Abstract
Retinoid X receptors (RXRs) are important transcriptional nuclear hormone receptors, acting as either homodimers or the binding partner for at least one fourth of all the known human nuclear receptors. Functional nongenomic effects of nuclear receptors are poorly understood; however, recently peroxisome proliferator-activated receptor (PPAR) γ, PPARβ, and the glucocorticoid receptor have all been found active in human platelets. Human platelets express RXRα and RXRβ. RXR ligands inhibit platelet aggregation and TXA2 release to ADP and the TXA2 receptors, but only weakly to collagen. ADP and TXA2 both signal via the G protein, Gq. RXR rapidly binds Gq but not Gi/z/o/t/gust in a ligand-dependent manner and inhibits Gq-induced Rac activation and intracellular calcium release. We propose that RXR ligands may have beneficial clinical actions through inhibition of platelet activation. Furthermore, our results demonstrate a novel nongenomic mode for nuclear receptor action and a functional cross-talk between G-protein and nuclear receptor signaling families.
Introduction
Retinoids, the common term for vitamin A metabolites, play important roles in development and cellular differentiation. Retinoid X receptors (RXRs) consist of a family of 3 nuclear receptor isoforms (α, β, and γ) activated by 9-cis-retinoic acid (9cRA),1–3 which act as transcription factors as either homodimers or the binding partner for at least one fourth of all the known human nuclear receptors.4,5 RXR isoforms have a distinct tissue distribution, RXRα being expressed in many tissues but particularly high in the liver, kidney, and skin; RXRβ is widespread, whereas RXRγ appears restricted to skeletal muscle and the heart.6,7
Platelets are widely understood to play critical roles in thrombosis, hemorrhage, inflammation, host defense, and tumor growth and metastasis.8,9 Although platelets are anucleated cell fragments, recent reports by us and others show that platelets express the steroid/nuclear receptors, peroxisome proliferator-activated receptor PPARγ,10 PPARβ/δ,11 and the glucocorticoid receptor.12 Activation of these receptors by their ligands inhibits platelet activation; however, no mechanisms have been described that can account for these rapid nongenomic actions of these nuclear receptors.
Materials and methods
Experiments with healthy volunteers were approved by the local research ethics committee (East London and City Health Authority).
Human platelet-rich plasma (PRP) and washed platelet preparations, aggregation,12 and intracellular calcium13 studies were as previously described. Meg-0 and HEK293 cells were from the European Collection of Cell Cultures (ECACC; Salisbury, United Kingdom). Human umbilical vein endothelial cells (HUVECs) were from PromoCell (Heidelberg, Germany), and cultured according to standard protocols. Membrane and cytosolic fractions were isolated using the QProteome cell fractionation kit (Qiagen; Crawley, United Kingdom). Western blot analysis and immunoprecipitation studies were carried out as previously described.14 Normal rabbit IgG and antibodies for RXRα, Gq, and Gi/o/t/z/gust were from Santa Cruz Biotechnology (San Diego, CA); PPARγ was from Biomol (Affinity Research Products, Exeter, United Kingdom); and RXRβ and RXRγ were from Abcam (Cambridge, United Kingdom). TXA2 release was measured by radioimmunoassay of its stable hydrolysis product TXB2.14 The Rac activation assay was performed according to the manufacturer's instructions (Upstate Biotechnology, Dundee, United Kingdom). Samples for Rac assays were incubated within the aggregometer, as in standard aggregation assays. Statistical analysis was performed using Prism 3.0.3 software (GraphPad, San Diego, CA). Image analysis was performed using ImageJ 1.32j software (W. S. Rasband, ImageJ, US National Institutes of Health, Bethesda, MD; http://rsb.info.nih.gov/ij/, 1997-2005).
Results and discussion
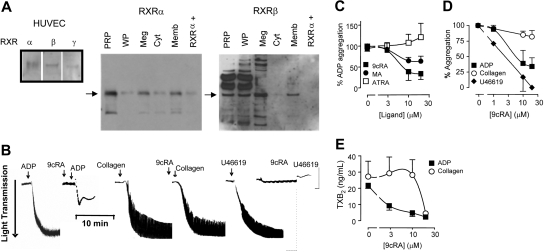
Our positive control HUVECs contain RXRα, RXRβ, and RXRγ. RXRα and RXRβ but not RXRγ are detected in freshly isolated human PRP, washed platelets, and the megakaryocyte cell line Meg-01 (Figure 1A). In platelets RXRα and RXRβ are present both in cytosolic and membrane fractions (Figures 1A and S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Despite the lack of nucleus, RXR ligands, 9cRA (Figure 1B-C),1–3 and the chemically distinct methoprene acid15 inhibit platelet aggregation to ADP (Figure 1C). In contrast, all-trans-retinoic acid, which is an isomer of 9cRA but not an activator of RXR, has no effect on platelet aggregation (Figure 1C). RXR ligands inhibit both the initial ADP receptor-mediated aggregation and the secondary phase sustained by the positive feedback of additional ADP and TXA2 produced by the activated platelets (Figures 1B and S1). Therefore, RXR is pharmacologically active in platelets and not just a cytoplasmic remnant of the megakaryocyte.
Figure 1.
Human platelets express functional retinoid X receptors. (A) Western blot analysis showing positive controls for RXR isoforms (α, β, and γ) in HUVECs, and the expression of RXRα and RXRβ in human platelet-rich plasma (PRP), washed platelets (WPs), megakaryoblast cell line Meg-01 (Meg), and the cytosol (Cyt) and membrane (Memb) fractions of PRP. In addition, a positive control peptide for RXRα (RXRα+) was also included. (B) Typical aggregometer traces showing the changes in light transmittance seen as platelets aggregate (in sequence from left to right) to ADP (4 μM; left panel), 9cRA (10μM) given 3 minutes prior to ADP; to collagen (1 μM), 9cRA (10 μM) given 3 minutes prior to collagen; and to the TXA2 mimetic U46619 (1 μM) and 9cRA (10μM) given 3 minutes prior to U46619. (C) RXR ligands 9cRA (■), and methoprene acid (MA; ●), but not the retinoic acid receptor ligand all-trans retinoic acid (ATRA; □) inhibit ADP-induced platelet aggregation. Figure represents the mean ± SEM changes in percent of ADP aggregation. (D) 9cRA (1-20 μM; 3-minute pretreatment) inhibits ADP-induced (2 or 4 μM; ■) and U46619-induced (1 μM; ♦), but not collagen-induced (1 μM; ○) platelet aggregation in a concentration-dependent manner. Figure represents the mean ± SEM changes in agonist-induced aggregation (titrated to approximately 85% of maximum). (E) 9cRA (1-20 μM; 3-minute pretreatment) more potently inhibits ADP-induced (2 or 4 μM; ■) and collagen-induced (1 μM; ○) platelet TXA2 secretion. Released TXA2 was measured in PRP at the end of aggregation assays (15 minutes) by using an assay for its stable metabolite, TXB2. Figure represents the mean ± SEM TXA2 release. Data represents result from at least 4 separate donors.
Any effects of RXR within 3 minutes must be via rapid protein-protein interactions. PPARγ does coimmunoprecipitate with RXR but does not mediate the inhibitory effect of 9cRA (Figure S2). Similar to ADP, 9cRA completely inhibits platelet aggregation induced by the TXA2 mimetic U46619 (Figure 1B,D). In contrast, 9cRA only weakly inhibits the secretory phase of aggregation induced by collagen (Figure 1B,D). Similarly, 9cRA strongly inhibits TXA2 release from the platelets stimulated by ADP, but only affects TXA2 stimulated by collagen at the highest concentration used (20 μM; Figure 1E). Collagen induces platelet aggregation through its GPVI receptor, linked to the PLCγ.9,10,16 In contrast, ADP and TXA2 receptors in platelets link predominantly to Gq and Gi, and Gq/G12/13 G proteins.9,10,16 Because 9cRA similarly blocks ADP- and TXA2-mediated platelet aggregation, our pharmacologic analysis suggests that 9cRA preferentially inhibits G protein-linked pathways.
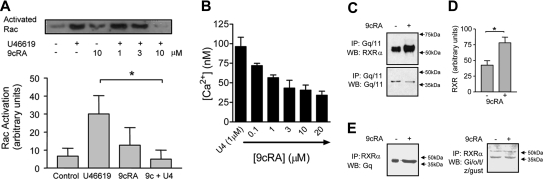
The small GTPase Rac is activated in the early stages of platelet stimulation. Rac is Gq-dependent, being absent in Gq-deficient mice.17 Independent of Rac, downstream of Gq, Ca2+ is released from intracellular stores. The TXA2 mimetic U46619 rapidly activates Rac after 30 seconds. A 3-minute pretreatment with 9cRA inhibits Rac activation induced by U46619 (Figure 2A); 9cRA alone has no effect. Similarly, U46619-induced Ca2+ release was inhibited by 9cRA in washed platelets. For RXR to mediate these effects, 9cRA must induce rapid protein-protein changes in the Gq-signaling cascade.
Figure 2.
RXR binds and inhibits Gq signaling in human platelets. (A) Top panel shows a typical Rac activation assay blot; lower panel shows densitometry measurements (arbitrary units) from 3 blots. Rac activation was measured at 30 seconds after U46619 stimulation. 9cRA was given as standard as a 3-minute pretreatment. For densitometry measurements vehicle pretreatment (control), U46619 (1 μM), 9cRA (10 μM), and 9cRA followed by U46619 (9c+U4; 9cRA 10 μM; U46619 1μM) are shown. *P < .05 by repeated measures 1-way ANOVA, followed by Bonferroni post test. (B) 9cRA (0.1-20 μM) inhibits U46619-induced (U4; 1 μM intracellular Ca2+ release in washed platelets. Figure represents the mean ± SEM changes in Ca2+ release (nM) in 4 × 108 platelets. (C) RXR binds Gq/11 in a ligand-dependent fashion. Human PRP was treated with 9cRA (10 μM) for 3 minutes. Platelet lysates were immunoprecipitated (IP) with anti-Gq/11 and Western blotted (WB) for RXRα (top panel) or Gq/11 (lower panel). (D) Densitometry measurements (arbitrary units) from 3 blots for RXRα expression detected after 9cRA (10 μM; 3 minutes) treatment and Gq/11 IP. *P < .05 by unpaired t test. (E) RXR binds Gq/11 but not Gi/o/t/z/gust in a ligand-dependent fashion. Human PRP was treated with 9cRA (10 μM) for 3 minutes. Platelet lysates were immunoprecipitated (IP) with anti-RXRα and Western blotted (WB) for Gq/11 (top panel) or Gi/o/t/z/gust (lower panel); arrow indicates 40-kDa band.
Many of the proteins that interact with nuclear receptors as coactivators contain a common LXXLL motif.6,18 Analysis of human Gq surprisingly indicated that it contains a single LXXLL motif in its n-terminal region (Table S1). Furthermore, unlike other G proteins, the whole Gq family (Gq, G11, G14, G15, and G16) contains this conserved LKLLL sequence (Table S1). Analysis of Gq-RXR by coimmunoprecipitation revealed that Gq/11 and RXR associate together under unstimulated conditions both in platelets (Figure 2C-E) and in HEK293 cells transfected with human Gq and RXRα (Figure S2). When stimulated with 9cRA for 3 minutes, the amounts of Gq/11 and RXR coimmunoprecipitating significantly increase (Figure 2C-E). In contrast, using an antibody that recognizes a variety of G proteins (Gi/o/t/z/gust), none of which contain an LXXLL motif and of which at least Gi and Gz are expressed in platelets, we found a weak association of proteins of the characteristic G-protein molecular weight of approximately 40 kDa with RXR under basal conditions, in some but not all experiments (Figure S3); however, no increase was observed in the presence of 9cRA (Figure 2E). We therefore propose this Gq-RXR–binding event induced by 9cRA, probably using the LXXLL motif, sequesters Gq, preventing its subsequent use in the activation of Rac and aggregation-dependent signaling pathways within the platelet.
RXR ligands are currently used topically for the treatment of cutaneous T-cell lymphoma and Kaposi sarcoma and show some efficacy at reducing the progression of atherosclerosis in apoE knockout mice.19 Because platelets play an important role in the pathogenesis of cancer and cardiovascular disease,8,9 our findings that RXR ligands inhibit platelet activation may help to explain the efficacy of these ligands. Although, the inhibition of platelet activation compared to agents such as prostacyclin is weak, the effects we see with 9cRA are of a similar order and potency to those of 10-μM aspirin (Figure S2).
Because it contains no nucleus, we propose that the platelet is an excellent human experimental model system to study nuclear receptor protein-protein interactions. In addition, to PPARγ,10 PPARβ/δ,11 the glucocorticoid receptor,12 and RXRs reported here we also find that PPARα and farnesoid X receptor (L.A.M. and D.B.-B., unpublished observations, March 2005) are also expressed in platelets, though as yet full mechanistic analysis of these receptors has not been performed. Functional, rapid nongenomic effects involving acute signaling pathways have been described for many nuclear receptor ligands,20,21 though as yet no clear mechanisms exist linking nuclear receptors to these events. The Gq family of signaling intermediates regulates many aspects of cellular signaling, homeostasis, and development.22 Our results show a functional cross-talk between RXR and Gq in platelets. The Gq family may therefore represent a novel family of nuclear receptor coregulators whose interactions with nuclear receptors can explain nongenomic signaling.
Acknowledgments
This work was supported by grants from the Barts and London Joint Research Board (XMNR), the Wellcome Trust (074361/Z/04/Z), the European Community FP6 funding (LSHM-CT-2004-0050333), and the British Heart Foundation (RG/05/007).
J.W. holds a British Heart Foundation Studentship (FS/04/075). D.B.B. is the recipient of a British Heart Foundation Basic Science Lectureship (BS/02/002).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: L.A.M., K.E.S., J.A.W., A.D., J.M.G., T.D.W., and D.B.B. designed the research; L.A.M., K.E.S., J.A.W., A.D., and D.B.B. performed research; L.A.M. and D.B.B. conceived the research; D.B.B., J.M.G., and T.D.W. supervised the experiments; and D.B.B. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David Bishop-Bailey, Cardiac, Vascular and Inflammation Research, William Harvey Research Institute, Barts and the London, Queen Mary University of London, Charterhouse Square, London EC1M 6BQ, United Kingdom; e-mail: d.bishop-bailey@qmul.ac.uk.
References
- 1.Mangelsdorf DJ, Ong ES, Dyck JA, Evans R. Nuclear receptor that identifies a novel retinoic acid response pathway. Nature. 1990;345:224–229. doi: 10.1038/345224a0. [DOI] [PubMed] [Google Scholar]
- 2.Heyman RA, Mangelsdorf DJ, Dyck JA, et al. 9-cis retinoic acid is a high affinity ligand for the retinoid X receptor. Cell. 1992;68:397–406. doi: 10.1016/0092-8674(92)90479-v. [DOI] [PubMed] [Google Scholar]
- 3.Levin AA, Sturzenbecker LJ, Kazmer S, et al. 9-cis retinoic acid stereoisomer binds and activates the nuclear receptor RXR alpha. Nature. 1992;355:359–361. doi: 10.1038/355359a0. [DOI] [PubMed] [Google Scholar]
- 4.Kliewer SA, Umesono K, Mangelsdorf DJ, Evans RM. Retinoid X receptor interacts with nuclear receptors in retinoic acid, thyroid hormone and vitamin D3 signalling. Nature. 1992;355:446–449. doi: 10.1038/355446a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ. Nuclear receptors and lipid physiology: opening the X-files. Science. 2001;294:1866–1870. doi: 10.1126/science.294.5548.1866. [DOI] [PubMed] [Google Scholar]
- 6.Heery DM, Kalkhoven E, Hoare S, Parker MG. A signature motif in transcriptional co-activators mediates binding to nuclear receptors. Nature. 1997;387:733–736. doi: 10.1038/42750. [DOI] [PubMed] [Google Scholar]
- 7.Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996;10:940–954. [PubMed] [Google Scholar]
- 8.Mangelsdorf DJ, Borgmeyer U, Heyman RA, et al. Characterization of three RXR genes that mediate the action of 9-cis retinoic acid. Genes Dev. 1992;6:329–344. doi: 10.1101/gad.6.3.329. [DOI] [PubMed] [Google Scholar]
- 9.Weyrich AS, Zimmerman GA. Platelets: signaling cells in the immune continuum. Trends Immunol. 2004;25:489–495. doi: 10.1016/j.it.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Akbiyik F, Ray DM, Gettings KF, et al. Human bone marrow megakaryocytes and platelets express PPARgamma, and PPARgamma agonists blunt platelet release of CD40 ligand and thromboxanes. Blood. 2004;104:1361–1368. doi: 10.1182/blood-2004-03-0926. [DOI] [PubMed] [Google Scholar]
- 11.Ali FY, Davidson SJ, Moraes LA, et al. Role of nuclear receptor signaling in platelets: antithrombotic effects of PPARbeta. FASEB J. 2006;20:326–328. doi: 10.1096/fj.05-4395fje. [DOI] [PubMed] [Google Scholar]
- 12.Moraes LA, Paul-Clark MJ, Rickman A, et al. Ligand-specific glucocorticoid receptor activation in human platelets. Blood. 2005;106:4167–4175. doi: 10.1182/blood-2005-04-1723. [DOI] [PubMed] [Google Scholar]
- 13.Cicmil M, Thomas JM, Leduc M, Bon C, Gibbins JM. PECAM-1 signalling inhibits the activation of human platelets. Blood. 2002;99:137–144. doi: 10.1182/blood.v99.1.137. [DOI] [PubMed] [Google Scholar]
- 14.Bishop-Bailey D, Pepper JR, Larkin SW, Mitchell JA. Differential induction of cyclooxygenase-2 in human arterial and venous smooth muscle: role of endogenous prostanoids. Arterioscler Thromb Vasc Biol. 1998;18:1655–1661. doi: 10.1161/01.atv.18.10.1655. [DOI] [PubMed] [Google Scholar]
- 15.Harmon MA, Boehm MF, Heyman RA, Mangelsdorf DJ. Activation of mammalian retinoid X receptors by the insect growth regulator methoprene. Proc Natl Acad Sci U S A. 1995;92:6157–6160. doi: 10.1073/pnas.92.13.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Savage B, Cattaneo M, Ruggeri ZM. Mechanisms of platelet aggregation. Curr Opin Hematol. 2001;8:270–276. doi: 10.1097/00062752-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Gratacap MP, Payrastre B, Nieswandt B, Offermanns S. Differential regulation of Rho and Rac through heterotrimeric G-proteins and cyclic nucleotides. J Biol Chem. 2001;276:47906–47913. doi: 10.1074/jbc.M104442200. [DOI] [PubMed] [Google Scholar]
- 18.McKenna NJ, O'Malley BW. Minireview: nuclear receptor coactivators—an update. Endocrinology. 2002;143:2461–2465. doi: 10.1210/endo.143.7.8892. [DOI] [PubMed] [Google Scholar]
- 19.Claudel T, Leibowitz MD, Fievet C, et al. Reduction of atherosclerosis in apolipoprotein E knockout mice by activation of the retinoid X receptor. Proc Natl Acad Sci U S A. 2001;98:2610–2615. doi: 10.1073/pnas.041609298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Losel RM, Falkenstein E, Feuring M, et al. Nongenomic steroid action: controversies, questions, and answers. Physiol Rev. 2003;83:965–1016. doi: 10.1152/physrev.00003.2003. [DOI] [PubMed] [Google Scholar]
- 21.Losel R, Wehling M. Nongenomic actions of steroid hormones. Nat Rev Mol Cell Biol. 2003;4:46–56. doi: 10.1038/nrm1009. [DOI] [PubMed] [Google Scholar]
- 22.Neves SR, Ram PT, Iyengar R. G protein pathways. Science. 2002;296:1636–1639. doi: 10.1126/science.1071550. [DOI] [PubMed] [Google Scholar]