Abstract
Background
The free-living amoeba Balamuthia mandrillaris may cause fatal encephalitis both in immunocompromised and in – apparently – immunocompetent humans and other mammalian species. Rapid, specific, sensitive, and reliable detection requiring little pathogen-specific expertise is an absolute prerequisite for a successful therapy and a welcome tool for both experimental and epidemiological research.
Results
A real-time polymerase chain reaction assay using TaqMan® probes (real-time PCR) was established specifically targeting the RNase P gene of B. mandrillaris amoebae. The assay detected at least 2 (down to 0.5) genomes of B. mandrillaris grown in axenic culture. It did not react with DNA from closely related Acanthamoeba (3 species), nor with DNA from Toxoplasma gondii, Leishmania major, Pneumocystis murina, Mycobacterium bovis (BCG), human brain, various mouse organs, or from human and murine cell lines. The assay efficiently detected B. mandrillaris DNA in spiked cell cultures, spiked murine organ homogenates, B. mandrillaris-infected mice, and CNS tissue-DNA preparations from 2 patients with proven cerebral balamuthiasis. This novel primer set was successfully combined with a published set that targets the B. mandrillaris 18S rRNA gene in a duplex real-time PCR assay to ensure maximum specificity and as a precaution against false negative results.
Conclusion
A real-time PCR assay for B. mandrillaris amoebae is presented, that is highly specific, sensitive, and reliable and thus suited both for diagnosis and for research.
Background
Balamuthiasis is a disease of humans and a variety of mammalian species caused by the free-living amoeba Balamuthia mandrillaris [1,2]. Its most important clinical manifestation is Balamuthia amoebic encephalitis (BAE), also described as granulomatous amebic encephalitis (GAE), in both immunocompromized hosts and in individuals apparently without immunological deficits [3,4]. B. mandrillaris is exquisitely encephalotropic and cytopathic [5], causing extensive brain tissue damage [6]. With worldwide around 150 identified cases to date [7], BAE is rare, however, with only 3 documented survivors [8,9], exceedingly lethal. Due to lack of practical experience, amoebic encephalitis is often mistaken for a brain tumour, viral or bacterial encephalitis, tuberculoma, or neurocysticercosis [3]. There exist no characteristic clinical symptoms, nor laboratory, or radiological findings diagnostic of BAE [10]. The high percentage of fatal BAE cases is also due to the high frequency of misdiagnosis and subsequent false, possibly even exacerbating, treatment strategies. At the moment, a proper diagnosis is possible only at autopsy, which is seldom done in most countries. Conversely, BAE is most likely severely under-diagnosed and thus reviewed by some authors as a genuine emerging [11] or at least potentially threatening [12] parasitic infection.
B. mandrillaris amoebae have recently been detected in soils both in the close vicinity of and distant from a BAE case [13,14]. Inhalation of contaminated dust or wounding contact with contaminated soils are the likely sources of infection. While infections of the skin and lungs may prevail for months, the infection of the central nervous system (CNS) can result in death in a matter of days [15]. As patients generally present first with neurological symptoms indicating that the CNS is already affected, rapid and dependable diagnosis is an absolutely prerequisite for any successful therapy.
Until recently, diagnosis of BAE relied on microscopical analysis of brain tissue (biopsy and necropsy), with indirect immunofluorescence (IIF) microscopy as the favoured detection method [2,16,17]. Apart from specific antiserum, this requires appreciable expertise, and a tissue sample of sufficient quality. PCR-based methods have the advantage of requiring much less pathogen-specific experience and can therefore be easily added to the diagnostic panel of any laboratory equipped with the basic tools and know-how. In addition, they are potentially more sensitive and specific, and can analyse larger sample volumes. Finally, they do not necessarily rely on intact pathogens and are more likely to detect an infection, i.e. pathogen-specific nucleic acid, in the blood or CSF, enabling less invasive diagnostic procedures.
PCR-based methods are being increasingly used for detecting known pathogenic amoebae [18-20]. To date, B. mandrillaris PCRs generally target ribosomal RNA (rRNA) gene sequences [17,21-24]. Even when quantification is not necessary, real-time PCR should be given preference over conventional PCR whenever possible, as the omission of post-amplification handling leads to quicker results and reduces the risk of amplicon contamination [24-26]. To identify false-positive results due to cross-reactivity and to ensure that novel variants or mutants in the target gene do not lead to false-negative results, the identification of microorganisms by PCR should involve the detection of at least 2 independent areas of the pathogen's genome in parallel. This becomes increasingly important with the microorganisms pathogenicity, when misdiagnosis bears grave consequences [27,28]. Accordingly, a real-time PCR assay was established specifically targeting the RNase P gene [29] of B. mandrillaris and tested in normal and spiked cell cultures, organ samples and patient material. Furthermore, this new assay was compared to a recently published real-time PCR targeting an 18S rRNA gene sequence of B. mandrillaris [24], and a duplex assay combining both was performed for optimal specificity and reliability.
Results and discussion
The aim of this communication is to introduce a real-time PCR-based assay for detecting infections with B. mandrillaris amoebae with maximum specificity, sensitivity, and reliability. For this, a partial RNase P gene sequence, published in GenBank, was found containing a region(nucleotide 251 to nucleotide 328) specific for B. mandrillaris as demonstrated by basic local alignment search tool (BLAST) analysis. PCR primers and a TaqMan probe were designed as described in Materials and Methods. The enzyme RNase P is responsible for generating the mature 5'-end of tRNA by a single endonucleolytic cleavage of their precursors. It is an essential, ubiquitous enzyme present in all cells and cellular compartments that synthesize tRNA: bacterial cells, Archaea, eukaryotic nuclei, mitochondria, and chloroplasts. All known RNase P enzymes are ribonucleoproteins containing an RNA subunit essential for catalysis [29]. Eukaryotic RNase P RNA contains a conserved core structure as well as regions of highly variable length and structure [30].
The ability of the designed RNase P primer set to detect B. mandrillaris DNA was evaluated with trophozoites (ca. 10% cysts) from axenic culture. In more than 30 experiments, less than 2 B. mandrillaris amoebae equivalents were dependably detected, in individual experiments down to 0.5. The RNase P primer set did not react with ≥ 500 pg DNA from 3 different Acanthamoeba species, other protozoa or mycobacteria, nor did it react with mammalian tissues or cell lines. It did, however, detect B. mandrillaris DNA in murine tissues spiked with B. mandrillaris amoebae (summarized in Table 1). The genus Acanthamoeba is closely related to Balamuthia [31] and 25-fold polyploid [32]. Assuming that a single B. mandrillaris cell contains 25 copies of the RNase P gene, a sensitivity limit of 2 amoebae or 50 gene copies seems reasonable.
Table 1.
Balamuthia mandrillaris RNase P genomic DNA detected by real-time PCR – specificity and detection limits
|
DNA [pg] a) |
Amoebae equivalents b) |
CT c) | |
| B. mandrillaris (axenic culture) | 5 – 50 | < 2 | 35 – 38 |
| Acanthamoeba hatchetti 2HH (axenic culture) | ≥ 500 | neg | ≥ 39 |
| A. lenticulata 72/2 (axenic culture) | ≥ 500 | neg | ≥ 39 |
| A. castellanii 1BU (axenic culture) | ≥ 500 | neg | ≥ 39 |
| Toxoplasma gondii (partially purified) | ≥ 500 | neg | ≥ 39 |
| Leishmania major (axenic culture) | ≥ 500 | neg | ≥ 39 |
| Pneumocystis murina (partially purified) | ≥ 500 | neg | ≥ 39 |
| Mycobacterium bovis (BCG) | ≥ 500 | neg | ≥ 39 |
| Murine macrophage RAW 264.7 cells (TIB 71) | ≥ 500 | neg | ≥ 39 |
| Human neuroblastoma Kelly cells (ACC 355) | ≥ 500 | neg | ≥ 39 |
| Brain tissue (mouse, ca. 15 mm3) | ≥ 500 | neg | ≥ 39 |
| Brain tissue spiked with 1–10 B.m. | n.d. | 1 – 10 | 33 – 38 |
| Lung, liver, spinal chord (mouse, ca. 15 mm3) | > 500 | neg | ≥ 39 |
| Lung tissue spiked with 10 B.m. | n.d. | 10 | 35–36 |
a) Minimal amounts of total DNA giving positive results (CT < 39). b) Minimal numbers of amoebae (calculated as amoeba DNA equivalents per PCR tube) giving positive results; neg = CT ≥ 39 for at least 500 ng total DNA. c) Range of CT values measured; neg = CT ≥ 39 or no DNA detected; n.d. = not done.
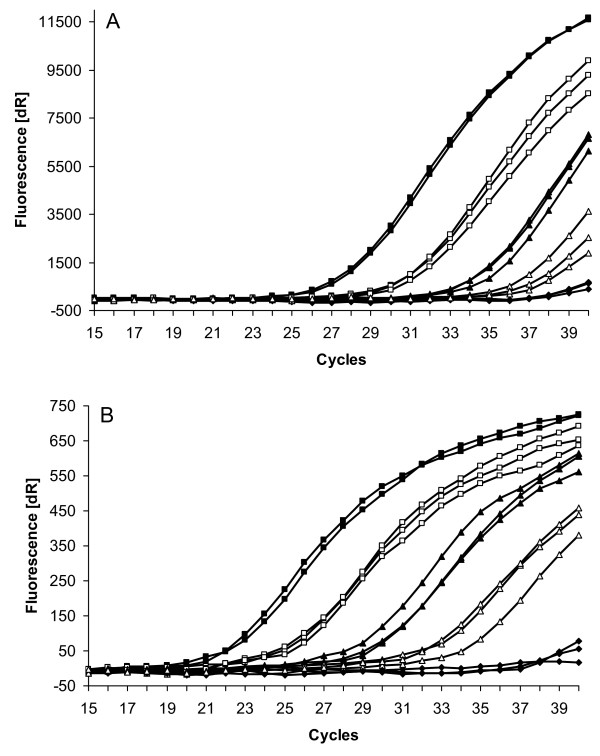
To establish assay sensitivity in the presence of host tissue, pieces of murine brain tissue were spiked with graded numbers of B. mandrillaris from axenic cultures (containing up to 10% cysts) and RNase P and 18S rRNA genes DNA detected in parallel by duplex real-time PCR. As show in Fig 1A, down to 0.5 amoeba equivalents could be detected when targeting both RNase P and 18S rRNA genomic DNA.
Figure 1.
Detection of B. mandrillaris RNase P (A)and 18S rRNA gene (B) DNA by duplex real-time PCR. Amplification curves of murine brain tissue (ca. 15 mm3) alone (closed diamonds) or spiked with 500 (closed squares), 50 (open squares), 5 (closed triangles), and 0,5 (open triangles) axenically cultured B. mandrillaris (calculated as amoeba DNA equivalents per PCR tube). Results from 3 separate experiments.
Our results targeting the RNase P gene revealed a similar sensitivity as those published by Qvarnstrom and colleagues for the 18S rRNA gene in a triplex real-time PCR [24]. In direct comparison (duplex real-time PCR), however, the 18S rRNA gene primer set (Fig. 1B) regularly was more sensitive by 2.5 to 4 cycles than the RNase P gene primer set (Fig. 1A). The 18S rRNA gene is 24-fold repetitive in A. castellanii [33]. Assuming B. mandrillaris to be 25-fold polyploid, one cell would contain 600 copies of the 18S rRNA gene. A 10-fold difference in gene copies normally results in a difference in sensitivity by 2–3 cycles in the real-time PCR. Thus the assumed 24-fold surplus of 18S rRNA genes per cell might well explain the somewhat lower sensitivity of the RNase P gene primer set.
These promising in vitro results encouraged testing stored material from experimentally infected animals. For example, a brain necropsy specimen that had been kept frozen at -70°C for over 3 years from an intranasally infected mouse that had shown neurological symptoms of BAE before death, and whose brains had been found to be infected with B. mandrillaris amoeba by IIF microscopy, also gave positive results by real-time PCR (Table 2). All specimens from noninfected mouse brains were negative.
Table 2.
B. mandrillaris RNase P genomic DNA in infected brain tissues detected by real-time PCR
|
DNA [pg] c) |
Amoebae equivalents d) |
CT e) | |
| Murine brain tissue from non-infected mice | > 500 | neg | ≥ 39 |
| Murine brain tissue from B.m.-infected mice a) | n.d. | n.d. | 30 |
| Human brain tissue from non-infected patients | n.d. | neg | ≥ 39 |
| Human brain tissue spiked with 50 B.m. | n.d. | 50 | 29 |
| Human brain tissue from BAE-patient 1 b) (1:100) | n.d. | n.d. | 30 |
| Human brain tissue from BAE-patient 2 b) (1:10) | n.d. | n.d. | 35 |
a) A highly symptomatic CD4+ T cell-deficient mouse 14 days post intranasal infection with B. mandrillaris amoebae. b) DNA extracted from 2 fresh BAE-patient brain biopsy samples ([23] Table 1: cases 4 and 5). c) Minimal amounts of total DNA giving positive results (CT < 39). d) Minimal numbers of amoebae (calculated as amoeba DNA equivalents per PCR tube) giving positive results; neg = CT ≥ 39 for at least 500 ng total DNA. e) Range of CT values measured; neg = CT ≥ 39 or no DNA detected; n.d. = not done.
Next, the real-time PCR assay was applied to fresh brain biopsy specimens from a tumor patient without or with added B. mandrillaris amoebae. Human brain tissue had no effect on the assay, as it neither produced false negative results nor inhibited the detection of B. mandrillaris DNA (Table 2). Finally, DNA from brain biopsies of 2 BAE patients [23] (Table 1: cases 4 and 5) were tested positive for B. mandrillaris RNase P genes (Table 2). Highly similar results were achieved with a second primer set batch, this time using Cy5/BHQ-2 for dye/quencher (data not shown). No differences in assay sensitivity were detected between B. mandrillaris DNA from human or baboon BAE cases, supporting the concept of a single genotype for B. mandrillaris [34].
Conclusion
This TaqMan real-time PCR assay using a primer set designed to target a B. mandrillaris-specific region of the RNase P gene, detects B. mandrillaris genomes in amoeba cultures, spiked tissues, and infected brain tissue with high specificity and sensitivity. In direct comparison with a published primer set that targets the 18S rRNA gene of B. mandrillaris, this assay is somewhat less sensitive, which can be explained by the different expected genome numbers per cell of the respective target genes. Both primer sets were successfully combined in a duplex real-time PCR assay to ensure maximum specificity and as a precaution against false negative results.
Methods
Mice
Female C57BL/6 wild-type (WT) and immunodeficient C57BL/6 rag1-/- (RAG) mice aged 8–12 weeks and bred at the Central Animal Facility (ZVZ), Federal Institute for Risk Assessment (BfR), Berlin, were kept in individually ventilated cages (Bio A.S., Ehret, Emmendingen, Germany) in the animal facility of the Robert Koch Institute. Housing materials, food and drink (both given ad libitum) were sterilized by autoclaving or irradiating before use, and all handling was performed in a class-II safety cabinet.
Cells, microorganisms, and clinical specimens
B. mandrillaris (CDC-V039; ATCC 50209) was grown in Chang's special medium as described [5]. Acanthamoeba hatchetti (strain 2HH; ATCC PRA-113), A. lenticulata (strain 72/2; ATCC 50705), and A. castellanii (strain 1BU; ATCC PRA-113) were kindly provided by Julia Walochnik, Clinical Institute of Hygiene, University of Vienna, Austria, and grown axenically in PYG medium as described [35]. Murine macrophage-like RAW 264.7 cells (ATCC TIB 71) and human neuroblastoma Kelly cells (DSMZ ACC 355) were grown in humidified, CO2-enriched (5%) normal atmosphere at 37°C in R10 consisting of RPMI 1640 medium (Gibco BRL-Life Technologies, Paisley, UK) supplemented with 10% (v/v) fetal calf serum (FCS; Boehringer Mannheim, Mannheim, Germany), 10 mM Na-pyruvate (Sigma-Aldrich, Steinheim, Germany), 100 IU/ml penicillin, and 100 μg/ml streptomycin (both Gibco). Leishmania major LV39 strain was grown at 25°C in R10. Pneumocystis murina organisms, originally kindly provided by E. Dei-Cas, INSERM Unit 42, Villeneuve d'Ascq, France, were contained in partially purified lung homogenates from experimentally infected RAG mice. Mycobacterium bovis BCG (Copenhagen) was grown at 37°C in Middlebrook medium containing 10% ADC enrichment (Becton Dickinson). PCR-tested Toxoplasma gondii (RH-strain) DNA and total DNA from the ileum of T. gondii-infected mice was kindly provided by O. Liesenfeld and U. Lohmann, Department of Microbiology and Hygiene, Charité University Medicine Berlin, Germany.
Balamuthia mitochondrial 16S rRNA gene-positive total DNA extracted from brain tissue of two reported BAE patients ([23] Table 1: cases 4 and 5) was kindly provided by S. Yagi, California Department of Health Services, Richmond, USA.
Extraction of DNA
DNA from normal human brain tissue, mouse tissues, cell lines, and amoebae was isolated by a modification of the UNSET procedure [36] as described by Walochnik and coworkers [19]. Briefly, tissue samples of approximately 2.5 × 2.5 × 2.5 mm or the indicated number of cells were solubilized at room temperature (RT) in 500 μl UNSET lysis buffer (8 M urea, 0.15 M NaCl, 2% SDS, 0.001 M EDTA, 0.1 M Tris/HCl; pH 7.5) by repeated (1–2 min) pipetting using a 1 ml disposable pipette tip until a quasi homogenous liquid was achieved. This was overlaid with 250 μl phenol and 250 μl chloroform (PC), and shaken gently (rocking table) for 3–5 h at RT. DNA was extracted by multiple PC-extraction followed by a single chloroform-extraction and precipitated in a 2.5-fold volume of 100% ethanol and a 0.1-fold volume of 3 M Na-acetate (pH 4.8 – 5.3) over night at -20°C, and then centrifuged for 30 min at 12,000 × g and 4°C. The supernatant was aspirated and the pellet washed with 2 ml of 70% ethanol, vortexed, and centrifuged for 5 min at 12,000 × g and 4°C. Alcohol was removed by aspiration and the open tubes placed in a laminar air-flow cabinet for ca. 10 min to evaporate the remaining alcohol before adding ca. 30 μl ddH2O (depending on the expected quantity of DNA). The DNA concentration was determined in a UV/Vis spectrophotometer (ND-1000, NanoDrop Technologies, Wilmington, USA). DNA from BCG was extracted as described by Somerville et al. [37].
Infection of mice with B. mandrillaris amoebae
Intranasal infection with B. mandrillaris (CDC-V039 was performed as described [38]. In short, 5 × 103 amoebae (~10% cysts) in 7.5 μl saline were injected into each nostril of CD4-depleted (experimental details will be published elsewhere) anesthetized mice using an Eppendorf pipette fitted with an ultra-micro tip (Eppendorf-Netheler-Hinz, Hamburg, Germany). Around 14 days later, when the mice had become severely ill, they were first anesthetized, then exsanguinated, and individual organs or ~15 mm3 blocks thereof shock-frozen in liquid nitrogen and stored at -70°C for over 3 years.
Real-time PCR assay
B. mandrillaris sequences published in GenBank include rRNA gene sequences and a partial RNase P gene sequence. A nucleotide-nucleotide BLAST analysis with the partial RNase P gene sequence (accession AF440362) revealed the region from nucleotide 251 to nucleotide 328 to be specific for B. mandrillaris. Particularly, this region was specific for B. mandrillaris also with respect to the Acanthamoeba sequences available in the nucleotide databases. The sequence of this specific DNA region was exported to the Primer Express software (Applied Biosystems, Foster City, CA, USA) for the design of the PCR primers and a TaqMan probe. The selected primers RNase P/FW (5'-GGC AGG TTC CGA GGA GAC A-3') and RNase P/RV (5'-GTG GCC TTG TGT ATT GAA CTT AAC ATT-3') amplify a 82 bp region and were used together with the FAM/TAMRA-labelled TaqMan probe RNase P/Probe (5'-Fam-TGG AAC CAT ACC TTG GGT GAC ACG ATG-Tamra-3').
For comparison and design of a duplex real-time PCR assay, the primers and probe recently published by Qvarnstrom et al. [24] were used, whereby the probe was labelled with HEX/BHQ: BalaF1451/FW (5'-TAA CCT GCT AAA TAG TCA TGC CAA T-3'), BalaR1621/RV (5'-CAA ACT TCC CTC GGC TAA TCA-3', and BalaP1582/Probe (5'-Hex-AG TAC TTC TAC CAA TCC AAC CGC CA-Bhq1-3').
The PCR reactions were performed using the ABsolute™ QPCR Mix from ABgene (Epsom, UK) according to the manufacturer's instructions in a total volume of 50 μl. The reaction mix contained 0.4 μM of each primer and 0.1 μM of the probe. PCR runs were performed in the Mx3005P real-time thermocycler from Stratagene (La Jolla, CA, USA) with one hold at 95°C for 15 min and 40 cycles at 95°C for 15 sec (denaturation) followed by 60°C for 1 min (annealing/elongation). The fluorescence data were collected at the end of the annealing/elongation step. Data were analyzed and plotted with the MxPro software from Stratagene.
Abbreviations
BAE: Balamuthia amoebic encephalitis; BCG: Bacille Calmette-Guérin; BLAST: basic local alignment research tool; CD: cluster of differentiation; CNS: central nervous system; CT: threshold cycle; IIF: indirect immunofluorescence; PCR: polymerase chain reaction.
Authors' contributions
AFK designed the project and wrote the manuscript. ER performed real-time PCR and analyzed the experimental data. AL designed the primer sets and analyzed the data.
Acknowledgments
Acknowledgements
We thank Prof. Frederick L. Schuster for his most generous mental support, Dr. Shigeo Yagi for biopsy DNA from balamuthiasis patients, Dr. Uwe Lohmann for DNA from T. gondii and T. gondii-infected mice, Dr. Julia Walochnik for Acanthamoeba species, Dr. Heinz Ellerbrok for helpful discussion, as well as Ulrike Laube and Petra Matzk for expert technical assistance. All experiments complied with current German law.
Contributor Information
Albrecht F Kiderlen, Email: KiderlenA@rki.de.
Elke Radam, Email: RadamE@rki.de.
Astrid Lewin, Email: LewinA@rki.de.
References
- Visvesvara GS, Martínez AJ, Schuster FL, Leitch GJ, Wallace SV, Sawyer TK, Anderson M. Leptomyxid ameba, a new agent of amebic meningoencephalitis in humans and animals. J Clin Microbiol. 1990;28:2750–2756. doi: 10.1128/jcm.28.12.2750-2756.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visvesvara GS, Schuster FL, Martínez AJ. Balamuthia mandrillaris, n.g., n.sp., agent of amebic meningoencephalitis in human and other animals. J Euk Microbiol. 1993;40:504–514. doi: 10.1111/j.1550-7408.1993.tb04943.x. [DOI] [PubMed] [Google Scholar]
- Schuster FL, Visvesvara GS. Free-living amoebae as opportunistic and non-opportunistic pathogens of humans and animals. Int J Parasitol. 2004;34:1001–1027. doi: 10.1016/j.ijpara.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Visvesvara GS, Moura H, Schuster FL. Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol Med Microbiol. 2007;50:1–26. doi: 10.1111/j.1574-695X.2007.00232.x. [DOI] [PubMed] [Google Scholar]
- Kiderlen AF, Tata PS, Özel M, Laube U, Radam E, Schäfer H. Cytopathogenicity of Balamuthia mandrillaris, an opportunistic causative agent of granulomatous amebic encephalitis. J Eukaryot Microbiol. 2006;53:456–463. doi: 10.1111/j.1550-7408.2006.00130.x. [DOI] [PubMed] [Google Scholar]
- Bakardjiev A, Azimi PH, Ashouri N, Ascher DP, Janner D, Schuster FL, Visvesvara GS, Glaser C. Amebic encephalitis caused by Balamuthia mandrillaris: report of four cases. Pediatr Infect Dis J. 2003;22:447–453. doi: 10.1097/01.inf.0000066540.18671.f8. [DOI] [PubMed] [Google Scholar]
- Schuster FL, Yagi S, Wilkins PP, Gavali S, Visvesvara GS, Glaser C. Balamuthia mandrillaris, agent of amebic encephalitis: detection of serum antibodies and antigenic similarity of isolates by enzyme immunoassay. J Eukaryot Microbiol. 2008;55:313–320. doi: 10.1111/j.1550-7408.2008.00333.x. [DOI] [PubMed] [Google Scholar]
- Deetz TR, Sawyer MH, Billman G, Schuster FL, Visvesvara GS. Successful treatment of Balamuthia amoebic encephalitis: presentation of 2 cases. Clin Infect Dis. 2003;37:1304–1312. doi: 10.1086/379020. [DOI] [PubMed] [Google Scholar]
- Jung S, Schelper RL, Visvesvara GS, Chang HT. Balamuthia mandrillaris meningoencephalitis in an immunocompetent patient. An unusual clinical course and a favorable outcome. Arch Pathol Lab Med. 2004;128:466–468. doi: 10.5858/2004-128-466-BMMIAI. [DOI] [PubMed] [Google Scholar]
- Martínez AJ, Guerra AE, García-Tamayo J, Céspedes G, Gonzáles-Alfonzo JE, Visvesvara GS. Granulomatous amebic encephalitis: a review and report of a spontaneous case from Venezuela. Acta Neuropathol. 1994;87:430–434. doi: 10.1007/BF00313614. [DOI] [PubMed] [Google Scholar]
- James SL. Emerging parasitic infections. FEMS Immunol Med Microbiol. 1997;18:313–317. doi: 10.1111/j.1574-695X.1997.tb01061.x. [DOI] [PubMed] [Google Scholar]
- Maciver SK. The threat from Balamuthia mandrillaris. J Med Microbiol. 2007;56:1–3. doi: 10.1099/jmm.0.47011-0. [DOI] [PubMed] [Google Scholar]
- Schuster FL, Dunnebacke TH, Booton GC, Yagi S, Kohlmeier CK, Glaser C, Vugia D, Bakardjiev A, Azimi P, Maddux-Gonzalez M, Martínez AJ, Visvesvara GS. Environmental isolation of Balamuthia mandrillaris associated with a case of amebic encephalitis. J Clin Microbiol. 2003;41:3175–3180. doi: 10.1128/JCM.41.7.3175-3180.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunnebacke TH, Schuster FL, Yagi S, Booton GC. Balamuthia mandrillaris from soil samples. Microbiology. 2004;150:2837–2842. doi: 10.1099/mic.0.27218-0. [DOI] [PubMed] [Google Scholar]
- Jayasekera S, Sissons J, Tucker J, Rogers C, Nolder D, Warhurst D, Alsam S, White JML, Higgins EM, Khan NA. Post-mortem culture of Balamuthia mandrillaris from the brain and cerebrospinal fluid of a case of granulomatous amoebic meningoencephalitis, using human brain microvascular endothelial cells. J Med Microbiol. 2004;53:1007–1012. doi: 10.1099/jmm.0.45721-0. [DOI] [PubMed] [Google Scholar]
- Kiderlen AF, Laube U, Radam E, Tata PS. Oral infection of immunocompetent and immunodeficient mice with Balamuthia mandrillaris amebae. Parasitol Res. 2007;100:775–782. doi: 10.1007/s00436-006-0334-5. [DOI] [PubMed] [Google Scholar]
- Tavares M, Correia da Costa JM, Carpenter SS, Santos LA, Afonso C, Aguiar A, Pereira J, Cardoso AI, Schuster FL, Yagi S, Sriram R, Visvesvara GS. Diagnosis of first case of Balamuthia amoebic encephalitis in Portugal by immunofluorescence and PCR. J Clin Microbiol. 2006;44:2660–2663. doi: 10.1128/JCM.00479-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilvington S, Beeching J. Development of a PCR for identification of Naegleria fowleri from the environment. Appl Environ Microbiol. 1995;61:3764–3767. doi: 10.1128/aem.61.10.3764-3767.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walochnik J, Haller-Schober EM, Kölli H, Picher O, Obwaller A, Aspök H. Discrimination between clinically relevant and nonrelevant Acanthamoeba strains isolated from contact lens-wearing keratitis patients in Austria. J Clin Microbiol. 2000;38:3932–3936. doi: 10.1128/jcm.38.11.3932-3936.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean RC, Hafez N, Tripathi S, Childress CG, Ghatak NR, Marciano-Cabral F. Identification of Acanthamoeba sp. in paraffin-embedded CNS tissue from an HIV+ individual by PCR. Diagn Microbiol Infect Dis. 2007;57:289–294. doi: 10.1016/j.diagmicrobio.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Booton GC, Carmichael JR, Visvesvara GS, Byers TJ, Fuerst PA. Genotyping of Balamuthia mandrillaris based on nuclear 18S and mitochondrial 16S rRNA genes. Am J Trop Med Hyg. 2003;68:65–69. [PubMed] [Google Scholar]
- Booton GC, Carmichael JR, Visvesvara GS, Byers TJ, Fuerst PA. Identification of Balamuthia mandrillaris by PCR assay using the mitochondrial 16S rRNA gene as a target. J Clin Microbiol. 2003;41:453–455. doi: 10.1128/JCM.41.1.453-455.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi S, Booton GC, Visvesvara GS, Schuster FL. Detection of Balamuthia 16S rRNA gene DNA in clinical specimens by PCR. J Clin Microbiol. 2005;43:3192–3197. doi: 10.1128/JCM.43.7.3192-3197.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qvarnstrom Y, Visvesvara GS, Sriram R, da Silva AJ. Multiplex real-time PCR assay for simultaneous detection of Acanthamoeba spp., Balamuthia mandrillaris, and Naegleria fowleri. J Clin Microbiol. 2006;44:3589–3595. doi: 10.1128/JCM.00875-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell AS, Ranford-Cartwright LC. Real-time quantitative PCR in parasitology. Trends Parasitol. 2002;18:337–342. [PubMed] [Google Scholar]
- Espy MJ, Uhl JR, Sloan LM, Buckwalter SP, Jones MF, Vetter EA, Yao JDC, Wengenack NL, Rosenblatt JE, Cockerill FR, III, Smith TF. Real-time PCR in clinical microbiology: applications for routine laboratory testing. Clin Microbiol Rev. 2006;19:165–256. doi: 10.1128/CMR.19.1.165-256.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellerbrok H, Nattermann H, Özel M, Beutin L, Appel B, Pauli G. Rapid and sensitive identification of pathogenic and apathogenic Bacillus anthracis by real-time PCR. FEMS Microbiol Lett. 2002;214:51–59. doi: 10.1111/j.1574-6968.2002.tb11324.x. [DOI] [PubMed] [Google Scholar]
- Leendertz FH, Yumlu S, Pauli G, Boesch C, Couacy-Hymann E, Vigilant L, Junglen S, Schenk S, Ellerbrok H. A new Bacillus anthracis found in wild chimpanzees and a gorilla from West and Central Africa. PLoS Pathog. 2006;2:e8. doi: 10.1371/journal.ppat.0020008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalan V, Vioques A, Altman S. RNase P: variations and uses. J Biol Chem. 2002;277:6759–6762. doi: 10.1074/jbc.R100067200. [DOI] [PubMed] [Google Scholar]
- Marquez SM, Harris JK, Kelley ST, Brown JW, Dawson SC, Roberts EC, Pace NR. Structural implications of novel diversity in eucaryal RNase P RNA. RNA. 2005;11:739–751. doi: 10.1261/rna.7211705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral Zettler LA, Nerad TA, O'Kelly CJ, Peglar MT, Gillevet PM, Silberman JD, Sogin ML. A molecular reassessment of the leptomyxid amoebae. Protist. 2000;151:275–282. doi: 10.1078/1434-4610-00025. [DOI] [PubMed] [Google Scholar]
- Byers TJ. Molecular biology of DNA in Acanthamoeba, Amoeba, Entamoeba, and Naegleria. Int Rev Cytol. 1986;99:311–341. doi: 10.1016/s0074-7696(08)61430-8. [DOI] [PubMed] [Google Scholar]
- Yang Q, Zwick MG, Paule MR. Sequence organization of the Acanthamoeba rRNA intergenic spacer: identification of transcriptional enhancers. Nucleic Acid Res. 1994;22:4798–4805. doi: 10.1093/nar/22.22.4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booton GC, Carmichael JR, Visvesvara GS, Byers TJ, Fuerst PA. Genotyping of Balamuthia mandrillaris based on nuclear 18S and mitochondrial 16S rRNA genes. Am J Trop Med Hyg. 2003;68:65–69. [PubMed] [Google Scholar]
- Walochnik J, Duchêne M, Seifert K, Obwaller A, Hottkowitz T, Wiedermann G, Eibl H, Aspöck H. Cytotoxic activity of alkylphosphocholines against clinical isolates of Acanthamoeba spp. Antimicrob Agents Chemother. 2002;46:695–701. doi: 10.1128/AAC.46.3.695-701.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugo ER, Stewart VJ, Gast RJ, Byers TJ. Purification of amoeba mtDNA using the UNSET procedure. In: Soldo AT, Lee JJ, editor. Protocols in Protozoology. Lawrence, Kans.: Allen Press; 1992. pp. D71–D72. [Google Scholar]
- Somerville W, Thibert L, Schwartzman K, Behr MA. Extraction of Mycobacterium tuberculosis DNA: a Question of Containment. J Clin Microbiol. 2005;43:2996–2997. doi: 10.1128/JCM.43.6.2996-2997.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiderlen AF, Laube U. Balamuthia mandrillaris, an opportunistic agent of granulomatous amebic encephalitis, infects the brain via the olfactory nerve pathway. Parasitol Res. 2004;94:49–52. doi: 10.1007/s00436-004-1163-z. [DOI] [PubMed] [Google Scholar]