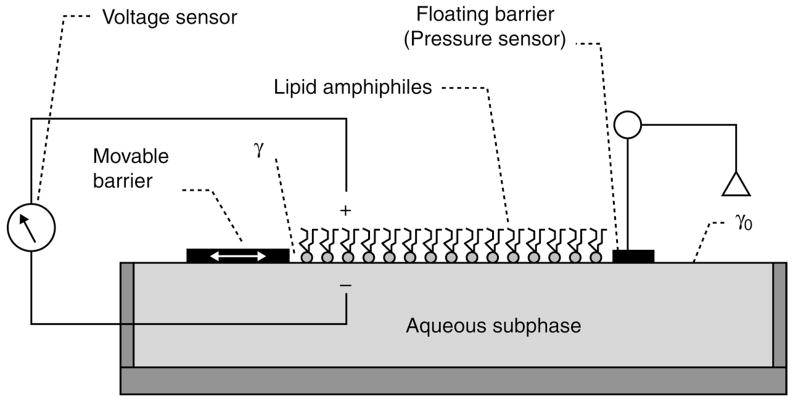
Fig. 1.
Langmuir-type surface balance for determining the surface pressure and surface potential as a function of average cross-sectional molecular area. γ is the surface tension of surface occupied by lipid amphiphile and γ0 is the surface tension of clean aqueous subphase.
Another consequence of the asymmetric orientation of lipid and water molecules at a gas–liquid interface is the generation of a sizeable (hundreds of millivolts) electrical potential perpendicular to the plane of the interface (3,10). Among the roles of this potential is its major contribution in determining the size and shape of lipid rafts (4,6). This dipole potential cannot be measured directly in each half of a bilayer, but like surface pressure, it is readily measured across monolayers using commercially available instrumentation. Its value (ΔV) is expressed as the difference in potential between a monolayer-containing interface and the aqueous phase in the absence of the monolayer,
For typical raft-forming lipids, the ends of the hydrocarbon moieties equivalent to the center of a bilayer, are at a positive potential relative to the aqueous phase. In a typical film balance (Fig. 1), surface pressure is measured continuously as a function of average molecular area. Control of the available surface area is provided by a movable barrier that can be swept along the subphase surface using computer-controlled motors. No leakage of the monolayer should occur underneath or around the barrier. This is best accomplished when the barrier is made of a hydrophobic material, which is rendered hydrophilic on its lower surface (see Note 6). The trough holding the subphase is usually made of Teflon® (see Note 6) and temperature is controlled by circulating water through channels located underneath the trough. Surface pressure is typically monitored by one of the two ways. In the first approach the surface tension is measured directly using a wettable plate, called a Wilhelmy plate, which is vertically suspended and partially immersed into the aqueous subphase. The downward force on the plate, which is attached to a calibrated microbalance, is then converted into surface tension (mN/m or dynes/cm) after taking into account the dimensions of the plate and contact angle (see Note 7). In the second approach, surface pressure is determined by measuring the translational force acting on a float separating the lipid monolayer-covered area from an adjacent surface free of lipids (Fig. 1). Teflon tape connected between the ends of the float and the trough provides a flexible seal for maintaining separation between the clean and monolayer-covered surfaces. A rigid wire, connected on one end to the float and on the other end to a torsion detection system mounted above the subphase, enables accurate detection of the minute translational movement of the float during compression/expansion sweeps of the movable barrier. The detection system used to determine surface pressure distinguishes a Wilhelmy-type film balance from a Langmuir-type film balance but the results obtained are equivalent.
During measurement of the surface pressure, it is possible to simultaneously record the interfacial potential as the movable barrier compresses the lipid monolayer. In reality, it is the potential difference that is measured between the clean water surface and a surface covered by lipid. Typically, an electrode containing either polonium or americium is used in order to ionize the air gap into an electrically conducting medium to achieve potentiometric recording (Fig. 1).
3.1. Setup Conditions for Film Balance
The film balance must be kept in a vibration-free environment to keep the subphase surface stable and wave-free. This can be accomplished by placing the trough on an active or passive vibration isolation table (e.g., Kinetic Systems Vibraplane®).
The trough should be filled so that the subphase level stands slightly above the rim of the trough (see Note 8). The subphase level is kept constant by enclosing the Langmuir trough with a plexiglass chamber that is continuously purged with purified, humidified argon or nitrogen gas. Avoiding subphase evaporation and setting the subphase level with high reproducibility increases the accuracy and reproducibility of isotherm measurements by ensuring a constant contribution of the meniscus to the total surface area.
Accurate surface pressure calibration of the film balance is essential for obtaining high-quality data. Calibration methods commonly used for surface balances equipped with Wilhelmy-type (direct measurement of surface tension) and Langmuir-type (surface pressure differences) detection systems have been carefully compared (11). Equilibrium spreading measurements using trioleoylglycerol, oleoylmethanol, trioctanoylglycerol, 1,3-dioleoylglycerol, and oleyl alcohol provide accurate calibration (see Note 9).
Accurate surface area calibration as a function of the position of the moving barrier is required to insure data that are independent of the number of molecules spread to form the monolayer. Because of geometric irregularities and meniscus curvature at that end of the trough, the moving barrier proportionality is not normally observed between barrier position and geometrically estimated area. To ensure proportionality, a calibration procedure has been described (12) (see Note 9).
Surface cleaning before and between runs is accomplished by multiple sweeps of the barrier across the empty surface combined with aspiration of the compressed surface (see Note 10).
Deposition of lipid onto the clean subphase surface is accomplished by dissolving it in volatile solvent and carefully applying a precise amount to the surface using a microsyringe (see Note 11).
Solvent combinations particularly effective for spreading phosphoglycerides, sterols, and sphingolipids commonly used for “raft” lipid studies are hexane/isopropanol/water (70:30:2.5). hexane:ethanol (9:1), and toluene/ethanol (5:6).
Complete evaporation of spreading solvent is needed before initiation of barrier compression. Hexane-based solvents (~50 μL) require 4–5 min to completely evaporate at room temperature.
Lipid films are compressed at a rate of ≤4 Å2/mol/min to minimize the occurrence of metastable phases.
Lipid stock concentrations must be known with great precision in order to accurately assess the total molecules of deposited lipid. For lipids containing phosphate, the Bartlett method is used (13). When the lipids contain no phosphate, quantitation is achieved by gravimetric determination using a microbalance (e.g., Cahn model 4700).
3.2. Acquisition of Monolayer Isotherms Using the Surface Balance
In its most basic configuration, the monolayer film balance measures the surface pressure as a function of available subphase surface area for a known number of molecules of lipid amphiphile. The measurement is performed at constant temperature and the resulting data is commonly referred to as a force-area or surface pressure-area (π-A) isotherm. An isotherm is usually recorded by reducing the available surface area, i.e., laterally compressing the film, with a barrier moving at a constant rate while continuously monitoring the surface pressure. During compression, the isotherm sometimes shows discontinuities that indicate transitions between monolayer phases differing with respect to their lateral packing features. The observed phase behavior of the monolayer is determined mainly by the physicochemical properties of the lipid amphiphile, the subphase temperature, and the subphase composition. The two most commonly observed monolayer states, the liquid-expanded and liquid-condensed monolayer states are analogous to the liquid-crystalline and gel states in bilayers, respectively (14). As with lipid bilayers, the monolayer-phase state for a particular lipid species depends on the length and unsaturation of the hydrocarbon chain and the bulkiness and charge state of the polar headgroup. An increase in the chain length increases the attraction between molecules causing the π-A isotherm to condense. In contrast, ionization of the lipid head groups induces repulsive forces tending to oppose phase transitions.
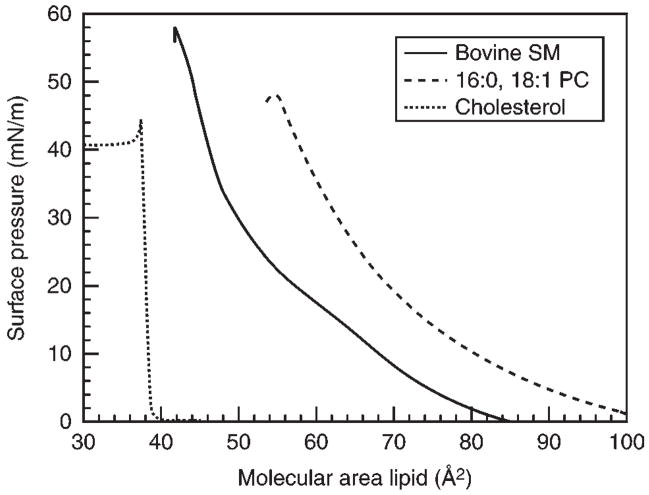
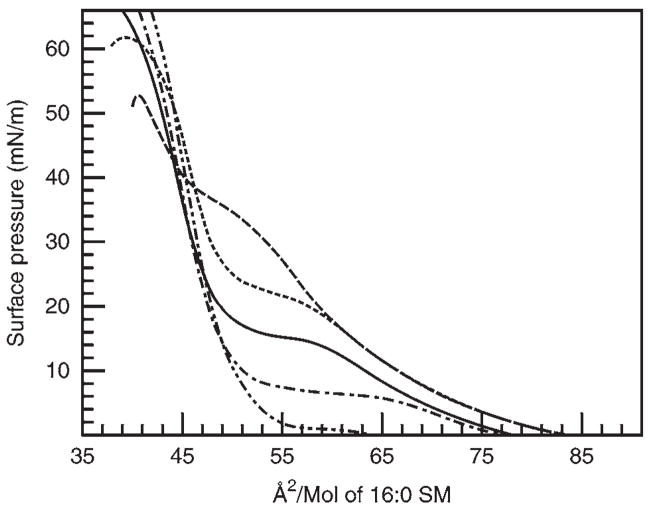
Representative isotherms collected at 24°C for different “raft” lipids, each in their pure state, are shown in Fig. 2. The SM isotherm shows liquid-expanded behavior at molecular areas between 84 and 64 Å2/mol, and then begins to pass through a transition to a condensed, chain-ordered state. The onset of this horizontal phase transition is very temperature-dependent (Fig. 3). As the temperature is increased the surface pressure value at which the horizontal transition phase occurs will increase and vice versa. The monolayer eventually reaches a collapse point, characterized by either a rapid decrease in the surface pressure or as a horizontal break in the isotherm (see Note 12). Markedly contrasting responses are displayed by POPC and cholesterol, which show only liquid-expanded and -condensed behavior, respectively, without any evidence of undergoing liquid-expanded-to-condensed phase transitions during compression at room temperature until achieving collapse (Fig. 2).
Fig. 2.
Representative π-A isotherms for “Raft” Lipids. Dotted line shows the condensed isotherm of cholesterol. Dashed line shows the liquid-expanded (fluid) isotherm of POPC. Solid line shows isotherm of bovine brain SM. The discontinuity occurring near 64 Å2/mol (14 mN/m) represents the onset of a 2D-phase transition from liquid-expanded-to-condensed behavior with monolayer collapse occurring near 42 Å2/mol.
Fig. 3.
The effect of temperature on 16:0 SM monolayers. Isotherms (from top to bottom by 2D-phase transition) were measured at 30, 24, 20, 15, and 10°C for SM monolayers containing palmitoyl acyl chains.
3.3. Preparation of Monolayers Containing Raft Lipid Mixtures
To prepare lipid mixtures for spreading on the film balance, aliquots of the different lipid stock solutions are combined to obtain the desired mole fraction of each lipid component in the mixture (see Note 13).
The total lipid applied to surface of the film balance should allow for spreading to a large average area per molecule after initial positioning of the compression barrier. A general rule of thumb for monolayer spreading is to position the barrier to provide a total surface area that exceeds the “lift off” area by at least 25 Å2/mol. For a given monolayer, the “lift off” area corresponds to the area per molecule when the surface pressure can be detected, i.e., π ≥ 1 mN/m. It must be determined empirically but is typically 75–125 Å2/mol.
As the barrier sweeps the surface and compresses the lipid film, it is desirable to achieve monolayer collapse before the total area gets too small. Doing so avoids mechanical limitations associated with the surface pressure-detecting system and the approaching barrier mounted to the edge of the trough and increases measurement accuracy. This can be accomplished by applying sufficient lipid to the surface so that monolayer collapse occurs at surface areas ≥25% of the total area available when compression is initiated.
3.4. Analyses of Monolayer Isotherms
π-A Isotherms of raft lipid mixtures differ substantially from those of the pure individual components. To obtain meaningful insights into the lateral packing in the mixed lipid films, various analyses have been developed.
3.4.1. Average Area Molecular vs Composition Analysis
A classic way to detect lateral interactions among lipids is to examine how changing composition affects the average molecular area within the mixed monolayer. The average molecular areas observed in the experimental mixtures are compared with the areas expected when one of the lipids replaces the other at specified mole fraction in the binary mix at a specified surface pressure. The expected areas are calculated by summing the molecular areas of the individual pure components, apportioned by mole fraction in the mixture, using the following equation:
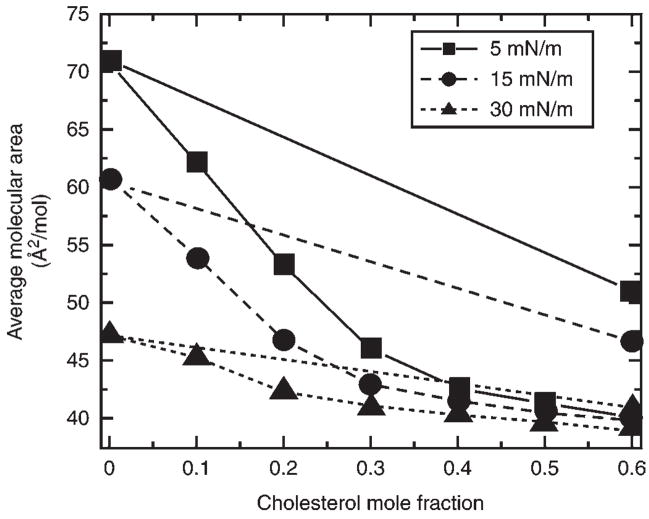
where Aav is the average molecular cross-sectional area in the mixed monolayer, X1 is the mole fraction of pure component 1, and A1 and A2 are the molecular areas of pure components 1 and 2 at identical surface pressures. Experimental average molecular areas that deviate negatively from the calculated molecular area additivity, as a function of lipid composition reflect cross-sectional area changes beyond what is expected by simply replacing a larger molecule with a smaller molecule (Fig. 4). Consequently, negative deviation from area additivity observed in the experimental mixture is often referred to as area condensation and suggests intermolecular accommodation between lipids comprising the mixed monolayer (see Note 14).
Fig. 4.
16:0 SM-cholesterol average molecular area vs composition analysis. Plots are shown for three different surface pressures in mN/m (5, squares; 15, circles; 30 triangles). The linear plots show the average molecular area obtained by calculation using the molecular areas of pure 16:0 SM and cholesterol, each apportioned by mole fraction. The nonlinear curves represent the experimentally observed areas for the mixtures. Negative deviation from ideal additivity (linear plots) shows the condensing or ordering effect of cholesterol.
A particularly relevant example of intermolecular accommodation involving lateral interactions of raft lipids is the “condensing effect” by cholesterol after mixing with PC or SM (see refs. 15–19, and references therein). The π-A isotherm of pure cholesterol is highly condensed (Fig. 2), showing almost no change in average molecular area with increasing surface pressure, and a cross-sectional molecular area of approx 36.8 Å2 at 30 mN/m, a surface pressure that approximates the membrane environment. This response shows that cholesterol is rigid and somewhat bulky compared with the cross-sectional area of an ordered hydrocarbon chain (~19 Å2). Mixing of the rigid cholesterol with phospholipids significantly reduces the number of possible conformations of the phospholipid acyl chains, thereby increasing chain order and decreasing phospholipid cross-sectional area. For this reason, the negative deviations from area additivity observed in the average molecular areas of cholesterol-PC and cholesterol-SM mixed monolayers can be expressed in terms of the apparent area change of the phospholipid using the following equation:
where PL condensation units are Å2/PL molecule, APL is the area per molecule of the pure phospholipid, Amix is the average area per molecule of the PL/Chol mixed monolayer, Achol is the area per molecule of the pure cholesterol, and Xchol is the mole fraction of cholesterol in the mixed monolayer. Application of this analysis to binary combinations of raft lipids (e.g., cholesterol and different molecular species of PC or SM) provides insights into how surface pressure and phospholipid phase state both affect the change in phospholipid cross-sectional area brought about by lateral interaction with cholesterol (17–19) (see Note 15).
3.4.2. Analysis of Lateral Compressibility
The surface compressibility (CS), i.e., lateral compressibility, of raft lipids, in either pure or mixed monolayers, can be obtained from π-A data using:
where A is the area per molecule at the indicated surface pressure (π). Mathematically, CS values represent the first derivative function, multiplied by the inverse area, and are calculated using routine software packages. When binary combinations of raft lipids are studied, ideal additivity can be modeled by apportioning the CS value for each lipid (as a pure entity) according to both molecular area fraction and mole fraction (19–21) (see Note 16). Thus, at a given constant surface pressure (π),
where X2 = (1 − X1) and CS is additive with respect to the product (CsiAi) rather than (Csi) for either ideal or completely nonideal mixing. Deviations of experimental values from calculated additivity provide evidence that the lipid components of the mixed monolayers are partially nonideally mixed (20), in analogous fashion to average area vs cholesterol composition plots.
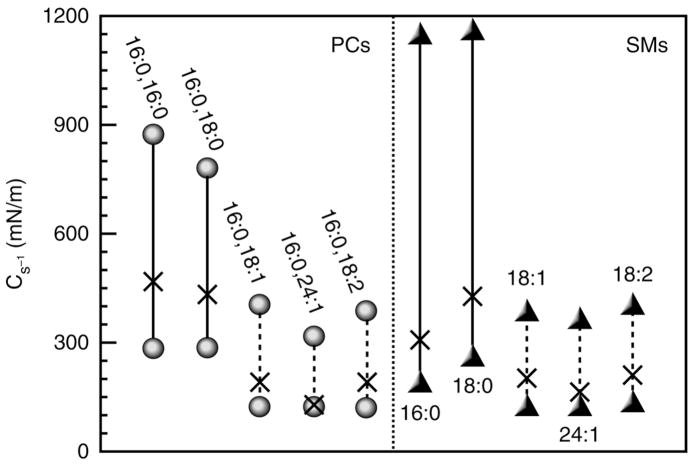
To facilitate comparison with elastic moduli of area compressibility values obtained in bilayer vesicles (22,23), the monolayer CS values can be expressed in reciprocal form (Cs−1), originally defined as the surface compressional modulus by Davies and Rideal (24). Thus, (Cs−1) data provide insights into the lateral packing elasticity, i.e., ease/resistance to lateral compression, within the monolayer. Because Cs−1 analyses utilize information available in the slopes of the isotherms, they are especially useful for evaluating the effects of cholesterol at high surface pressures (see Note 17). Moreover, compared with area condensations, Cs−1 values are more responsive to subtle changes in lipid structure during lateral interaction with cholesterol (Fig. 5). For more detailed descriptions of the mechanoelastic properties of model membranes (22,23,25) and the application of Cs−1 analysis to “raft lipids” and their mixtures, readers are referred to refs. 19 and 26–30.
Fig. 5.
Changes in surface compressional moduli (Cs−1) induced on mixing of equimolar cholesterol with PC or SM. Lower symbols represent the Cs−1 values of the pure lipids in the absence of cholesterol. Upper symbols represent the experimentally observed Cs−1 values for binary mixtures with equimolar cholesterol. The X along each line represents the ideal Cs−1 values calculated based on additivity of each pure lipid component in the binary mixtures. All Cs−1 values were determined at a surface pressure of 30 mN/m. The notation X:Y refers to the acyl chain length in carbon atoms (X) and the number of cis-double bonds (Y) present in the different molecular species of PC or SM.
3.4.3. Interfacial Potential
For lipids that exhibit fluid π-A isotherms, the dipole potential changes linearly with the 2D concentration, is 1/A of lipid amphiphile, irrespective of the surface pressure. From the slope and intercept of this line, two contributions to the potential can be obtained (10,12). One is the dipole moment, μ⊥, which arises from the vectorial component of lipid dipoles and associated water normal to the interface. Note that if the lipid carries a formal charge, like diacyl phosphatidylserine or sulfated glycolipids, the charge and its associated counter-ions in solution will also contribute to the value of μ⊥ (see Note 18). The second parameter, ΔV0, is a constant potential difference, relative to water that arises from the epitaxial ordering of all interfacial water by the presence of the lipid-amphiphile in the liquid-expanded and more condensed monolayer phases.
Experimentally, in a lipid mixture showing no monolayer phase transition in its π-A isotherm, the corresponding ΔV − 1/A isotherm is linear (31), similar to that of pure lipid amphiphiles (see Note 19). Thus, an apparent value for each of the two parameters can be obtained for any mixture. These can be compared with ideal values for mixtures of ΔV0 and μ⊥ calculated from those of the constituent lipids. Ideal values of the parameters for mixtures have been shown to apportion on the basis of the area fraction and mole fraction of each constituent in the mixture, respectively (31,32). Comparison of apparent parameter values in mixtures with predicted ideal values can reveal interactions not evident from area or compressibility analysis (31).
4. Notes
Argon or nitrogen gas is cleaned by passage through a seven-stage series filtration setup consisting of an Alltech-activated charcoal gas purifier, a LabClean filter, and a series of Balston disposable filters consisting of two adsorption (carbon) and three filter units (93 and 99.99% efficiency at 0.1 μm).
Water is purified by reverse osmosis, activated charcoal adsorption, mixed-bed de-ionization, and then passage through a Milli-Q UV Plus System (Millipore Corp., Bedford, MA), and filtration through a 0.22-μm Millipak 40 membrane.
Solvent and buffer purity can be assessed by dipole potential measurements using a 210Poionizing electrode (33).
Primulin is a particularly sensitive and nonspecific detection spray for analyzing lipid purity after thin-layer chromatography. Plates are sprayed with a 0.001% solution of primulin, dissolved in acetone:water (4:1), and viewed under long-wave ultraviolet light after evaporation of the acetone from the thin-layer plate.
Solvents particularly effective for storing lipids as stock solutions for long periods (>1 yr) at −20°C without any evidence of degradation are hexane:isopropanol:water (70:30:2.5), toluene:ethanol (1:1), and hexane:ethanol (9:1).
Occasional etching of Teflon surfaces that are exposed to water with a solution of metallic sodium/naphthalene (Chemgrip Treating Agent, Norton Performance Plastics, Wayne, NJ) increases their wettability and decreases nondesired adsorption with some proteins and lipids (34,35).
When using the Wilhelmy plate method for determination of surface tension, the material comprising the plate must be carefully selected to ensure accuracy and avoid artifacts. Momsen et al. (11) have shown that nichrome used as a wire configuration provides many advantages over other materials (e.g., platinum, glass, and filter paper) used in the more common plate configuration. An example of the difficulties and artifacts encountered using the Wilhelmy plate method is provided in studies of lignin monolayers (36).
Highly reproducible setting of the subphase level is accomplished by slightly overfilling the trough and then reducing the subphase level using a firmly mounted aspirator device consisting of a narrow-gauge tube (e.g., hypodermic needle and tubing attached to a vacuum source). As the subphase level decreases, loss of contact with the sipper needle ensures uniform and reproducible setting of the subphase level.
Surface pressure and area calibration of the surface balance can be accomplished with great precision using procedures detailed in Momsen et al. (11) and Smaby and Brockman (12).
Some lipids tend to be more difficult to clean from the surface by simple repetitive sweeps of the barrier. In such instances, repeatedly spreading a fluid-phase lipid (e.g., POPC) and having the barrier/aspirator system sweep it off the surface usually helps remove the residue.
To achieve highly reproducible spreading of lipid samples, the film balance can be equipped with a modified high-pressure liquid chromatography auto-injector for deposition of lipids onto the surface. This also allows for multiple samples to be run in succession when combined with automated cleaning sweeps of the barrier between runs (37).
For a comprehensive review of the monolayer properties of various glycosylated and nonglycosylated sphingolipids, readers are referred to Maggio et al. (38).
To obtain accurate and reproducible mixing, Hamilton digital syringes are used.
The magnitude of the area condensation should not be used as a sole measure of lateral affinity of cholesterol for different membrane lipids, as is sometimes found in the literature. One must also consider the lipid packing density (area/molecule) before and after mixing with cholesterol to arrive at meaningful conclusions. For further discussion, see ref. 18.
Monolayers at high surface pressures (e.g., π ≥ 30 mN/m) approximate the bio-membrane environment (39). However, accurate quantitation of small changes in absolute lipid area at high surface pressures is extremely challenging and beyond the practical capabilities of most surface balances. To circumvent this challenge, investigators sometimes extrapolate to the biomembrane situation using data obtained at low surface pressures because the area condensations are much larger and more easily measured. Such extrapolations are prone to inherent caveats because of the complex and nonlinear relationship that exists between the area condensation and surface pressure for phospholipids with different acyl structures. For further discussion, see ref. 19.
Derivation of the relationships governing the surface compressibility functions in binary mixtures of lipids show that both the mole fraction and area fraction contributions of each pure species must be considered when calculating ideal additivity response (19,20).
For π-A isotherms, approx 1200 data points to construct an isotherm are routinely collected. To calculate a Cs−1 value, a 100-point sliding window that utilizes every fourth π-A data point before advancing the window one point is used. The reliability of the window size for the calculated Cs−1 values is checked by reducing the window size two- and five-fold. The resulting Cs−1 values are used to construct Cs−1 vs average molecular area plots. High Cs−1 values correspond to low lateral elasticity among packed lipids forming the monolayer. The standard errors of the Cs−1 values are about 2%. At 30 mN/m pure cholesterol forms highly condensed monolayers characterized by a Cs−1 value near 1540 mN/m; whereas liquid-expanded POPC has a Cs−1 value near 122 mN/m. The Cs−1 values of PC-cholesterol and SM-cholesterol mixed monolayers are strongly affected by PC and SM acyl structure. When mixed with equivalent high-cholesterol mole fractions, PCs and SMs with saturated acyl chains have much higher Cs−1 values (lower lateral elasticity) than PCs (and SMs) with unsaturated acyl chains consistent with cis-double bonds acting as interfacial “springs” that mitigate the capacity of cholesterol to reduce lateral elasticity (Fig. 5). For further discussion, see refs. 19 and 26–29.
The charge contribution to the dipole moment can be almost completely eliminated by raising the pI of the subphase buffer (e.g., including ≥100 mM NaCl).
With highly condensed films consisting of pure raft lipids, for example, cholesterol or ceramide, the analysis of dipole potential within the context of constituent components, μ⊥ and ΔV0, becomes complicated because of the small area change associated with large changes in surface potential. In such instances, useful information can still be obtained from simple comparison of dipole potentials without separate component analysis of μ⊥ and ΔV0. A recent example involves ceramide and a series of related structural analogs (40). The presence of the 4–5 trans-double bond or a triple bond in the sphingosine backbone of ceramide was found to make a large contribution to the dipole potential of the molecule. Interestingly, the biological activities of ceramide and its analogs are largely lost if the double bond is moved even one carbon atom along the sphingosine chain, a change that substantially alters the molecular dipole potential (40).
5. Epilogue
Over the past two decades, surface balances have evolved from free-standing instruments for direct measurement of lipid lateral interactions (π-A) isotherm into basic platforms used in concert with other technological approaches.
Supported monolayers/bilayers: supported monolayers or even multilayers can be deposited stepwise onto a solid substrate surface by passing it through a lipid monolayer while keeping the surface pressure constant (41,42). Such films have been used in combination with atomic force microscopy to study raft lipids mixtures (43,44).
Epifluorescence microscopy: mounting of a surface balance onto the stage of an epifluorescence microscope enables the distribution patterns of trace amounts of lipids containing covalently attached reporter fluorophores to be monitored within the lipid monolayer. Because the lipid fluorophores tend to partition nonuniformly among monolayer regions of differing lateral packing density, their lateral distribution patterns can be visualized (2,4–6,45,46). This technology has been especially useful for definitive detection of critical points and domains among various lipid mixtures involving cholesterol and has facilitated construction of phase diagrams for ternary mixtures of “raft” lipids (47–49). For more on the application of epifluorescent microscropy to model membranes, see Chapter 12 in this book by Sarah Veatch.
Fluorescence spectroscopy: surface balances can now be equipped with laser and fiberoptic technology to directly measure lipid fluorophore intensity in lipid monolayers (50). Monolayer studies of BODIPY-PC indicate that this technology holds promise for providing nanoscale insights into the nonideal mixing behavior of “raft” lipid mixtures, thus considerably increasing resolution compared with epifluorescence microscopy. Moreover, this same technology now provides a means for assessing the real-time adsorption of proteins to lipid monolayers from the subphase by monitoring the increase in the intrinsic emission intensity of tryptophan/tyrosine (or covalently attached extrinsic fluorophores) at the monolayer surface (51,52).
Acknowledgments
We extend our thanks to Bill Momsen, Maureen Momsen, Nancy Mizuno, Jan Smaby, Dmitry Malakov, and Craig Jones for their many contributions to the advancement of monolayer technology used in the study of lipid–lipid and lipid–protein interactions. We are grateful to NIGMS45928, NHLBI49180, and The Hormel Foundation for their past, present, and future support.
References
- 1.Gorter E, Grendel F. On bimolecular layers of lipoids on the chromocytes of the blood. J Exp Med. 1925;41:439–443. doi: 10.1084/jem.41.4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rice PA, McConnell HM. Critical shape transitions of monolayer lipid domains. Proc Natl Acad Sci USA. 1989;86:6445–6448. doi: 10.1073/pnas.86.17.6445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Möhwald H. Phospholipid and phospholipid-protein monolayers at the air/water interface. Annu Rev Phys Chem. 1990;41:441–476. doi: 10.1146/annurev.pc.41.100190.002301. [DOI] [PubMed] [Google Scholar]
- 4.McConnell HM. Structures and transitions in lipid monolayers at the air-water interface. Annu Rev Phys Chem. 1991;42:171–195. [Google Scholar]
- 5.Keller SL, Pitcher WH, III, Huestis WH, McConnell HM. Red blood cells lipids form immiscible liquids. Phys Rev Lett. 1998;81:5019–5022. [Google Scholar]
- 6.McConnell HM, Vrljic M. Liquid-liquid immiscibility in membranes. Annu Rev Biophys Biomol Struct. 2003;32:469–492. doi: 10.1146/annurev.biophys.32.110601.141704. [DOI] [PubMed] [Google Scholar]
- 7.Silvius JR. Role of cholesterol in lipid raft formation: lessons from lipid model systems. Biochim Biophys Acta. 2003;1610:174–183. doi: 10.1016/s0005-2736(03)00016-6. [DOI] [PubMed] [Google Scholar]
- 8.Simons K, Vaz WLC. Model systems, lipid rafts, and cell membranes. Annu Rev Biophys Biomol Struct. 2004;332:269–295. doi: 10.1146/annurev.biophys.32.110601.141803. [DOI] [PubMed] [Google Scholar]
- 9.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 10.Brockman HL. Dipole potential of lipid membranes. Chem Phys Lipids. 1994;73:57–79. doi: 10.1016/0009-3084(94)90174-0. [DOI] [PubMed] [Google Scholar]
- 11.Momsen WE, Smaby JM, Brockman HL. The suitability of nichrome for measurement of gas-liquid interfacial tension by the Wilhelmy method. J Colloid Interface Sci. 1990;135:547–552. [Google Scholar]
- 12.Smaby JM, Brockman HL. Surface dipole moments of lipids at the argon-water interface: Similarities among glycerol-ester-based lipids. Biophys J. 1990;58:195–204. doi: 10.1016/S0006-3495(90)82365-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartlett GR. Phosphorus assay in column chromatography. J Biol Chem. 1959;234:466–468. [PubMed] [Google Scholar]
- 14.Kaganer VM, Möhwald H, Dutta P. Structural and phase transitions in Langmuir monolayers. Rev Mod Phys. 1999;71:779–819. [Google Scholar]
- 15.Leathes JB. Role of fats in vital phenomena. Lancet. 1925;208:853–856. [Google Scholar]
- 16.Phillips MC. The physical state of phospholipids and cholesterol in monolayers, bilayers, and membranes. Prog Surf Membr Sci. 1972;5:139–221. [Google Scholar]
- 17.Smaby JM, Brockman HL, Brown RE. Cholesterol’s interfacial interactions with sphingomyelins and phosphatidylcholines: Hydrocarbon chain structure determines the magnitude of condensation. Biochemistry. 1994;31:9135–9142. doi: 10.1021/bi00197a016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smaby JM, Momsen M, Kulkarni VS, Brown RE. Cholesterol-induced interfacial area condensations of galactosylceramides and sphingomyelins with identical acyl chains. Biochemistry. 1996;35:5696–5704. doi: 10.1021/bi953057k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smaby JM, Momsen MM, Brockman HL, Brown RE. Phosphatidyl-choline acyl unsaturation modulates the decrease in interfacial elasticity induced by cholesterol. Biophys J. 1997;73:1492–1505. doi: 10.1016/S0006-3495(97)78181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ali S, Smaby JM, Brockman HL, Brown RE. Cholesterol’s interfacial interactions with galactosylceramides. Biochemistry. 1994;33:2900–2906. doi: 10.1021/bi00176a020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X-M, Momsen MM, Smaby JM, Brockman HL, Brown RE. Sterol structure and sphingomyelin acyl chain length modulate lateral packing elasticity and detergent solubility in model membranes. Biophys J. 2003;85:3788–3801. doi: 10.1016/S0006-3495(03)74794-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evans E, Needham D. Physical properties of surfactant bilayer membranes: thermal transitions, elasticity, rigidity, cohesion, and colloidal interactions. J Phys Chem. 1987;91:4219–4228. [Google Scholar]
- 23.Needham D. In: Permeability and Stability of Lipid Bilayers. Disalvo EA, Simon SA, editors. CRC Press; Boca Raton, FL: 1995. pp. 49–76. [Google Scholar]
- 24.Davies JT, Rideal EK. Interfacial Phenomena. 2. Academic Press; New York: 1963. p. 265. [Google Scholar]
- 25.Behroozi F. Theory of elasticity in two dimensions and its application to Langmuir-Blodgett films. Langmuir. 1996;12:2289–2291. [Google Scholar]
- 26.Smaby JM, Kulkarni VS, Momsen M, Brown RE. The interfacial elastic packing interactions of galactosylceramides, sphingomyelins, and phosphatidylcholines. Biophys J. 1996;70:868–877. doi: 10.1016/S0006-3495(96)79629-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X-M, Momsen MM, Brockman HL, Brown RE. Lactosylceramide: Effect of acyl chain structure on phase behavior and molecular packing. Biophys J. 2002;83:1535–1546. doi: 10.1016/S0006-3495(02)73923-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li X-M, Momsen MM, Smaby JM, Brockman HL, Brown RE. Cholesterol decreases the interfacial elasticity and detergent solubility of sphingomyelins. Biochemistry. 2001;40:5954–5963. doi: 10.1021/bi002791n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhai X, Li X-M, Momsen MM, Brockman HL, Brown RE. Lactosylceramide: Lateral interactions with cholesterol. Biophys J. 2006 doi: 10.1529/biophysj.106.084921. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allende D, Vidal A, McIntosh TJ. Jumping to rafts: Gatekeeper role of bilayer elasticity. Trends Biochem Sci. 2004;29:325–330. doi: 10.1016/j.tibs.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 31.Ali S, Brockman HL, Brown RE. Structural determinants of miscibility in surface films of galactosylceramide and phosphatidylcholine: Effect of unsaturation in the galactosylceramide acyl chain. Biochemistry. 1991;30:11,198–11,205. doi: 10.1021/bi00111a002. [DOI] [PubMed] [Google Scholar]
- 32.Smaby JM, Brockman HL. Characterization of lipid miscibility in liquid-expanded monolayers at the gas-liquid interface. Langmuir. 1992;8:563–570. [Google Scholar]
- 33.Smaby JM, Brockman HL. A simple method for estimating surfactant impurities in solvents and subphases used for monolayer studies. Chem Phys Lipids. 1991;58:249–252. doi: 10.1016/0009-3084(91)90099-w. [DOI] [PubMed] [Google Scholar]
- 34.Rye RR. Electron irradiation of poly(tetrafluoroethylene): Effect on adhesion and comparison with X-rays. Langmuir. 1990;6:338–344. [Google Scholar]
- 35.Kulkarni VS, Brown RE. Interactions of phospholipid bilayer vesicles with monomolecular films at the air-water interface. Thin Solid Films. 1994;244:869–873. [Google Scholar]
- 36.Constantino CJL, Dhanabalan A, Oliveira ON., Jr Experimental artifacts in the surface pressure measurement for lignin monolayers in Langmuir troughs. Rev Sci Instr. 1999;70:3674–3680. [Google Scholar]
- 37.Brockman HL, Smaby JM, Jarvis DE. Automation of surface cleaning and sample addition for surface balances. J Phys E. 1984;17:351–353. [Google Scholar]
- 38.Maggio B, Carrer DC, Fanani ML, Oliveira RG, Rosetti CM. Interfacial behavior of glycosphingolipids and chemically related sphingolipids. Curr Opin Colloid Interface Sci. 2004;8:448–458. [Google Scholar]
- 39.Marsh D. Lateral pressure in membranes. Biochim Biophys Acta. 1996;1286:183–223. doi: 10.1016/s0304-4157(96)00009-3. [DOI] [PubMed] [Google Scholar]
- 40.Brockman HL, Momsen MM, Brown RE, et al. The 4,5-double bond of ceramide regulates its dipole potential, elastic properties, and packing behavior. Biophys J. 2004;87:1722–1731. doi: 10.1529/biophysj.104.044529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petty MC. Langmuir-Blodgett Films: An Introduction. Cambridge University Press; 1996. [Google Scholar]
- 42.Sackmann E. Supported membranes: Scientific and practical approaches. Science. 1996;271:43–48. doi: 10.1126/science.271.5245.43. [DOI] [PubMed] [Google Scholar]
- 43.Yuan C, Furlong J, Burgos P, Johnston LJ. The size of lipid rafts: an atomic force microscopy study of ganglioside GM1 domains in sphingomyelin/DOPC/cholesterol membranes. Biophys J. 2002;82:2526–2535. doi: 10.1016/S0006-3495(02)75596-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yuan C, Johnston LJ. Atomic force microscopy studies of ganglio-side GM1 domains in phosphatidylcholine and phosphatidylcholine/cholesterol bilayers. Biophys J. 2001;81:1059–1069. doi: 10.1016/S0006-3495(01)75763-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Von Tscharner V, McConnell HM. An alternative view of phospholipid phase behavior at the air-water interface. Microscope and film balance studies. Biophys J. 1981;36:409–419. doi: 10.1016/S0006-3495(81)84740-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weis RM, McConnell HM. Two-dimensional chiral crystals of phospholipid. Nature (London) 1984;310:47–49. doi: 10.1038/310047a0. [DOI] [PubMed] [Google Scholar]
- 47.Veatch SL, Keller SL. Organization in lipid membranes containing cholesterol. Phys Rev Lett. 2002;89:268101-1–268101-4. doi: 10.1103/PhysRevLett.89.268101. [DOI] [PubMed] [Google Scholar]
- 48.Radhakrishnan A, McConnell HM. Critical points in charged membranes containing cholesterol. Proc Natl Acad Sci USA. 2002;99:13,391–13,396. doi: 10.1073/pnas.212522699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stottrup BL, Stevens DS, Keller SL. Miscibility of ternary mixtures of phospholipids and cholesterol in monolayers and application to bilayer systems. Biophys J. 2005;88:269–276. doi: 10.1529/biophysj.104.048439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dahim M, Mizuno NK, Li X-M, Momsen WE, Momsen MM, Brockman HL. Physical and photophysical characterization of a BODIPY phosphatidylcholine as a membrane probe. Biophys J. 2002;83:1511–1524. doi: 10.1016/S0006-3495(02)73921-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Momsen WE, Mizuno NK, Lowe ME, Brockman HL. Real-time measurement of solute partitioning to lipid monolayers. Anal Biochem. 2005;346:139–149. doi: 10.1016/j.ab.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 52.Hoang KC, Malakhov D, Momsen WE, Brockman HL. Open, microfluidic flow cell for studies of interfacial processes at gas-liquid interfaces. Anal Chem. 2006;78:1657–1664. doi: 10.1021/ac051772m. [DOI] [PubMed] [Google Scholar]