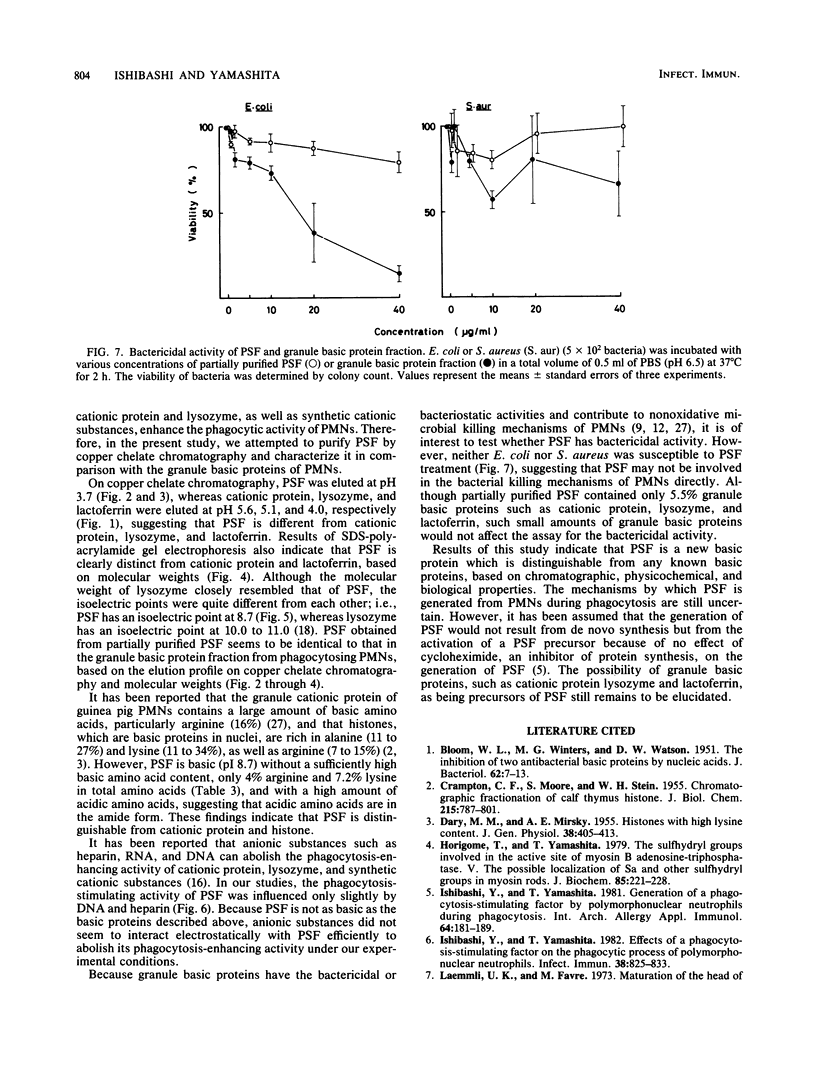
Abstract
Phagocytosis-stimulating factor (PSF) was purified by copper chelate chromatography and characterized in comparison with basic proteins in the granule of polymorphonuclear neutrophils. By copper chelate chromatography, PSF was eluted at pH 3.7; whereas cationic protein, lysozyme, and lactoferrin were eluted at pH 5.6, 5.1, and 4.0, respectively. Purified PSF has an approximate molecular weight of 16,000 and an isoelectric point at 8.7, which differ from those of basic proteins, such as cationic protein, lysozyme, and lactoferrin. Anionic substances such as DNA and heparin did not influence the phagocytosis-stimulating activity of PSF, whereas that of the granule basic protein fraction from resting polymorphonuclear neutrophils was abolished. PSF had little bactericidal activity against Escherichia coli and Staphylococcus aureus, whereas the granule basic protein fraction from resting PMNs had strong bactericidal activity against E. coli and weak activity against S. aureus. These results indicate that PSF is a basic protein which is distinguishable from cationic protein, lysozyme, and lactoferrin.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLOOM W. L., WINTERS M. G., WATSON D. W. The inhibition of two antibacterial basic proteins by nucleic acids. J Bacteriol. 1951 Jul;62(1):7–13. doi: 10.1128/jb.62.1.7-13.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRAMPTON C. F., MOORE S., STEIN W. H. Chromatographic fractionation of calf thymus histone. J Biol Chem. 1955 Aug;215(2):787–801. [PubMed] [Google Scholar]
- DALY M. M., MIRSKY A. E. Histones with high lysine content. J Gen Physiol. 1955 Jan 20;38(3):405–413. doi: 10.1085/jgp.38.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horigome T., Yamashita T. The sulfhydryl groups involved in the active site of myosin B adenosinetriphosphatase. V. The possible localization of Sa and other sulfhydryl groups in myosin rods. J Biochem. 1979 Jan;85(1):221–228. doi: 10.1093/oxfordjournals.jbchem.a132315. [DOI] [PubMed] [Google Scholar]
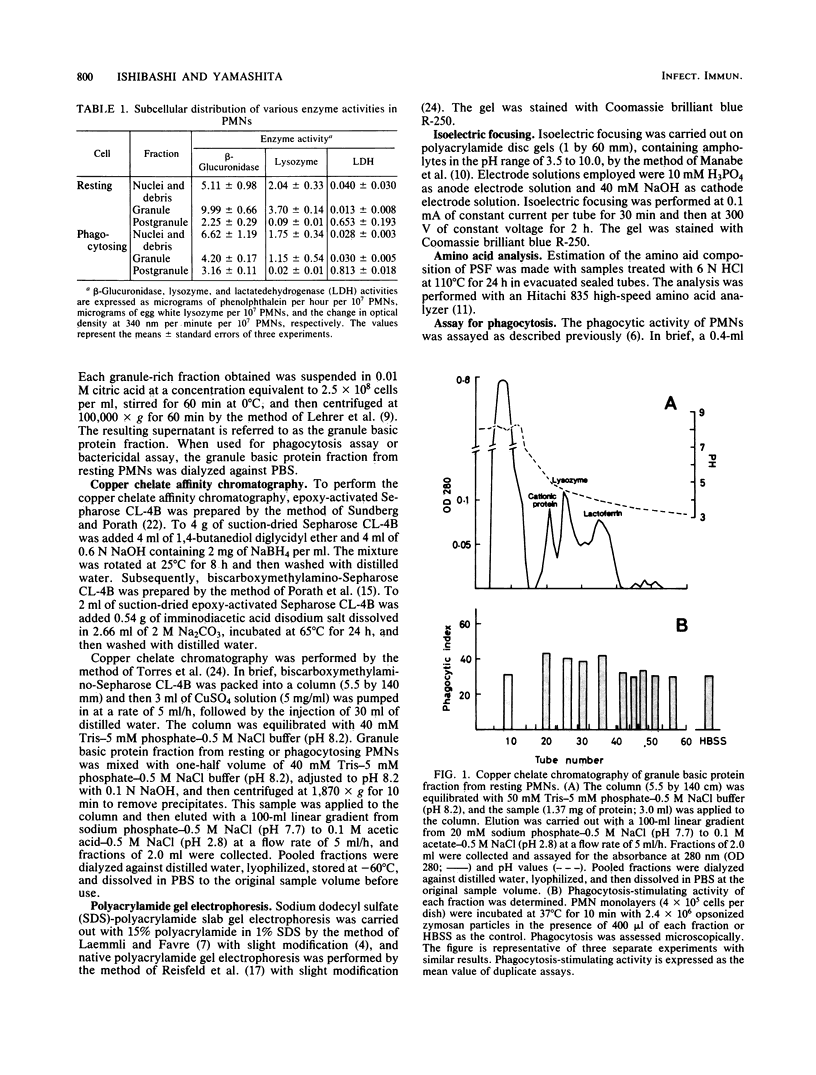
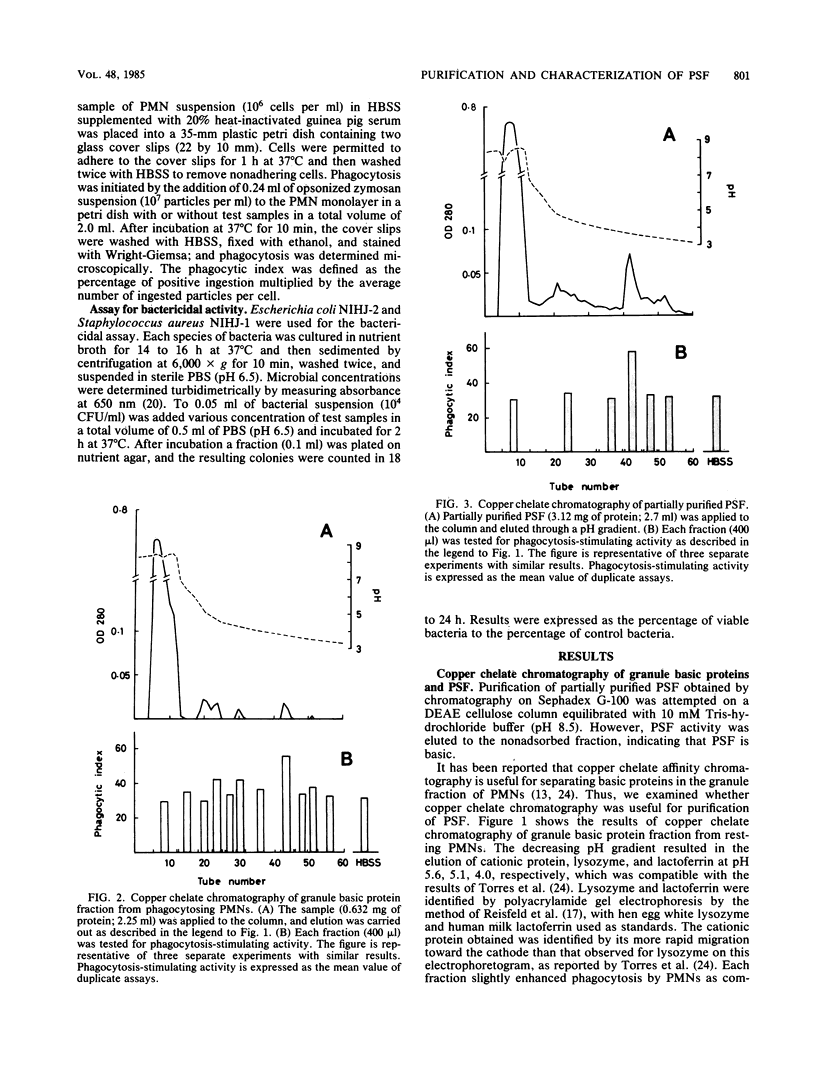
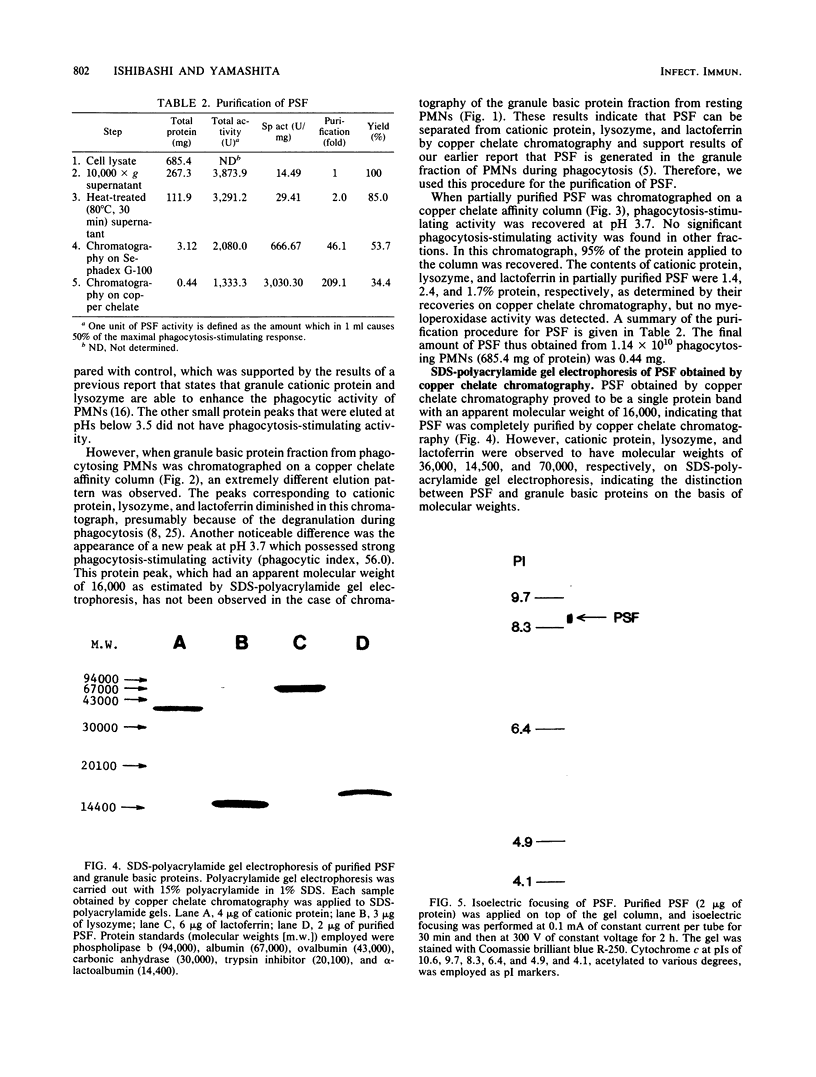
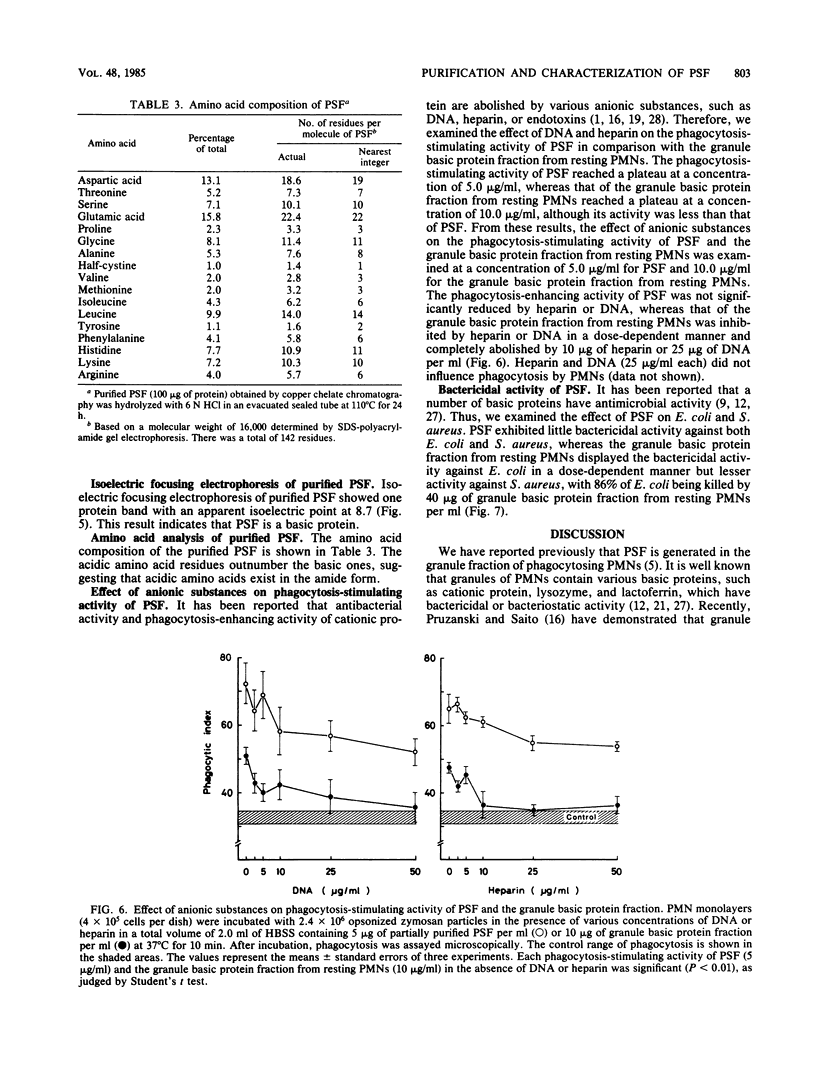
- Ishibashi Y., Yamashita T. Effects of a phagocytosis-stimulating factor on the phagocytic process of polymorphonuclear neutrophils. Infect Immun. 1982 Dec;38(3):825–833. doi: 10.1128/iai.38.3.825-833.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi Y., Yamashita T. Generation of a phagocytosis-stimulating factor by polymorphonuclear neutrophils during phagocytosis. Int Arch Allergy Appl Immunol. 1981;64(2):181–189. doi: 10.1159/000232690. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Leffell M. S., Spitznagel J. K. Fate of human lactoferrin and myeloperoxidase in phagocytizing human neutrophils: effects of immunoglobulin G subclasses and immune complexes coated on latex beads. Infect Immun. 1975 Oct;12(4):813–820. doi: 10.1128/iai.12.4.813-820.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Ladra K. M., Hake R. B. Nonoxidative fungicidal mechanisms of mammalian granulocytes: demonstration of components with candidacidal activity in human, rabbit, and guinea pig leukocytes. Infect Immun. 1975 Jun;11(6):1226–1234. doi: 10.1128/iai.11.6.1226-1234.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manabe T., Hayama E., Okuyama T. Microscale multisample two-dimensional electrophoresis of proteins in human serum, cerebrospinal fluid, and urine. Clin Chem. 1982 Apr;28(4 Pt 2):824–827. [PubMed] [Google Scholar]
- Oram J. D., Reiter B. Inhibition of bacteria by lactoferrin and other iron-chelating agents. Biochim Biophys Acta. 1968 Dec 23;170(2):351–365. doi: 10.1016/0304-4165(68)90015-9. [DOI] [PubMed] [Google Scholar]
- Oseas R. S., Allen J., Yang H. H., Baehner R. L., Boxer L. A. Rabbit cationic protein enhances leukocyte adhesiveness. Infect Immun. 1981 Aug;33(2):523–526. doi: 10.1128/iai.33.2.523-526.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson P. K., Gekker G., Shapiro R., Freiberg M., Keane W. F. Polyamino acid enhancement of bacterial phagocytosis by human polymorphonuclear leukocytes and peritoneal macrophages. Infect Immun. 1984 Feb;43(2):561–566. doi: 10.1128/iai.43.2.561-566.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porath J., Carlsson J., Olsson I., Belfrage G. Metal chelate affinity chromatography, a new approach to protein fractionation. Nature. 1975 Dec 18;258(5536):598–599. doi: 10.1038/258598a0. [DOI] [PubMed] [Google Scholar]
- Pruzanski W., Saito S. The influence of natural and synthetic cationic substances on phagocytic activity of human polymorphonuclear cells. An alternative pathway of phagocytic enhancement. Exp Cell Res. 1978 Nov;117(1):1–13. doi: 10.1016/0014-4827(78)90421-4. [DOI] [PubMed] [Google Scholar]
- REISFELD R. A., LEWIS U. J., WILLIAMS D. E. Disk electrophoresis of basic proteins and peptides on polyacrylamide gels. Nature. 1962 Jul 21;195:281–283. doi: 10.1038/195281a0. [DOI] [PubMed] [Google Scholar]
- Righetti P. G., Caravaggio T. Isoelectric points and molecular weights of proteins. J Chromatogr. 1976 Apr 21;127(11):1–28. doi: 10.1016/s0021-9673(00)98537-6. [DOI] [PubMed] [Google Scholar]
- SPITZNAGEL J. K. The effects of mammalian and other cationic polypeptides on the cytochemical character of bacterial cells. J Exp Med. 1961 Dec 1;114:1063–1078. doi: 10.1084/jem.114.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stendahl O., Tagesson C., Magnusson K. E., Edebo L. Physiochemical consequences of opsonization of Salmonella typhimurium with hyperimmune IgG and complement. Immunology. 1977 Jan;32(1):11–18. [PMC free article] [PubMed] [Google Scholar]
- Strominger J. L., Ghuysen J. M. Mechanisms of enzymatic bacteriaolysis. Cell walls of bacteri are solubilized by action of either specific carbohydrases or specific peptidases. Science. 1967 Apr 14;156(3772):213–221. doi: 10.1126/science.156.3772.213. [DOI] [PubMed] [Google Scholar]
- Sundberg L., Porath J. Preparation of adsorbents for biospecific affinity chromatography. Attachment of group-containing ligands to insoluble polymers by means of bifuctional oxiranes. J Chromatogr. 1974 Mar 13;90(1):87–98. doi: 10.1016/s0021-9673(01)94777-6. [DOI] [PubMed] [Google Scholar]
- Takamori K., Yamashita T. Biochemical properties of polymorphonuclear neutrophils from venous blood and peritoneal exudates of rabbits. Infect Immun. 1980 Aug;29(2):395–400. doi: 10.1128/iai.29.2.395-400.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres A. R., Peterson E. A., Evans W. H., Mage M. G., Wilson S. M. Fractionation of granule proteins of granulocytes by copper chelate chromatography. Biochim Biophys Acta. 1979 Feb 26;576(2):385–392. doi: 10.1016/0005-2795(79)90413-6. [DOI] [PubMed] [Google Scholar]
- Van Snick J. L., Masson P. L., Heremans J. F. The involvement of lactoferrin in the hyposideremia of acute inflammation. J Exp Med. 1974 Oct 1;140(4):1068–1084. doi: 10.1084/jem.140.4.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T., Imaizumi N., Yuasa S. Effect of endocellular cryoprotectant upon polymorphonuclear neutrophil function during storage at low temperature. Cryobiology. 1979 Apr;16(2):112–117. doi: 10.1016/0011-2240(79)90020-8. [DOI] [PubMed] [Google Scholar]
- Zeya H. I., Spitznagel J. K. Cationic proteins of polymorphonuclear leukocyte lysosomes. I. Resolution of antibacterial and enzymatic activities. J Bacteriol. 1966 Feb;91(2):750–754. doi: 10.1128/jb.91.2.750-754.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeya H. I., Spitznagel J. K. Cationic proteins of polymorphonuclear leukocyte lysosomes. II. Composition, properties, and mechanism of antibacterial action. J Bacteriol. 1966 Feb;91(2):755–762. doi: 10.1128/jb.91.2.755-762.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]




