Abstract
Strongyloides stercoralis is a human intestinal parasite which may lead to complicated strongyloidiasis in immunocompromised. Here, a case of complicated strongyloidiasis in a patient with chronic lymphocytic leukemia is reported. Presence of numerous S. stercoralis larvae in feces and sputum confirmed the diagnosis of hyperinfection syndrome in this patient. Following recovery of filariform larvae from agar plate culture of the stool, the isolate was characterized for the ITS1 region of ribosomal DNA gene by nested-PCR and sequencing. Albendazole therapy did not have cure effects; and just at the beginning of taking ivermectin, the patient died. The most important clue to prevent such fatal consequences is early diagnosis and proper treatment.
Keywords: Strongyloides stercoralis, hyperinfection, chronic lymphocytic leukemia, ITS1, ribosomal DNA
INTRODUCTION
Strongyloides stercoralis is an intestinal nematode of humans with worldwide distribution which may also infects dogs, cats, and monkeys. This parasite is most common in tropical and subtropical regions [1,2]. The transmission can happen by autoinfection, which occurs when larvae in the intestine develop precociously to the infective 3rd stage larvae (L3) and invade the intestinal wall. In this case the disease may remain chronic for several years [3].
Strongyloidiasis presents 3 possible outcomes in the infected hosts: (a) self-cure by S. stercoralis elimination; (b) the chronic form supported by autoinfection; and (c) hyperinfection or the form disseminated by larvae in ectopic sites. These factors are highly dependent on the host immune responses and the evasive capacity of the parasite [4,5]. In human strongyloidiasis, the chronic infection, when limited to the gastrointestinal tract, is clinically non-apparent in most cases. Hyperinfection and dissemination can lead to the death of individuals with proteincaloric malnutrition, cancer, renal transplant, systemic lupus erythematosus, acquired immunodeficiency syndrome (AIDS), or tuberculosis as well as alcoholics or those receiving treatment with immunosuppressor drugs [3,6,7]. Here, a case of fatal strongyloidiasis in a patient with chronic lymphocytic leukemia (CLL) is described and a molecular diagnosis of isolate is presented.
CASE RECORD
In August 2006, a 74-yr-old male with a history of CLL and originally a resident of Khouzestan Province (southwestern Iran), was admitted to Al-Zahra Medical Centre in Isfahan, Iran. The patient suffered from intestinal and respiratory disorders including abdominal pain, cough, sputum discharge, and chest pain, as well as weakness, dizziness, and anemia for several years. He was taking prednisolone and ciprofloxacin during previous 2 months. Laboratory examinations at the time of hospitalization showed hemoglobin 8.1 g/dL, the leucocyte count 37,800/mm3, the platelet count 54,000/mm3, and an erythrocyte sedimentation rate of 80 mm/hr. There were no blasts or eosinophils in the peripheral smear. Liver function tests showed serum bilirubin 0.7 mg/dL, and alanine and aspartate aminotransferases 20 and 65 U/L, respectively. Alkaline phosphatase was 188 U/L, and albumin 3.1 g/dL. Serum phosphorus, calcium, creatinine, potassium, and sodium were 3.3 mg/dL, 8 mg/dL, 0.8 mg/dL, 3.2 mEq/L, and 122 mEq/L, respectively. In a direct smear and formalin-ether examinations of the diarrheic stool sample, numerous rhabditiform larvae of S. stercoralis were present, while in sputum smear and agar plate culture of the stool both rhabditiform and filariform larvae (Fig. 1) were observed. The detection of numerous larvae in both the stool and sputum confirmed Strongyloides hyperinfection in this patient. No other parasite or pathogenic bacteria were found by either stool examination or culture. Albendazole therapy (400 mg twice daily for 3 days) did not have cure effects; and just at the beginning of putting on ivermectin, the patient died.
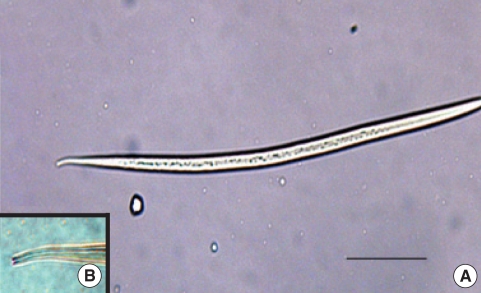
Fig. 1.
A filariform larva of Strongyloides stercoralis. (A) Whole view of the larva, (B) Nutched tail of the larva. Scale bar = 100 µm.
S. stercoralis filariform larvae recovered from agar plate culture were preserved in 75% ethanol solution and kept at ambient temperature for subsequent molecular processing. Total genomic DNA was extracted from filariform larvae using the QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) spin column method following the manufacturer's instructions. For molecular typing of S. stercoralis a nested-PCR and partial sequencing of the ITS1 region of the rDNA gene (approximately 680 bp) was performed using the primer set mentioned by Nilforoushan et al. [8]. The sequences of these 2 primer set was as follows: SS-Forward: 5'-GTA ACAAGG TTT TCG TAG GTG A-3', SS-Reverse: 5'-ATT TAG TTT CTT TTC CTC CGC TT-3'.
Negative control (water instead of DNA template) was included in each amplification run. The PCR product was analyzed by electrophoresis in a 1.5% agarose gel and visualized after ethidium bromide staining (Fig. 2). The secondary PCR product was sequenced by SEQLAB company (Germany) with an ABI 3100 genetic analyzer. Blasting the sequence showed that the amplicon was definitely S. stercoralis. The sequence determined in this study has been registered in the GenBank database under accession number EF653264.
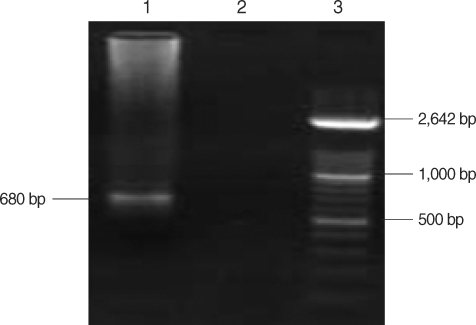
Fig. 2.
Molecular diagnosis of Strongyloides stercoralis by a nested PCR based on rDNA gene. Lane 3, molecular weight marker XIV; lane 2, negative control; lane 1, patient isolate.
DISCUSSION
Strongyloides is unique among the parasitic infections because of its ability to autoinfect the host without soil or intermediate host. The detection of increased number of larvae in the stool and/or sputum is a hallmark of hyperinfection. It is often complicated by infections caused by gut flora that gain access to intestinal sites, presumably through ulcers induced by the filariform larvae [6]. Strongyloidiasis can occur without any symptoms, or as a potentially fatal hyperinfection or disseminated infection. Th2 cell-mediated immunity, humoral immunity and mucosal immunity have been shown to have protective effects against this parasitic infection especially in animal models. Any factors that suppress these mechanisms (such as intercurrent immune suppression or glucocorticoid therapy) could potentially trigger hyperinfection or disseminated infection which could be fatal [9].
Immunosuppressed patients are at the greatest risk because impaired cellular and humoral immunity alter parasite proliferation, resulting in increased parasitic burden and possible dissemination to other organs. In this accelerated phase of autoinfection, enormous numbers of larvae are released into the venous circulation and disseminate throughout the body. This potentially fatal phenomenon has been described not only in patients with AIDS but also in patients being treated with corticosteroids and/or other immunosuppressive drugs [10]. Unfortunately, fecal larval output does not correlate with the adult worm burden and may underestimate the severity of the infection.
In the case presented here, the patient was a resident of Khouzestan Province, an endemic area for S. stercoralis with warm climate. He suffered from intestinal and respiratory disorders for several years; however, his infectivity with S. stercoralis was undiagnosed till his hospitalization. In fact, altered immunity due to the chronic CLL in this patient is most probably the primary reason for acceleration of S. stercoralis proliferation leading to hyperinfection syndrome involving both pulmonary and gastrointestinal systems. Although, in both the stool and sputum direct smears of the patient, enormous S. stercoralis larvae were present, and no eosinophilia was found in the peripheral blood. Therefore, in some cases of hyperinfection syndrome and disseminated strongyloidiosis, eosinophilia could not be found in spite that eosinophilia is the most important laboratory finding in patients infected with S. stercoralis [9,11].
In summary, individuals with immunodeficiency states, particularly with cell-mediated immunity defect, hematological malignancy, steroid usage, malnutrition, diabetes, and organ transplantation, are predisposed to this infection. Early diagnosis and timely therapy in case of hyperinfection syndrome can have a marked impact on the disease outcome. In fact, recognition of risk factors for infection, including immune compromise, habitation in endemic areas, and prisoner-of-war status, may enhance detection by prompting stool studies or endoscopy. Because clinical symptoms and endoscopic findings are non-specific, a high level of suspicion is required for diagnosis [6]. Regular stool screening of high risk patients is necessary to minimize the risk of hyperinfection and prevention of fatal consequences as the case presented here.
ACKNOWLEDGEMENTS
We thank Mrs. Jalali Zand N, Mrs. Sayyad Talaie Z, Mr. Rahimi A, and Mr. Falakimoghadam MH for their cooperation in this study. This research was financially supported by the Tehran University of Medical Sciences and Health Services, Grant No: 132/10600.
References
- 1.Grove DI. Human strongyloidiasis. Adv Parasitol. 1996;38:251–309. doi: 10.1016/s0065-308x(08)60036-6. [DOI] [PubMed] [Google Scholar]
- 2.Lam CS, Tong MK, Chan KM, Siu YP. Disseminated strongyloidiasis: a retrospective study of clinical course and outcome. Eur J Clin Microbiol Infect Dis. 2006;25:14–18. doi: 10.1007/s10096-005-0070-2. [DOI] [PubMed] [Google Scholar]
- 3.Concha R, Harrington WJ, Rogers AI. Intestinal strongyloidiasis recognition, management and determinants of outcome. J Clin Gastroenterol. 2005;39:203–211. doi: 10.1097/01.mcg.0000152779.68900.33. [DOI] [PubMed] [Google Scholar]
- 4.Grove DI, Heenan PJ, Northern C. Persistent and disseminated infections with Strongyloides stercoralis in immunossupressed dogs. Int J Parasitol. 1983;13:483–490. doi: 10.1016/s0020-7519(83)80012-5. [DOI] [PubMed] [Google Scholar]
- 5.Goncalves ALR, Macado MG, Goncalves-Pires MRF, Ferreira-Junior A, Silva DAO, Costa-Cruz JM. Evaluation of strongyloidiasis in kennel dogs and keepers by parasitological and serological assays. Vet Parasitol. 2007;147:132–139. doi: 10.1016/j.vetpar.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 6.Keiser PB, Nutman TB. Strongyloides stercoralis in the immunocompromised population. Clin Microbiol Rev. 2004;17:208–217. doi: 10.1128/CMR.17.1.208-217.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oliveira LCM, Ribeiro CT, Mendes DM, Oliveira TC, Costa-Cruz JM. Frequency of Strongyloides stercoralis infection in alcoholics. Mem Inst Oswaldo Cruz. 2002;97:119–121. doi: 10.1590/s0074-02762002000100021. [DOI] [PubMed] [Google Scholar]
- 8.Nilforoushan MR, Mirhendi H, Rezaie S, Rezaian M, Meamar AR, Kia EB. A DNA-Based Identification of Strongyloides stercoralis Isolates from Iran. Iranian J Publ Health. 2007;36:16–20. [Google Scholar]
- 9.Vadlamudi RS, Chi DS, Krishnaswamy G. Intestinal strongyloidiasis and hyperinfection syndrome. Clin Mol Allergy. 2006;4:8. doi: 10.1186/1476-7961-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarangarajan R, Ranganathan A, Belmonte AH, Tchertkoff V. Strongyloides stercoralis infection in AIDS. AIDS Patient Care STDS. 1997;11:407–414. doi: 10.1089/apc.1997.11.407. [DOI] [PubMed] [Google Scholar]
- 11.Fardet L, Genereau T, Poirot JL, Guidet B, Kettaneh A, Cabane J. Severe strongyloidiasis in corticostrroid-treated patients: Case series and literature review. J Infect. 2007;54:18–27. doi: 10.1016/j.jinf.2006.01.016. [DOI] [PubMed] [Google Scholar]