Abstract
The aim of this study was to investigate the difference in the serum malondialdehyde (MDA), glutathione (GSH), and nitric oxide (NO) levels between normal and T. gondii-infected patients. To this end, MDA, GSH, and NO levels in the sera of 37 seropositive patients and 40 participants in the control group were evaluated. In Toxoplasma ELISA, IgG results of the patient group were 1,013.0 ± 543.8 in optical density (mean ± SD). A statistically significant difference was found between patients and the control group in terms of MDA, GSH, and NO levels. A decrease in GSH activity was detected, while MDA and NO levels increased significantly. Consequently, it is suggested that the use of antioxidant vitamins in addition to a parasite treatment shall prove useful. The high infection vs control ratio of MDA and NO levels probably suggests the occurrence as a mechanism of tissue damage in cases of chronic toxoplasmosis. Moreover, it is recommended that the patient levels of MDA, GSH, and NO should be evaluated in toxoplasmosis.
Keywords: Toxoplasma gondii, malondialdehyde, glutathione, nitric oxide
Toxoplasma gondii is a highly frequent obligate intracellular protozoan parasite. It is reported that about one-third of the world population is infected with T. gondii, and the disease has asymptomatic progress in 90% of the patients with sound immune systems [1,2]. Toxoplasmosis can cause serious pathologies including hepatitis, pneumonia, blindness, and severe neurological disorders. These types of diseases are seen particularly in people with weak immune systems [3,4]. Yet, the pathogenic mechanisms in healthy people could not be explained completely.
It is assumed that the malondialdehyde (MDA) arising from the lipid peroxidation is an indicator of the oxidative stress in tissue and cells. Lipid peroxidase is a derivative enzyme of feeble unsaturated fatty acid which is produced as a result of decomposition of a set of complex components [5]. Glutathione (GSH), an endogen-originated peptide which can be synthesized in the liver without need for genetic data, is made up of glutamic acid, cysteine and glycine amino acids, and is an important antioxidant. It defends the cells against oxidative damage by undergoing reaction with free radicals and peroxidase [6]. The activity of inducible nitric oxide synthase (INOS) enzyme is independent of calcium and is active as long as there is argine involved. In this case it can catalyze nitric oxide (NO) synthesis at long-termed and high concentration. Most of the basic antioxidants have been examined in protozoans and helminths [7-9]. The aim of the present study was to investigate the difference of MDA, GSH, and NO levels in sera of normal and T. gondii-infected patients (IgG seropositive but without symptoms).
Prior to the study the approval of the ethical council was obtained and only those patients voluntary to donate samples were included. The materials were examined in the Parasitology and Biochemistry Laboratory in Faculty of Medicine at Inonu University, Malatya, Turkey during 2005-2007. The sera from 37 T. gondii seropositive patients and 40 participants in the control group were checked for MDA, GSH, and NO levels. The age of the experimental group was 30.6 ± 5.5 yr on average with 3 males and 34 females. The all-man participants in the control group aged 40.3 ± 5.8 yr on average. Serum samples for the control group were obtained from healthy people who had referred to the different Departments of Inonu University/Medical Faculty for a regular check-up and from students or employees of the University.
All of the subjects fasted after midnight before blood collection in the next morning. Given the fact that increases in MDA levels can be observed in parasitic diseases, the participants in both patients and control groups were examined for their intestinal parasites using native lugol, cellophaned perianal region material, and sedimentation methods. The cyst hydatid, in addition, was evaluated using manual indirect hemagglutination (IHA) test and indirect fluorescent antibody test (IFAT). The experimental group was formed out of those patients found to be T. gondii seropositive using ELISA and IFAT tests alone. Besides, among T. gondii seropositive patients, those with parasites detected in their feces, using a hormonal medication, smoking, and drinking alcohol were excluded from the study since they are likely to give rise to differences in MDA, GSH, and NO levels. Also ELISA and IFAT tests were used in order to detect anti-T. gondii antibodies. The brands of the kits used in ELISA and IFAT tests are Meddens and Zeus, respectively. All of the samples were found IgM seronegative. Among volunteers in the control group, only those who had no parasitic infections, no smoking or drinking habits, and who did not take any hormonal medication were included in the study. Those patients found to be positive in ELISA and IFAT tests were invited once again and informed about the study results. Then, blood samples of 5 ml were taken from those willing. Next the sera were separated from the samples and kept under -20℃ until analysis.
All venous blood samples were taken between 8:00 and 9:00 a.m. after 12 hr of fasting and collected in polystyrene tubes and vacutainers containing heparin. The tubes were centrifuged at 500 × g for 15 min. The serum was then removed and stored at -20℃ until analysis. Serum MDA levels were determined using the Uchiyama-Mihara method [6]. Briefly, 1 ml of 0.6% thiobarbituric acid solution, 3 ml 1% phosphoric acid, and 0.5 ml of 10% tissue homogenate were put into a tube. This mixture was heated in boiled water for 45 min. An extract was obtained by adding 4 ml of n-butanol in cooled tubes and then MDA passing into the serum was measured using spectrophotometer at 532 nm wave-length. The serum MDA amount was measured in nmol/L. Glutathione designation was calculated in µmol/L after evaluating the yellow product at 410 nm wave-length which was observed as a result of the reaction of sulphydryl groups with the Elman reagent [10]. Colored compound produced, when the nitrite formed though NO system activity (considering the total amount of nitrite) as a result of cadmium-reduction of nitrate (NO3-) to nitrite (NO2-) was reacted with Griess reactive, was measured at 545 nm by a spectrophotometer. The results were given in µmol/L.
The data were given in means and standard errors, and the normality test was done with Shapiro-Wilk method. Also independent samples t-test was used for the statistical analysis. P < 0.05 was considered statistically significant.
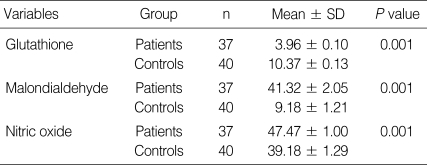
Descriptive statistics on the MDA level, GSH, and NO activities were shown in Table 1. The anti-T. gondii ELISA IgG results of the patient group were 1,013.0 ± 543.8 in optical density (mean ± SD). The ELISA IgG values were found negative for the control group. Anti-T. gondii IFAT results measuring 1/16 and more in the patient group were considered positive (Table 1). Statistically significant differences were found between the patients and the control group in terms of MDA, GSH, and NO parameters (P < 0.05).
Table 1.
Descriptive statistics of glutathione, malondialdehyde, and nitric oxide between seropositive patients and healthy controls
T. gondii is an obligate intracellular parasite frequently occurring in cystic forms in humans. T-cells in cellular immune systemcontrols the growth of the parasite. Moreover, macrophages, fibroblasts, astrocytes, and microglial cells in the brain are the cells capable of restricting the proliferation of toxoplasmosis. Also it is known that the major anti-parasitic effector mechanisms stimulate the production of NO by activating indolamine 2,3-dioxygenase (IDO) activity and the INOS [11,12]. TNF-α, a cytokine secreted from the activated macrophage and monocytes, is responsible for the toxoplasmocidal effects of macrophages via IFN-γ and necessary for the synthesis of NO, which can suppress the proliferation of toxoplasmosis, from macrophages. In a previous study, the serum TNF-α level in patients with acute toxoplasmosis was found higher than the level in patients with chronic toxoplasmosis and in healthy people of the control group. It was reported that this parasite has enzymes which produce free oxygen radicals such as superoxide and hydrogen peroxide [4,11]. It was suggested that it can play a role in the pathogenesis of acute toxoplasmosis. NO is the product of arginine metabolism and one of the most effective O2-free toxins. There are also previous studies reporting an increase in the NO level in parasitic diseases [1,12]. It can be stated that the NO level increases as a defensive mechanism to protect the patient against the harmful effects of the parasite.
One of the products of the lipid peroxidation is MDA. MDA arises from peroxidation of fatty acids containing 3 or more double bonds. The MDA product can cause the cross-linkage of membrane elements by affecting the ion exchange from cell membranes, which gives way to aftermaths including a change in ion permeability and enzyme activity. In a previous study, it was reported that MDA increases in Toxoplasma seropositive patients [13]. Again in this study, an increase in the MDA level was detected. It was reported that increased MDA activity in lymphocytes and erythrocytes in the dust-mite positive or skin test positive group shows the presence of the oxidative stress in patients with dust-mite infestation [14].
The potentially harmful effects of reactive oxygen species are controlled by the cellular antioxidant defense system. GSH is an important constituent of intracellular protective mechanisms against a number of noxious stimuli including oxidative stress. It plays a role in preventing the transformation of hemoglobin into methemoglobin due to oxidation. Moreover, it maintains the sulfhydryl (-SH) groups in proteins in a reduced state and protects these groups against oxidation [15]. In the present study, the significant decrease found in GSH activity in the patient group can be explained with the oxidative stress caused by lipid peroxidation and depletion in the GSH level, which is an endogen antioxidant. Thus, it is suggested that antioxidant vitamins (E and C) increase the GSH activity (one of the cell-protective factors) [16].
As far as we know, there is no previous research which studied MDA and NO levels, and GSH activities together in Toxoplasma seropositive patients. A decrease in the GSH activity in Toxoplasma seropositive patients indicates a decline in the response to oxidative stresses. The increase of MDA, on the other hand, refers to the increase of lipid peroxidation. The results of our study strongly suggest that one of the main reasons for high MDA levels in Toxoplasma seropositive patients could be a decreased activity of the defense system protecting tissues from free radical damage. It was concluded that an increase in the NO level can be associated with the stimulation of the cell mediated immune system.
References
- 1.Kang KM, Lee GS, Lee JH, Choi IW, Shin DW, Lee YH. Effects of INOS inhibitor on IFN-γ production and apoptosis of splenocytes in genetically different strains of mice infected with Toxoplasma gondii. Korean J Parasitol. 2004;42:175–183. doi: 10.3347/kjp.2004.42.4.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deorari AK, Broor S, Maitreyi RS, Agarwal D, Kumar H, Paul VK, Singh M. Incidence, clinical spectrum and outcome of intrauterine infections in neonates. J Trop Pediatr. 2000;46:155–159. doi: 10.1093/tropej/46.3.155. [DOI] [PubMed] [Google Scholar]
- 3.Yazar S, Kilic E, Saraymen R, Sahin I. Serum malondialdehyde leves in Toxoplasma seropositive patients. Ann Saudi Med. 2003;23:413–415. doi: 10.5144/0256-4947.2003.413. [DOI] [PubMed] [Google Scholar]
- 4.Nishikawa Y, Kawasa O, Vielemeyer O, Suzuki H, Joiner KA, Xuar X, Nagasawa H. Toxoplasma gondii infection induces apoptosis in noninfected macrophages: role of nitric oxide and other soluble factors. Parasite Immunol. 2007;29:375–385. doi: 10.1111/j.1365-3024.2007.00956.x. [DOI] [PubMed] [Google Scholar]
- 5.Koltas IS, Yucebilgic G, Bilgin R, Parsak CK, Sakman G. Serum malondialdehyde level in patients with cystic echinococcosis. Saudi Med J. 2006;27:1703–1705. [PubMed] [Google Scholar]
- 6.Uchiyama M, Mihara M. Determination of malondialdehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978;34:271–278. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- 7.Gensert JM, Ratan RR. The metabolic coupling of arginine metabolism to nitric oxide generation by astrocytes. Antioxid Redox Signal. 2006;8:5–6. doi: 10.1089/ars.2006.8.919. [DOI] [PubMed] [Google Scholar]
- 8.Aladag A, Turkoz Y, Ozerol IB. Nitric oxide and its neurophysiopathological effects. Turk J Med Sci. 2000;20:107–111. [Google Scholar]
- 9.Ding M, Kwok LY, Schluter D, Clayton C, Soldati D. The antioxidant systems in Toxoplasma gondii and the role of cytosolic catalase in defence against oxidative injury. Mol Microbiol. 2004;51:47–61. doi: 10.1046/j.1365-2958.2003.03823.x. [DOI] [PubMed] [Google Scholar]
- 10.Fairbanks V, Klee GG. Biochemical aspects of hematology. In: Tietz NW, editor. Textbook of Clinical Chemistry. Philadelphia, USA: W.B. Saunders Co; 1986. pp. 1532–1534. [Google Scholar]
- 11.Rozenfeld C, Martinez R, Figueiredo RT, Bozza MT, Lima FR, Pires AL, Silva PM, Bonomo A, Lannes-Vieira J, De Souza W, Moura-Neto V. Soluble factors released by Toxoplasma gondii-infected astrocytes down-modulate nitric oxide production by gamma interferon-activated microglia and prevent neuronal degeneration. Infect Immun. 2003;71:2047–2057. doi: 10.1128/IAI.71.4.2047-2057.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daubener W, Posdziech V, Hadding U, Mackenzie CR. Inducuble anti-parasitic effector mechanisms in human uropethelial cells: tryptophan degradation vs. NO production. Med Microbiol Immunol. 1999;187:143–147. doi: 10.1007/s004300050086. [DOI] [PubMed] [Google Scholar]
- 13.Yazar S, Kilic E, Saraymen R, Ozbilge H. Serum malondialdehyde levels in patients infected with plasmodium. West Indian Med. 2004;53:147–149. [PubMed] [Google Scholar]
- 14.Atambay M, Karabulut AB, Aycan OM, Kılıc E, Yazar S, Saraymen R, Karaman U, Daldal N. Dust-mites: effect on lipid peroxidation. Natl Med J India. 2006;19:75–77. [PubMed] [Google Scholar]
- 15.Akkus I. Effects of free radicals and pathophysiological. Konya, Turkey: Mimoza Publisher: 32; 1995. pp. 1–76. ISBN 975-543-038-5. [Google Scholar]
- 16.Mercan U. The importance of free radicals in toxicology. YYU J Vet Faculty. 2004;15:91–96. [Google Scholar]