Abstract
Introduction
Progesterone receptors (PRs) are present in many breast tumors, and their levels are increased by certain endocrine therapies. They can be used as targets for diagnostic imaging and radiotherapy.
Method
16α,17α-[(R)-1’-α-(5-[76Br]bromofurylmethylidene)dioxyl]-21-hydroxy-19-norpregn-4-ene-3,20-dione ([76Br]3), a PR ligand with relative binding affinity (RBA) = 65 and log Po/w = 5.09 ± 0.84, was synthesized via a two-step reaction and its tissue biodistri0bution and metabolic stability were evaluated in estrogen-primed immature female Sprague-Dawley rats.
Results
[76Br]3 was synthesized in 5% overall yield with specific activity being 200~1250 Ci/mmol. [76Br]3 demonstrated high PR-mediated uptake in the target tissue uterus (8.72 ± 1.84 %ID/g at 1 h) that was reduced by a blocking dose of unlabeled progestin R5020, but the non-specific uptake in blood and muscle (2.11 ± 0.14 and 0.89 ± 0.16 %ID/g at 1 h, respectively) was relatively high. [76Br]3 was stable in whole rat blood in vitro, but it was not stable in vivo due to the fast metabolism that occurred in the liver, resulting in the formation of a more polar radioactive metabolite and free [76Br]bromide. The level of free [76Br]bromide in blood remained high during the experiment (2.11 ± 0.14 %ID/g at 1 h and 1.52 ± 0.24 %ID/g at 24 h). The tissue distribution of [76Br]3 at 1 and 3 hours was compared with that of the 18F analogs, [18F]FFNP 1 and ketal 2.
Conclusion
[76Br]3 may have potential for imaging PR positive breast tumors at early time points, but it is not suitable for imaging at later times or for radiotherapy.
Keywords: progesterone receptor, Br-76 radiolabeling, PET breast tumor imaging, radiotherapy
1. Introduction
Steroid receptors are found in a number of endocrine-responsive cancers, estrogen receptors (ERs) and progesterone receptors (PRs) in many breast tumors and androgen receptors (ARs) in most prostate cancers. These receptors serve as targets for endocrine therapies of these cancers, but they also can be used as targets for diagnostic imaging and radiotherapy. Diagnostic imaging can be achieved by the administration of a suitably radiolabeled ligand that accumulates in the receptor-positive tumor, where it can be detected and quantified by imaging. Such images can sometimes be used to predict whether hormone therapy will be effective [1–4]. In a related manner, a hormone receptor ligand labeled with a radionuclide (e.g., an Auger electron emitting isotope) that accumulates in a tumor through a receptor-mediated uptake process can deliver a cytotoxic dose of high linear energy transfer (LET) radiation selectively to the tumor cells, ablating the tumor while limiting widespread radiation toxicity. Therefore, the development of such hormone receptor ligands for both diagnostic imaging and radiotherapy is a promising area of research.
Diagnostic imaging of breast and prostate tumors by positron emission tomography (PET) is well established and has been achieved using steroids labeled with fluorine-18, such as 16α-[18F]fluoroestradiol (FES) [3,4] for breast tumor imaging and 16β-[18F]fluoro-5α-dihydrotestosterone (FDHT) [5,6] for prostate cancer imaging. Although a number of steroids labeled with bromine and iodine radioisotopes have been prepared [7–12] and studied, especially in terms of their potential for selective radiotherapy, their use in imaging studies, particularly in humans, has been more limited [12]. Nevertheless, previous studies have established the feasibility of using various Auger electron-emitting isotopes for selective cellular therapy [13–23].
Bromine-76 decays with a significant amount of positron emission (57% positron and 43% electron capture), a characteristic that allows for diagnostic PET imaging to be used to complement the use of this isotope in radiotherapy. It can conveniently be produced via the 76Se(p,n)76Br reaction on the majority of medical cyclotrons [24]. 76Br has a half life of 16.2 hours, which is long enough to permit target tissue-selective distribution while being sufficiently short so that the bulk of the dose can be delivered to the tissue prior to metabolism and elimination of the radiopharmaceutical. Because the natural abundance of bromine is low, organic compounds can be labeled with very high specific activity (SA), which is a general requirement for the study of receptor-binding related biological processes.
In principle, a ligand based on binding to either ER or PR might be used in applications of breast tumor imaging and therapy in an untreated patient. However, a PR-based radioligand has some potential advantages over an ER-based one: (1) there is a better correlation between PR status and hormonal responsiveness than there is with ER status [25–29]; (2) a PR-based ligand could be used after the initiation of anti-estrogen hormonal therapy, whereas an ER-based one would not be useful when tumor ER is saturated by the hormonal agent [30]. Moreover, (3) PR-based ligands may benefit from the increased PR levels induced by the transient agonistic effect of tamoxifen during the initial course of tamoxifen treatment of breast tumor [31–33].
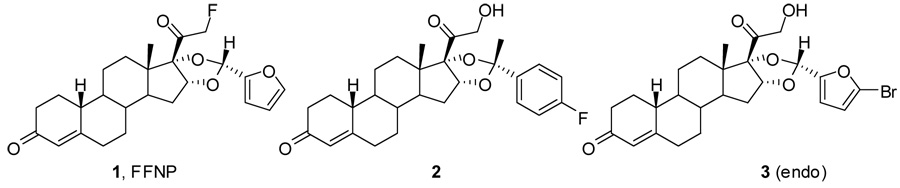
A series of bromine-and iodine-substituted 16α,17α-dioxolane progestins have been synthesized [34] based on the very promising PR ligand, fluoro furanyl norprogesterone (FFNP) 1 [35] and its analog 2 [36]. Among this series, 16α,17α-[(R)-1’-α-(5-bromofurylmethylidene)dioxyl]-21-hydroxy-19-norpregn-4-ene-3,20-dione (3) appeared to be the most promising one with good relative binding affinity to PR (RBA = 65, relative to R5020) and moderate lipophilicity (log Po/w = 5.09 ± 0.84) [34]. In this report, we describe the 76Br radiolabeling of 3, the in vivo biodistribution and metabolic stability studies of 3 in estrogen-primed immature female Sprague-Dawley rats as a potential agent for breath tumor imaging and radiotherapy.
2. Materials and methods
2.1 General
76Br was produced at the Washington University cyclotron facility by the 76Se(p,n)76Br nuclear reaction on a 76Se-enriched Cu2Se target. 76Br was recovered via a modified dry distillation method [24]. The radionuclide was in the form of [76Br]NH4Br in 0.6 M NH4OH. The solution was filtered through a C-18 Sep-Pak light cartridge (Waters Corp.) and blown down to dryness under very mild N2 flow at 130°C. High performance liquid chromatography (HPLC, Dionex, Sunnyvale, CA, USA) was performed with an ultraviolet detector operating at 231 nm and a radioactive detector. Agilent Zorbax SB-C18 250 × 4.6 mm 5 µm analytical column and Agilent Zorbax SC-C18 250 × 9.4 mm 5 µm semi-preparative column were used for analysis and preparative purification respectively. Acetonitrile and water were used as the HPLC mobile phase. RadioTLC was performed using a Bioscan System 2000 imaging scanner (Bioscan, Washington, DC, USA). All chemicals and solvents were obtained from common commercial sources. Compound 4 [37] and 6 [34,38] were synthesized according to previously published literature. All animal experiments were conducted in compliance with the Guidelines for the Care and Use of Research Animals published by the Animal Studies Committee of Washington University in St. Louis, School of Medicine.
2.2 Radiochemistry
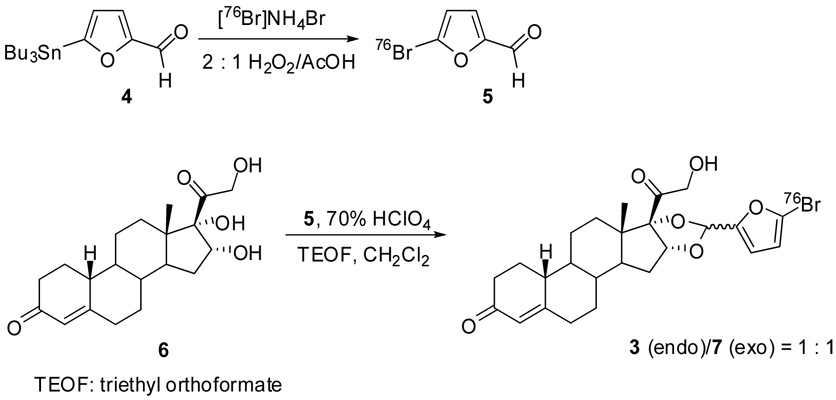
~10 mCi [76Br]NH4Br was dried in a 5 mL Wheaton V vial at 130°C under very gentle N2 flow. At ambient temperature, 20 µL acetic acid and 0.5 mg 4 in 50 µL methanol were added, and the solution was vortexed before addition of 100 µL 2 : 1 hydrogen peroxide/acetic acid (pre-mixed for at least 4 hours). Then the reaction mixture was vortexed to homogeneity. Upon the completion of the reaction in 30 minutes (> 80% incorporation according to radio-TLC analysis: silica plate (EMD Cat. 15341–5), 1 : 1 ethyl acetate/hexanes, Rf = 0.8), the reaction was quenched by the addition of 2 mL water. The reaction mixture was extracted with 0.7 mL × 2 dichloromethane, which was loaded onto a column (I.D. 0.5 cm, from bottom to top: 2 cm MgSO4, 4 cm silica, 2 cm Na2SO4, and 1 cm sand, filled with dichloromethane). [76Br]5 was eluted with dichloromethane, and the eluent was collected in 1 mL portions. The two portions containing the majority of 76Br radioactivity (3.5 mCi, 35% isolated yield) were transferred to a 10 mL Pyrex screw-capped tube containing 1.2 mg 6, and the solution was concentrated to 1.0 mL under a flow of N2 at ambient temperature. Then 18.0 µL 1 : 1 triethyl orthoformate (TEOF)/CH2Cl2 was added followed by the addition of 2.0 µL 70% HClO4. After the solution was vortexed, the color of the reaction changed to pale pink, deepening slightly in color at 30 minutes, at which point more than 80% of [76Br]5 was converted to[76Br]3 (2 : 1 ethyl acetate/hexanes, Rf = 0.4). The reaction mixture was first quenched by the addition of small amount of NaHCO3 (right after the tube was opened to the air) and then it was passed through a light alumina Sep-Pak (Waters Corp.), followed by rinsing with 2 mL dry dichloromethane. 2 mCi of radioactivity was collected in dichloromethane, which was subsequently removed under a flow of N2 at ambient temperature. The dried radioactivity was reconstituted in 100 µL dimethyl formide, 700 µL acetonitrile and 1200 µL water and injected for HPLC purification. The reverse phase HPLC purification (Agilent Zorbax SC-C18 250 × 9.4 mm 5 µm) was carried out using 47.5% acetonitrile 52.5% water at a flow rate of 3 mL/min and UV at 231 nm. Radioactivity was monitored by a radioactivity detector (Bioscan, B-FC-3200), and 600 µCi [76Br]3 was collected at 25.6 minutes. The HPLC collection of [76Br]3 was diluted in 30 mL water, and the solution was passed through a C18 classic Sep-Pak (preconditioned with 6 mL methanol and 12 mL water) to trap the activity. After the Sep-Pak was rinsed with 10 mL water, [76Br]3 was eluted with 1 mL ethanol. The ethanol solution of [76Br]3 was concentrated in order to make a final solution of 1.2 mL of 15% ethanol and 85% saline. Specific activity was determined to be more than 400 Ci/mmol (EOS) using reverse phase HPLC (Agilent Zorbax SB-C18 250 × 4.6 mm 5 µm) applying 60% acetonitrile and 40% water as solvents at a flow rate of 1.5 mL/min and UV at 231 nm.
2.3 In vitro stability study
An in vitro stability study was carried out in isolated heparinized whole rat blood (Sprague-Dawley rats, mature). The whole blood (5 mL) was incubated with ~3 µCi [76Br]3 in 20 µL ethanol for 5 min, 30 min, 1 h and 2 h at 37°C. An aliquot of blood was treated with 3 volumes of ethanol at each time point, and the lysed sample was centrifuged to separate the supernatant from the pellet. The radioactivity in the supernatant and the pellet was counted separately on a Beckman Gamma 8000 well counter. The radioactive species in the supernatant was analyzed by Silica TLC using 2 : 1 ethyl actate/hexanes as the developing solvent (Rf = 0.4 for [76Br]3) and co-elution with non-radioactive 3.
2.4 In vivo metabolic stability study
The metabolism of [76Br]3 was evaluated in estrogen-primed immature female Sprague-Dawley rats (24 h and 3 h estradiol treatment before injection, n = 1) by injection of 30 µCi of [76Br]3 in 15% ethanol/saline via the tail vein. The rats were sacrified at 1 min, 1 h, 3 h, and 24 h post-injection and blood, muscle, liver, and uterus were collected. Tissue samples were homogenized in 1 mL buffer at room temperature. The supernatant and pellet were separated by centrifugation and kept in ice to prevent degradation of the samples. The radioactive species in the supernatant was analyzed by silica gel TLC using Bioscan system 2000 imaging scanner. The samples were co-spotted with non-radioactive 3 and TLC was developed in ethyl acetate (Rf = 0, 0.6 for [76Br]bromide and [76Br]3 respectively). The radioactivity in the supernatant and the pellet was counted separately as mentioned above.
2.5 In vivo tissue biodistribution studies
PR levels in the uteri of immature female Sprague-Dawley rats (25 days old, ~50 g) were induced by daily subcutaneous injections for 1 or 2 days of 5 µg of estradiol in 0.1 mL of 20% ethanol/80% sunflower seed oil. The experiments were begun 24 h (or 3 h) after the last injection. The HPLC purified [76Br]3 was injected in 100~150 µL 15% ethanol/saline via the tail vein of 2% Isoflurane anesthetized rats. At the specified time points post-injection, the rats were sacrificed by cervical dislocation, and blood, tissues and organs were removed, weighed, and counted in a Beckman Gamma 8000 counter. To determine whether the uptake was mediated by a high-affinity, limited-capacity system, one set of animals was co-injected with [76Br]3 together with 18 µg of R5020 in order to fully occupy the progesterone receptors.
3. Results
3.1 Radiochemistry
[76Br]3 was synthesized by two steps: aromatic electrophilic substitution of the tributyltin precursor 4 to afford [76Br]5 using 2 : 1 hydrogen peroxide/acetic acid as oxidizing agent and acetalization of progestin 16α,17α,21-triol 6 with [76Br]5 in the presence of 70% HClO4 and TEOF to afford an endo isomer 3 and an exo isomer 7 in 1 : 1 ratio (Fig. 2). The yields for both the incorporation of [76Br]bromide and the acetalization of 6 were up to 80%, but the overall yield of [76Br]3 was only 5% (not decay corrected). [76Br]3 was purified by reversed phase C18 HPLC and confirmed by co-elution with non-radioactive 3. The specific activity was determined by HPLC to be from 200 to 1250 mCi/µmol at the end of synthesis.
3.2 In vitro stability study
[76Br]3 was stable in HPLC solvent overnight, showing no sign of radiolysis due to the high energy radiation of the 76Br isotope. The in vitro stability study was carried out in isolated whole rat blood. Around 75% of the radioactivity was separated into the supernatant from the pellet over the period of 2 hours, indicating little binding of [76Br]3 to cells or cellular proteins in the blood. According to the radioTLC analysis of the supernatant, [76Br]3, confirmed by co-elution with co-spotted non-radioactive 3 on TLC, was the only radioactive species observed during the experiment (2 hours), indicating good in vitro stability of [76Br]3 in rat blood.
3.3 In vivo metabolic stability study
An in vivo metabolic stability study was carried out in estrogen-primed immature female Sprague-Dawley rats, the same animal model used in the tissue biodistribution (Table 1). Around 90%, 90%, 80%, and 75% of radioactivity was found in the supernatants from uterus, muscle, blood, and liver extraction samples during the experiment (24 h), indicating little binding of [76Br]3 to cells or cellular proteins in the animal. There was slightly less recovery of radioactivity from blood and liver. At 1 minute after administration of [76Br]3, metabolism was observed in the liver: 58.5% radioactivity in the supernatant remained as [76Br]3 (Rf = 0.6), 27% as [76Br]bromide (Rf = 0) and 14.5% as a more polar metabolite (8, Rf = 0.3, structure unknown); no metabolism was observed in the blood, muscle and uterus supernatants at 1 minute. At 1 h post-injection, 31.5% of the radioactivity in the liver supernatant was observed to be [76Br]3; the other radioactivity was the more polar metabolite 8 and [76Br]bromide; 66.5% of the radioactivity in the blood supernatant was [76Br]bromide, with only 9% as [76Br]3 and 24.5% as the more polar metabolite 8; 60% and 80% radioactivity in the muscle and uterus supernatants remained as [76Br]3, respectively, with the rest being [76Br]bromide. At 3 h post-injection, 19.5% of activity was still [76Br]3 in the liver supernatant, but the majority of radioactivity in the blood supernatant was free [76Br]bromide; the amount of [76Br]3 in the muscle and uterus supernatants remained high (68% and 67.5%, respectively). At 24 hours post-injection, the amount of radioactivity in liver and muscle was under the detection limit for radio-TLC analysis; free[76Br]bromide was the only radioactivity observed in the blood supernatant; there was still 40% as [76Br]3 in the uterus supernatant with the rest as [76Br]bromide.
Table 1.
Fraction of 76Br species in various tissues of estrogen-primed immature female rats
| Fraction of [76Br] species (%)a, b |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Uterus | Blood | Muscle | Liver | |||||||||||||
| Time | 1 m | 1 h | 3 h | 24 h | 1 m | 1 h | 3 h | 24 h | 1 min | 1 h | 3 h | 24 h | 1 m | 1 h | 3 h | 24 h |
| [76Br]3 | 100 | 80 | 67.5 | 40 | 100 | 9 | 9 | 0 | 100 | 60 | - | - | 58.5 | 31.5 | 19.5 | - |
| [76Br]8 | 0 | 0 | 14.5 | 0 | 0 | 24.5 | 0 | 0 | 0 | 0 | - | - | 14.5 | 33.5 | 16 | - |
| [76Br]Br− | 0 | 20 | 17 | 60 | 0 | 66.5 | 91 | 100 | 0 | 40 | - | - | 27 | 35 | 63 | - |
| Extraction (%) | 94 | 93.5 | 90 | 88 | 85.5 | 79.5 | 80 | 79 | 90.5 | 82.5 | 86 | 89 | 83 | 73.5 | 74 | 76 |
Determined by radio-TLC: silica plate/ethyl acetate, Rf = 0.6, 0.3, 0 for 3, 8 (the more polar metabolite), and bromide, respectively
-: Under detection limit.
3.4 Tissue biodistribution studies
0.5~40 µCi HPLC-purified [76Br]3 at a specific activity of 200~1250 mCi/µmol was injected into estrogen-primed immature female Sprague-Dawley rats. Table 2 shows the %ID/g uptake of radioactivity data using 7 µCi [76Br]3 at a specific activity of more than 400 mCi/µmol. Initially, at 15 min post-injection, the highest uptake was observed in the uterus (8.22 ± 0.6 %ID/g), which is the most PR-rich target organ; the uptake in liver and muscle was 4.22 ± 0.87 and 1.38 ± 0.23 %ID/g, respectively. At 1h post-injection, uptake in the uterus remained high (8.72 ± 1.84 %ID/g), while up to 50% of the radioactivity in liver and muscle was washed out; the activity in blood was similar to that at 15 min. At 3 h post-injection, uptake in the uterus was reduced to 5.52 ± 1.84 %ID/g, and the uptake in liver and muscle was reduced further. However, the activity in blood remained constant. At 24 h post-injection, all tissue activities were reduced to background, with the highest activity in blood.
Table 2.
Tissue biodistribution of [76Br]3 in estrogen-primed immature female rats
| Percent injected dose/g ± SDan = 4 |
||||
|---|---|---|---|---|
| 15 min (n = 3) | 1 h | 3 h | 24 h | |
| Blood | 2.51 ± 0.58 | 2.11 ± 0.14 | 2.24 ± 0.38 | 1.52 ± 0.24 |
| Liver | 4.22 ± 0.87 | 2.01 ± 0.37 | 1.49 ± 0.26 | 0.60 ± 0.09 |
| Spleen | 1.34 ± 0.02 | 1.43 ± 0.82 | 1.07 ± 0.12 | 0.74 ± 0.15 |
| Kidney | 4.73 ± 0.84 | 2.96 ± 0.96 | 1.72 ± 0.21 | 1.01 ± 0.18 |
| Muscle | 1.38 ± 0.23 | 0.89 ± 0.16 | 0.71 ± 0.11 | 0.45 ± 0.11 |
| Uterus | 8.22 ± 0.63 | 8.72 ± 1.84 | 5.52 ± 1.84 | 1.18 ± 0.22 |
| Uterus/blood | 3.40 ± 0.83 | 4.11 ± 0.73 | 2.42 ± 0.36 | 0.77 ± 0.04 |
| Uterus/muscle | 6.08 ± 1.03 | 9.82 ± 1.57 | 7.67 ± 1.30 | 2.66 ± 0.32 |
SD: standard deviation.
24 h and 3 h estradiol treatment before injection of [76Br]3; 7 µCi/100 µL 15% ethanol/saline; Specific activity: > 400 mCi/µmol
To determine whether the uptake was mediated by a high-affinity, limited-capacity system, a blocking study was carried out by co-injection of 2~3 µCi [76Br]3 at a specific activity of 1250 mCi/µmol together with 18 µg R5020 (RBA = 100) to fully occupy the progesterone receptors; the results are shown in Table 3. The uptake in uterus and ovaries of the blocked animals was reduced 54% and 42%, respectively, lowering to the level seen in non-target tissues, indicating that the uptake of [76Br]3 in the uterus is mediated by binding to PR. No obvious differences in activity levels were observed in blood, liver, spleen and muscle between the blocked and non-blocked animals, and the uptake values reported in Table 3 are similar to those in Table 2 at 1 and 3 h post-injection.
Table 3.
Tissue biodistribution of [76Br]3 in estrogen-primed immature female rats: blocking study
| Percent injected dose/g ± SDan = 4 |
||||
|---|---|---|---|---|
| 1 h | 1 h (block) | 3 h | 18 h | |
| Blood | 2.10 ± 0.48 | 1.89 ± 0.18 | 1.76 ± 0.08 | 1.19 ± 0.19 |
| Liver | 2.39 ± 0.19 | 2.18 ± 0.21 | 1.18 ± 0.15 | 0.53 ± 0.07 |
| Spleen | 0.98 ± 0.08 | 0.95 ± 0.11 | 0.88 ± 0.05 | 0.59 ± 0.08 |
| Kidney | 2.32 ± 0.36 | 3.37 ± 0.48 | 1.31 ± 0.04 | 0.70 ± 0.12 |
| Muscle | 0.86 ± 0.10 | 0.81 ± 0.13 | 0.60 ± 0.09 | 0.41 ± 0.11 |
| Uterus | 5.26 ± 0.35 | 2.43 ± 0.34 | 2.69 ± 0.20 | 0.71 ± 0.14 |
| Ovaries | 3.54 ± 0.56 | 2.06 ± 0.44 | 1.91 ± 0.38 | 0.64 ± 0.12 |
| Uterus/blood | 2.65 ± 0.88 | 1.28 ± 0.12 | 1.54 ± 0.17 | 0.60 ± 0.04 |
| Uterus/muscle | 6.21 ± 1.12 | 3.01 ± 0.24 | 4.58 ± 0.65 | 1.74 ± 0.14 |
SD: standard deviation.
3 h estradiol treatment before injection of [76Br]3; 2~3 µCi/100µL 15% ethanol/saline specific activity: 1250 mCi/µmol
To establish that the uptake was not limited by undetected PR-binding non-radioactive impurities, 0.8, 2 and 40 µCi [76Br]3 at a specific activity of 200 mCi/µmol was injected into a set of animals, and the tissue biodistribution was determined at 1 h post-injeciton. The results are shown in Table 4. The uptake in the organs or tissues tested showed no statistical difference from the ‘low’ to the ‘high’ dose administration at 1 h post-injection. Also, these biodistribution data are similar to those reported in Table 2 and 3. These results indicate that the effective specific activity of the HPLC-purified [76Br]3 is not low and that PR-binding impurities are not a limiting factor in the biodistribution of [76Br]3.
Table 4.
Tissue biodistribution of [76Br]3 in estrogen-primed immature female rats: dose study
| Percent injected dose/g ± SDan = 4 |
|||
|---|---|---|---|
| 1 h (0.8 µCi) | 1 h (2 µCi) | 1 h (40 µCi)) | |
| Blood | 2.16 ± 0.55 | 1.83 ± 0.16 | 1.86 ± 0.40 |
| Liver | 2.26 ± 0.28 | 2.07 ± 0.36 | 1.99 ± 0.45 |
| Spleen | 1.10 ± 0.15 | 0.93 ± 0.05 | 1.07 ± 0.32 |
| Kidney | 2.60 ± 0.99 | 1.84 ± 0.21 | 2.38 ± 0.98 |
| Muscle | 0.70 ± 0.06 | 0.78 ± 0.16 | 0.87 ± 0.42 |
| Uterus | 6.00 ± 1.44 | 5.42 ± 1.14 | 5.26 ± 1.04 |
| Ovaries | 3.35 ± 0.42 | 2.83 ± 0.28 | 3.36 ± 0.66 |
| Uterus/blood | 2.79 ± 0.21 | 2.97 ± 0.64 | 2.96 ± 1.06 |
| Uterus/muscle | 8.65 ± 2.15 | 7.23 ± 2.19 | 7.16 ± 3.67 |
SD: standard deviation.
48h and 24 h estradiol treatments before injection of [76Br]3; 100µL 15% ethanol/saline; specific activity: 200 mCi/µmol
4. Discussion
Unlike the extensive imaging studies that have been performed using ER radio-ligands [39], the study of PR ligands as imaging agents or for radiotherapy has been limited. Nevertheless, the most promising PR ligand for PET imaging of breast cancer reported so far is fluoro furanyl norprogesterone (FFNP, 1), the 18F-labeled form of which is undergoing clinical imaging investigations at Washington University, School of Medicine. FFNP has a high relative binding affinity to PR, low nonspecific binding, and a high binding selectivity index [35, 40]. In tissue biodistribution studies, [18F]FFNP demonstrated high PR-selective uptake in the principal PR target organs, uterus and ovaries, and relatively low uptake in non-PR target tissues, such as fat and bone [35]. In addition, FFNP is expected to be more stable towards the metabolism of the C-20 ketone by steroid dehydrogenases, because it is protected by the bulk of the 16α,17α-furanyl group, a group onto which a bromine can be introduced easily. The favorable pharmacokinetic and pharmacodynamic features of FFNP meet the requirements for a successful ligand for imaging or therapy [40]. Therefore, 16α,17α-[(R)-1’-α-(5-bromofurylmethylidene)dioxyl]-21-13 hydroxy-19-norpregn-4-ene-3,20-dione (3) was developed, because when labeled with bromine-76, it has potential as a PR ligand for imaging and radiotherapy based on the favorable characteristics of FFNP (1) and its predecessor ketal 2.
The synthesis of the tributyltin precursor for direct labeling of [76Br]3 via electrophilic radiobromination failed to afford the desired product. Therefore, the two-step synthesis of [76Br]3 was adopted from the previously reported method [36]. The radiobromination of 5 proceeded very well to afford more than 80% incorporation using 1 : 1 hydrogen peroxide/acetic acid (premixed 4 hours); however, the isolated yield was low because of the instability of the furfural group towards acid and low recovery from the silica gel column. The acetalization was a challenging step [36]. The reaction was optimized to afford up to 80% conversion of [76Br]5 using 9 µL TEOF, 2 µL 70% HClO4 in 1 mL dichloromethane. The major problem with this acetalization reaction was the rapid formation of a more polar radioactive unknown, the formation of which was also observed in the previously reported method [36]. It was found necessary to separate the desired product from the reagents before it became converted to the unknown byproduct completely. After Alumina Sep-Pak filtration, the desired products were very stable as in a dichloromethane solution. The low overall yield of [76Br]3 was partially due to the formation of the two isomers, endo 3 and exo 7, in addition to workup conditions that were not fully optimized. The specific activity was determined by HPLC, and while impurities coeluting with [76Br]3 might have lowered the effective specific activity of this compound, tissue distribution studies indicated that the effective specific activity of [76Br]3 was not a limiting factor.
Metabolic stability is an issue facing the development of PR-based steroid ligands [41–43]. The C-20 ketone in progestins is at risk for reduction to a C-20 hydoxy group, giving a compound with very low affinity for PR that is inactive in vivo and would thus be unsuitable as a progestin radiotracer imaging agent. The in vitro stability test of [76Br]3 in whole rat blood showed that it was stable, presumably because the C-20 ketone was protected by the 16α,17α-dioxolane group in 3. However, in vivo metabolic stability studies of [76Br]3 in estrogen-primed immature female Sprague-Dawley rats demonstrated that it underwent rapid metabolism in the liver, giving two metabolites, free [76Br]bromide and a more polar unidentified metabolite 8 that were evident as early as 1 minute. The structure of the more polar unknown 8 was not determined, but is likely to be the C-20 hydroxy analog of 3, based on its greater hydrophilicity and reports of this conversion in compounds with similar structure [42]. Free [76Br]bromide appeared to be the final metabolite in vivo.
Despite the rapid metabolism of [76Br]3 in the liver, as early as 1 minute post injection, there appeared to be no appearance of metabolites of [76Br]3 in the blood in vivo (also, as noted above, no metabolism was observed in whole rat blood at 2 hours in vitro). Nevertheless, by 1-hour post-injection, when [76Br]3 had undergone extensive metabolism in liver, there were only low levels of unconverted [76Br]3 in the blood and high level of metabolites. At later times, metabolites also began to appear in muscle and uterus, although there was substantial retention of unconverted [76Br]3 remained in the uterus, where it is presumably protected from metabolism by its binding to PR. The target tissue protection of receptor binding radiopharmaceuticals has been noted before with ER ligands [43–45]. The rapid metabolism we have observed is not surprising, because liver is known to be the major site of steroid metabolism [46] and the C-21 hydroxy group in [76Br]3 might facilitate the reduction of the C-20 ketone.
Tissue distribution studies of [76Br]3 demonstrated high uptake in the target tissues, uterus and ovaries, that was shown to be PR specific by selective displacement by a blocking dose of the potent progestin R5020. Uptake in non-PR target tissues, however, was not low, particularly at early times. This high, initial non-specific uptake is likely due to the relatively high lipophilicity of 3 [40] compared with that of FFNP 1 (Table 5). At later times, the metabolites of [76Br]3, especially free [76Br]bromide, are likely contributors to the non-target tissue activity because within 1 hour post-injection, these species constitute the majority of the circulating activity. It is of note that [76Br]bromide is known to distribute rapidly [47] and non-specifically [48] and to be retained in blood for long periods of time [47].
Table 5.
Comparison of the tissue biodistribution of 18F and 76Br labeled progestins in estrogen-primed immature female rats
| Percent injected %ID/g ± SDa |
||||||
|---|---|---|---|---|---|---|
| 1 (1 h) | 2 (1 h) | 3 (1 h) | 1 (3 h) | 2 (3 h) | 3 (3 h) | |
| Blood | 0.38 ± 0.08 | 0.26 ± 0.02 | 2.11 ± 0.14 | 0.19 ± 0.07 | 0.10 ± 0.02 | 2.24 ± 0.38 |
| Liver | 2.40 ± 0.18 | 4.25 ± 0.39 | 2.01 ± 0.37 | 1.65 ± 0.25 | 1.36 ± 0.22 | 1.49 ± 0.26 |
| Kidney | 1.66 ± 0.32 | 1.42 ± 0.08 | 2.96 ± 0.96 | 0.73 ± 0.26 | 0.45 ± 0.10 | 1.72 ± 0.21 |
| Muscle | 0.59 ± 0.08 | 0.44 ± 0.04 | 0.89 ± 0.16 | 0.26 ± 0.08 | 0.16 ± 0.09 | 0.71 ± 0.11 |
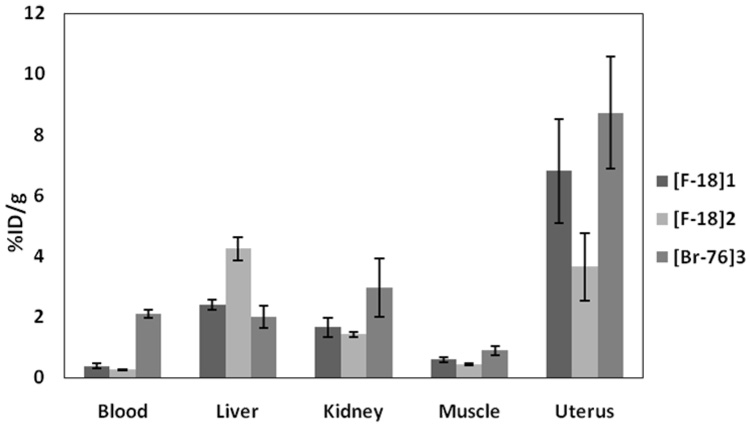
| Uterus | 6.80 ± 1.70 | 3.65 ± 1.11 | 8.72 ± 1.84 | 7.83 ±1.41 | 3.86 ± 0.99 | 5.52 ± 1.84 |
| Uterus/blood | 18.2 ± 4.9 | 14.0 ± 4.2 | 4.11 ± 0.73 | 48.0 ± 24.4 | 38.0 ± 8.3 | 2.42 ± 0.36 |
| Uterus/muscle | 11.6 ± 3.3 | 8.20 ± 2.5 | 9.82 ± 1.57 | 32.2 ± 10.9 | 27.9 ± 9.5 | 7.67 ± 1.30 |
| RBA (%)b | 190 | 240 | 65 | |||
| Log Po/wc | 3.87 | 4.92 | 5.09 ± 0.84d | |||
| SAe | > 1200* | 409 | > 400 | |||
Despite the high initial uptake of [76Br]3 by non-target tissues, the radioactivity in uterus was retained very well from 1 minute to 1 hour, thereafter washing out more slowly than was observed in non-target tissues. This selective retention of uterine activity following [76Br]3 injection is further evidence that a large proportion of its interaction in the uterus is due to the presence of PR. Furthermore, at 1 hour post-injection, the ratio of activity in the uterus compared to muscle following [76Br]3 injection was comparable to that of FFNP (Table 5), and a good ratio was maintained out to 3 hours, declining, not surprisingly by 18–24 hours. Uterus to blood ratios were lower, however, presumably because of the increasing fraction of free [76Br]bromide that was being retained in the blood. The selective uptake and retention of [76Br]3 in target tissues is impressive, considering that its PR binding affinity is relatively low, being 65 (compared to 100 for R5020 and 190 for FFNP; Table 5) and the declining levels of circulating [76Br]3 that results from its rapid metabolism in liver. The bromofuranyl group in [76Br]3 appears to be a metabolic liability that is responsible for the rapid production of free [76Br]bromide. Thus, metabolism may render imaging agents that contain this functionality ineffective and cause therapeutic agents to become harmful to non-target tissues from a dosimetry point of view.
It is instructive to compare [76Br]3 with FFNP 1 and the C-21 hydroxy ketal analog 2 (the predecessor of FFNP) in terms of their in vivo biodistribution (Table 5 and Figure 1). Among them, 3 has the lowest relative binding affinity but the highest lipophilicity (log Po/w). Interestingly, the uterus uptake of [76Br]3 was comparable to that of 1 and even better than that of 2 at 1 hour post-injection, but the blood and muscle uptake at 1 hour post-injection was much higher than those of 1 and 2 because of the lipophilicity of [76Br]3. The known routes of metabolism and excretion of steroids are liver and kidney. The liver uptake was the lowest for 3, but the kidney uptake was very high. The similarities between the uptake of [76Br]3 and FFNP 1 at 1 hour post-injection diminish with time (compare 1 h and 3 h), because of the extensive metabolism of [76Br]3. Thus, while the uterine activity of compounds 1 and 2 remain the same, and their uterus to blood and muscle ratios increase with time, these values decrease with [76Br]3. Thus, it is likely that compound 1 has greater metabolic stability than [76Br]3, perhaps the result of the C-21 fluorine further stabilizing the C-20 ketone [49].
Figure 1.
Comparison of the tissue biodistribution of [18F]1, [18F]2, and [76Br]3 at 1 h post-injection
In summary, [76Br]3 has high PR-mediated uptake in the target tissues, however, the high uptake in blood and muscle may cause problems for imaging and radiotherapy. The metabolic stability of 1 and 2 was not studied, so no direct comparison can be made between them and [76Br]3. However, the stability of [76Br]3 is comparable to that of 16α-[18F]fluoroestradiol (FES) [50], suggesting that [76Br]3 has some potential for PET imaging of PR in breast tumors.
5. Conclusion
16α,17α-[(R)-1’-α-(5-[76Br]bromofurylmethylidene)dioxyl]-21-hydroxy-19-norpregn-4-ene-3,20-dione ([76Br]3) was successfully synthesized, and its tissue biodistribution and metabolic stability were evaluated in estrogen-primed immature female Sprague-Dawley rats. [76Br]3 demonstrated high PR-mediated uptake in the target tissues that was blocked by excess unlabeled progestin, but the activity in blood and muscle was also relatively high because of the high lipophilicity of [76Br]3 and the subsequent formation of metabolites. [76Br]3 was metabolized quickly in the liver, but not in blood, to form a more polar radioactive metabolite and free [76Br]bromide. Despite the favorable initial biodistribution of [76Br]3, these subsequent metabolism events render this compound unsuitable as an imaging agent at later time points and for radiotherapy applications that would require specific and prolonged target tissue retention.
Scheme 1.
Progestin 16α,17α-dioxolanes
Scheme 2.
Scheme for the radiolabeling of [76Br]3
Acknowledgment
This work was supported by the DOE (DE FG02 86ER60401 to JAK and DE FG02-84ER-60218 to MJW) and the NIH (PHS 2R01 CA25836 to JAK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dehdashti F, Flanagan FL, Mortimer JE, Katzenellenbogen JA, Welch MJ, Siegel BA. Positron emission tomographic assessment of "metabolic flare" to predict response of metastatic breast cancer to antiestrogen therapy. Eur J Nucl Med. 1999;26:51–56. doi: 10.1007/s002590050359. [DOI] [PubMed] [Google Scholar]
- 2.Mortimer JE, Dehdashti F, Siegel BA, Trinkaus K, Katzenellenbogen JA, Welch MJ. Metabolic flare: indicator of hormone responsiveness in advanced breast cancer. J Clin Oncol. 2001;19:2797–2803. doi: 10.1200/JCO.2001.19.11.2797. [DOI] [PubMed] [Google Scholar]
- 3.Mortimer JE, Dehdashti F, Siegel BA, Katzenellenbogen JA, Fracasso P, Welch MJ. Positron emission tomography with 2-[18F]fluoro-2-deoxy-D-glucose and 16α-[18F]fluoro-17β-estradiol in breast cancer: Correlation with estrogen receptor status and response to systemic therapy. Clin Cancer Res. 1996;2:933–939. [PubMed] [Google Scholar]
- 4.Jonson SD, Bonasera TA, Dehdashti F, Cristel ME, Katzenellenbogen JA, Welch MJ. Comparative breast tumor imaging and comparative in vitro metabolism of 16α-[18F]fluoroestradiol-17β and 16β-[18F]fluoromoxestrol in isolated hepatocytes. Nucl Med Biol. 1999;26:123–130. doi: 10.1016/s0969-8051(98)00079-1. [DOI] [PubMed] [Google Scholar]
- 5.Larson SM, Morris M, Gunther I, Beattie B, Humm JL, Akhurst TA, et al. Tumor localization of 16β-18F-fluoro-5α-dihydrotestosterone versus 18F-FDG in patients with progressive, metastatic prostate cancer. J Nucl Med. 2004;45:366–373. [PubMed] [Google Scholar]
- 6.Dehdashti F, Picus J, Michalski JM, Dence CS, Siegel BA, Katzenellenbogen JA, et al. Positron tomographic assessment of androgen receptors in prostatic carcinoma. Eur J Nucl Med Mol Imaging. 2005;32:344–350. doi: 10.1007/s00259-005-1764-5. [DOI] [PubMed] [Google Scholar]
- 7.McElvany KD, Welch MJ, Katzenellenbogen JA. Radiobrominated estrogen receptor-binding radiopharmaceuticals for breast tumor imaging. Nucl Med Biol Adv, Proc World Congr 3rd. 1983;4:3610–3613. [Google Scholar]
- 8.Senderoff SG, McElvany KD, Carlson KE, Heiman DF, Katzenellenbogen JA, Welch MJ. Methodology for the synthesis and specific activity determination of 16α-[77Br]-bromoestradiol-17β and 16α-[77Br]-bromo-11β-methoxyestradiol-17β, two estrogen receptor-binding radiopharmaceuticals. Int J Appl Radiat Isot. 1982;33:545–551. doi: 10.1016/0020-708x(82)90010-2. [DOI] [PubMed] [Google Scholar]
- 9.Katzenellenbogen JA, McElvany KD, Senderoff SG, Carlson KE, Landvatter SW, Welch MJ. 16α-[77Br]bromo-11β-methoxyestradiol-17β: a gamma-emitting estrogen imaging agent with high uptake and retention by target organs. J Nucl Med. 1982;23:411–419. [PubMed] [Google Scholar]
- 10.Katzenellenbogen JA, Senderoff SG, McElvany KD, O'Brien HA, Jr, Welch MJ. 16α-[bromine-77]bromoestradiol-17β: a high specific-activity, gamma-emitting tracer with uptake in rat uterus and induced mammary tumors. J Nucl Med. 1981;22:42–47. [PubMed] [Google Scholar]
- 11.Landvatter SW, Katzenellenbogen JA, McElvany KD, Welch MJ. (2R*,3S*)-1-[125I]Iodo-2,3-bis(4-hydroxyphenyl)pentane ([125I]iodonorhexestrol) and (2R*,3S*)-1-[77Br]Bromo-2,3-bis(4-hydroxyphenyl)pentane ([77Br]bromonorhexestrol), two gamma-emitting estrogens that show receptor-mediated uptake by target tissues in vivo. J Med Chem. 1982;25:1307–1312. doi: 10.1021/jm00353a007. [DOI] [PubMed] [Google Scholar]
- 12.McElvany KD, Katzenellenbogen JA, Shafer KE, Siegel BA, Senderoff SG, Welch MJ. 16 alpha-[77Br]bromoestradiol: dosimetry and preliminary clinical studies. J Nucl Med. 1982;23:425–430. [PubMed] [Google Scholar]
- 13.Bronzert DA, Hochberg RB, Lippman ME. Specific cytotoxicity of 16 alpha-[125I]iodoestradiol for estrogen receptor-containing breast cancer cells. Endocrinology. 1982;110:2177–2182. doi: 10.1210/endo-110-6-2177. [DOI] [PubMed] [Google Scholar]
- 14.Bloomer WD, McLaughlin WH, Milius RA, Weichselbaum RR, Adelstein SJ. Estrogen receptor-mediated cytotoxicity using iodine-125. J Cell Biochem. 1983;21:39–45. doi: 10.1002/jcb.240210106. [DOI] [PubMed] [Google Scholar]
- 15.McLaughlin WH, Pillai KM, Edasery JP, Blumenthal RD, Bloomer WD. [125I]iodotamoxifen cytotoxicity in cultured human (MCF-7) breast cancer cells. J Steroid Biochem. 1989;33:515–519. doi: 10.1016/0022-4731(89)90035-6. [DOI] [PubMed] [Google Scholar]
- 16.McLaughlin WH, Milius RA, Pillai KMR, Edasery JP, Blumenthal RD, Bloomer WD. Cytotoxicity of receptor-mediated 16a-[125I]iodoestradiol in cultured MCF-7 human breast cancer cells. J Natl Cancer Inst. 1989;81:437–440. doi: 10.1093/jnci/81.6.437. [DOI] [PubMed] [Google Scholar]
- 17.Epperly MW, Damodaran KM, McLaughlin WH, Pillai KM, Bloomer WD. Radiotoxicity of 17-alpha-[125I]iodovinyl-11-beta-methoxyestradiol in MCF-7 human breast cancer cells. J Steroid Biochem Mol Biol. 1991;39:729–734. doi: 10.1016/0960-0760(91)90373-d. [DOI] [PubMed] [Google Scholar]
- 18.Beckmann MW, Scharl A, Rosinsky BJ, Holt JA. Breaks in DNA accompany estrogen-receptor-mediated cytotoxicity from 16-alpha[125I]iodo-17 beta-estradiol. J Cancer Res Clin Oncol. 1993;119:207–214. doi: 10.1007/BF01624432. [DOI] [PubMed] [Google Scholar]
- 19.DeSombre ER, Mease RC, Hughes A, Harper PV, DeJesus OT, Friedman AM. Bromine-80m-labeled estrogens: Auger electron-emitting, estrogen receptor-directed ligands with potential for therapy of estrogen receptor-positive cancers. Cancer Res. 1988;48:899–906. doi: 10.2172/6347502. [DOI] [PubMed] [Google Scholar]
- 20.DeSombre ER, Shafii B, Hanson RN, Kuivanen PC, Hughes A. Estrogen receptor-directed radiotoxicity with Auger electrons: specificity and mean lethal dose. Cancer Res. 1992;52:5752–5758. [PubMed] [Google Scholar]
- 21.Schwartz JL, Mustafi R, Hughes A, DeSombre ER. DNA and chromosome breaks induced by iodine-123-labeled estrogen in Chinese hamster ovary cells. Radiat Res. 1996;46:151–158. [PubMed] [Google Scholar]
- 22.DeSombre ER, Hughes A, Landel CC, Greene G, Hanson R, Schwartz JL. Cellular and subcellular studies of the radiation effects of Auger electron-emitting estrogens. Acta Oncologica. 1996;35:833–840. doi: 10.3109/02841869609104034. [DOI] [PubMed] [Google Scholar]
- 23.Kassis AI, Adelstein SJ, Haydock C, Sastry KSR, McElvany KD, Welch MJ. Lethality of Auger electrons from the decay of bromine-77 in the DNA of mammalian cells. Radiat Res. 1982;90:362–373. [PubMed] [Google Scholar]
- 24.Tang L. Radionuclide Production and Yields at Washington University School of Medicine. Q J Nucl Med Mol Imag. [in press] [PubMed] [Google Scholar]
- 25.Cui X, Schiff R, Arpino G, Osborne CK, Lee AV. Biology of progesterone receptor loss in breast cancer and its implications for endocrine therapy. J Clin Oncol. 2005;23:7721–7735. doi: 10.1200/JCO.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Osborne CK, Schiff R, Arpino G, Lee AS, Hilsenbeck VG. Endocrine responsiveness: understanding how progesterone receptor can be used to select endocrine therapy. Breast. 2005;14:458–465. doi: 10.1016/j.breast.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 27.Horowitz KB. The Structure and Function of Progesterone Receptors in Breast Cancer. J Steroid Biochem. 1987;27:447–457. doi: 10.1016/0022-4731(87)90339-6. [DOI] [PubMed] [Google Scholar]
- 28.Santen R, Manni A, Harvey H, Redmond C. Endocrine treatment of breast cancer in women. Endocrine Rev. 1990;11:221–265. doi: 10.1210/edrv-11-2-221. [DOI] [PubMed] [Google Scholar]
- 29.Gelbfish GA, Davison AL, Kopel S, Schreibmen B, Gelbfish JS, Degenshein GA, et al. Relationship of Estrogen and Progesterone Receptors to Prognosis in Breast Cancer. Ann Surg. 1988;207:75–79. doi: 10.1097/00000658-198801000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Furr BJA, Jordan VC. The Pharmacology and Clinical Uses of Tamoxifen. Pharmacol Ther. 1984;25:127–205. doi: 10.1016/0163-7258(84)90043-3. [DOI] [PubMed] [Google Scholar]
- 31.Noguchi S, Miyauchi K, Nishizawa Y, Koyama H. Induction of Progesterone Receptor with Tamoxifen in Human Breast Cancer with Special Reference to its Behavior over Time. Cancer. 1988;61:1345–1349. doi: 10.1002/1097-0142(19880401)61:7<1345::aid-cncr2820610712>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 32.Howell A, Harland RNL, Barnes DM, Baildam AD, Wilkinson MJS, Hayward E, et al. Endocrine Therapy for Advanced Carcinoma of the Breast: Relationship Between the Effect of Tamoxifen upon Concentrations of Progesterone Receptor and Subsequent Response to Treatment. Cancer Res. 1987;47:300–304. [PubMed] [Google Scholar]
- 33.Namer M, Lalanne C, Baulieu E. Increase of Progesterone Receptor by Tamoxifen as a Hormonal Challenge Test in Breast Cancer. Cancer Res. 1980;40:1750–1752. [PubMed] [Google Scholar]
- 34.Zhou D, Carlson KE, Katzenellenbogen JA, Welch MJ. Bromine-and iodine-substituted 16α,17α-dioxolane progestins for breast tumor imaging and radiotherapy: synthesis and receptor binding affinity. J Med Chem. 2006;49:4737–4744. doi: 10.1021/jm060348q. [DOI] [PubMed] [Google Scholar]
- 35.Buckman BO, Bonasera TA, Kirschbaum KS, Welch MJ, Katzenellenbogen JA. Fluorine-18-labeled progestin 16α,17α-dioxolanes: development of high-affinity ligands for the progesterone receptor with high in vivo target site selectivity. J Med Chem. 1995;38:328–337. doi: 10.1021/jm00002a014. [DOI] [PubMed] [Google Scholar]
- 36.Kochanny MJ, VanBrocklin HF, Kym PR, Carlson KE, O'Neil JP, Bonasera TA, et al. Fluorine-18 labeled progestin ketals: synthesis and target tissue uptake selectivity of potential imaging agents for receptor-positive breast tumors. J Med Chem. 1993;36:1120–1127. doi: 10.1021/jm00061a002. [DOI] [PubMed] [Google Scholar]
- 37.Denat F, Gaspard-Iloughmane H, Dubac J. An easy one-pot synthesis of Group 14 C-metalated 2(or 3)-furan-and thiophenecarboxaldehydes. Synthesis. 1992;10:954–956. [Google Scholar]
- 38.Vijaykumar D, Wang M, Kirschbaum KS, Katzenellenbogen JA. An Efficient Route for the Preparation of a 21-Fluoro Progestin-16α,17α-Dioxolane, a High-Affinity Ligand for PET Imaging of the Progesterone Receptor. J Org Chem. 2002;67:4904–4910. doi: 10.1021/jo020190r. [DOI] [PubMed] [Google Scholar]
- 39.Van Den Bossche B, Van de Wiele C. Receptor imaging in oncology by means of nuclear medicine: current status. J Clin Oncol. 2004;22:3593–3607. doi: 10.1200/JCO.2004.10.216. [DOI] [PubMed] [Google Scholar]
- 40.Katzenellenbogen JA, Heiman DF, Carlson KE, Lloyd JE. In vivo and in vitro Steroid Receptor Assays in the Design of Estrogen Radiophamaceuticals. In: Eckelman WC, editor. Receptor-Binding Radiotracers. Vol. 1. Boca Raton, FL: CRC; 1982. pp. 93–126. [Google Scholar]
- 41.Dehdashti F, McGuire AH, Van Brocklin HF, Siegel BA, Andriole DP, Griffeth LK, et al. Assessment of 21-[18F]fluoro-16 alpha-ethyl-19-norprogesterone as a positronemitting radiopharmaceutical for the detection of progestin receptors in human breast carcinomas. J Nucl Med. 1991;32:1532–1537. [PubMed] [Google Scholar]
- 42.Verhagen A, Studeny M, Luurtsema G, Visser GM, De Goeij CC, Sluyser M, et al. Metabolism of a [18F]fluorine labeled progestin (21-[18F]fluoro-16 alpha-ethyl-19-norprogesterone) in humans: a clue for future investigations. Nucl Med Biol. 1994;7:941–952. doi: 10.1016/0969-8051(94)90083-3. [DOI] [PubMed] [Google Scholar]
- 43.Pomper MG, VanBrocklin H, Thieme AM, Thomas RD, Kiesewetter DO, Carlson KE, et al. 11β-Methoxy-, 11β-Ethyl- and 17α-Ethynyl-Substituted 16α-Fluoroestradiols: Receptor-Based Imaging Agents with Enhanced Uptake Efficiency and Selectivity. J Med Chem. 1990;33:3143–3155. doi: 10.1021/jm00174a009. [DOI] [PubMed] [Google Scholar]
- 44.VanBrocklin HF, Pomper MG, Carlson KE, Welcη MJ, Katzevellenbogen JA. Preparation and Evaluation of 17-Ethynyl-substituted 16α-[18F]Fluoroestradiols: Selective Receptor-based PET Imaging Agents. Nucl Med Biol. 1992;19:363–374. doi: 10.1016/0883-2897(92)90122-f. [DOI] [PubMed] [Google Scholar]
- 45.VanBrocklin HF, Rocque PA, Lee HV, Carlson KE, Katzenellenbogen JA. 16β-[18F]Fluoromoxestrol: A Potent, Metabolically Stable Positron Emission Tomography Imaging Agent for Estrogen Receptor Positive Human Breast Tumors. Life Sciences. 1993;53:811–819. doi: 10.1016/0024-3205(93)90503-u. [DOI] [PubMed] [Google Scholar]
- 46.Hanson RN. The Influence of Structure Modification on the Metabolic Transformations of Radiolabeled Estrogen Derivatives. In: Nunn A, editor. Radiopharmaceutical: Chemistry and Pharmacology. New York: Marcel Dekker; 1992. pp. 333–364. [Google Scholar]
- 47.Soremark R, Ullberg S. Distribution of bromide in mice. An autoradiographic study with Br-82. Int J Appl Radiat Isot. 1960;8:192–197. [Google Scholar]
- 48.Lee H, Finck BN, Jones LA, Welch MJ, Mach RH. Synthesis and evaluation of a bromine-76-labeled PPARγ antagonist 2-bromo-5-nitro-N-phenylbenzamide. Nucl Med Biol. 2006;33:847–854. doi: 10.1016/j.nucmedbio.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 49.Müller K, Faeh C, Diederich F. Fluorine in pharmaceuticals: looking beyond intuition. Science. 2007;317:1881–1886. doi: 10.1126/science.1131943. [DOI] [PubMed] [Google Scholar]
- 50.Mathias CJ, Welch MJ, Katzenellenbogen JA, Brodack JW, Kilbourn MR, Carlson KE, et al. Characterization of the uptake of 16α-([18F]fluoro)-17β-estradiol in DMBA-induced mammary tumors. Int J Rad Appl Instrum B. 1987;14:15–25. doi: 10.1016/0883-2897(87)90156-5. [DOI] [PubMed] [Google Scholar]