Abstract
It is recognized that both whey protein and essential amino acids (EAA) are stimuli for muscle protein anabolism. The aim of the present study was to determine if the effects of whey protein ingestion on muscle protein accrual in elderly are due solely to its constituent EAA content. Fifteen elderly humans were randomly assigned to ingest a bolus of either 15 g of whey protein (WY), 6.72 g of essential amino acids (EAA), or 7.57 g of non-essential amino acids (NEAA). We utilized the leg arterio-venous model to measure the leg phenylalanine balance (PB), which is an index of muscle protein accrual. PB (nmol·min−1·kg lean leg mass−1) during the 3.5 hours following the bolus ingestion improved in the WY (−216 ± 14 vs −105 ± 19; P < .05) but not in the EAA (−203 ± 21 vs −172 ± 38; P > .05) or NEAA groups (−203 ± 19 vs −204 ± 21; P > .05). The insulin response (ulU·ml−1 210 min−1) during the same period was lower in both the NEAA (48 ± 40) and EAA (213 ± 127) when compared to the WY (1073 ± 229; P < .05). In conclusion, whey protein ingestion improves skeletal muscle protein accrual through mechanisms that are beyond those attributed to its essential amino acid content. This finding may have practical implications for the formulation of nutritional supplements to enhance muscle anabolism in older individuals.
Keywords: protein metabolism, intact protein, free amino acids, protein balance, protein supplement, humans
1. Introduction
The use of nutritional approaches to increase protein accrual in muscle by regulating the rates of protein synthesis (i.e. increase) and breakdown (i.e. decrease) has received great attention in recent years. Such research has provided an insight into ways to improve muscle protein accrual, and it is now recognized that increasing plasma amino acid availability is a key factor to promote muscle protein anabolism [1]. Approaches to improve muscle protein anabolism are particularly important for individuals at risk for muscle loss, such as the elderly [2, 3]. In these individuals, nutritional supplementation provides a practical approach to increase the availability of plasma amino acids.
Published reports have focused on various forms of either dietary proteins [4, 5] or amino acid mixtures [6–8] in order to increase plasma amino acid availability and improve muscle protein anabolism in elderly. It has been shown that postprandial protein gains in elderly are greater following ingestion of whey protein (rapidly digested protein) than casein (slowly digested protein) [5], presumably due to the rapid increase in plasma amino acids with whey protein. Rapid increase in plasma amino acids is also observed following ingestion of free amino acids, and it is known that ingestion of a balanced amino acid mixture in the elderly stimulates muscle protein anabolism [9]. Moreover, it has been shown that this effect is a result of the essential amino acids (EAA) in the mixture [10], which comprise ~ 50% of the total nitrogen and calories found in whey protein. Such evidence, in addition to showing that acute stimulation of muscle protein synthesis is approximately two-fold greater following 6 g of essential amino acids compared to 6 g of a balanced mixture [11], has lead research to focus on the role of EAA, including that of specific EAA, such as leucine [6, 12, 13], in stimulating muscle protein anabolism in the elderly. However, there are practical considerations associated with supplement cost and palatability that would support the choice of intact protein, such as whey protein, over EAA, if the same anabolic benefits were to be achieved with either supplement.
Bolus ingestions of either whey protein [14] or EAA [7, 8] are known to acutely improve muscle protein balance in the elderly. We have recently shown that ingestion of 15 g of EAA more than doubles muscle protein balance in the elderly when compared to that of the ingestion of 15 g of whey protein [14], which would support a greater importance of the EAA (as opposed to whey protein) in improving muscle protein accretion in the elderly. This latter evidence would further suggest that ingestion of only the EAA part of whey protein (~ 7 g of EAA) may confer similar benefits with respect to protein accretion in muscle in elderly as that of ~15 g of whey protein.
The objective of this study was to quantify muscle protein accrual in elderly in response to ingestion of 15 g of a whey protein and compare it to that following ingestion of mixtures of the EAA, as well as the non-essential amino acids (NEAA), found in the 15 g of whey protein. Such information describes the specific role of amino acids in whey protein in the stimulation of muscle protein accrual, and also has practical implications with respect to the formulation of nutritional supplements to optimize muscle protein anabolism in the elderly. On the basis of our previous results we hypothesized that the whey protein ingestion will improve muscle protein accrual and that this improvement will be similar to that following ingestion of the EAA mixture, while NEAA will not significantly alter muscle protein accrual. Muscle protein accrual was evaluated in the postabsorptive state and following ingestion of the above mixtures using the leg arterio-venous model.
2. Methods and materials
2.1 Subjects
Fifteen elderly subjects (60–85 years old) that were living independently with no limit in ambulation were included in this study. None of the subjects was habitually physically active. Five subjects ingested whey protein (WY), another five ingested essential amino acids (EAA), while the other five ingested non-essential amino acids (NEAA). Subjects in the three groups were matched with respect to physical characteristics, which are presented in Table 1. Female subjects were not on estrogen replacement therapy. Body composition and leg lean mass were determined using dual-energy x-ray absorptiometry.
Table 1.
Characteristics of three groups of elderly subjects that ingested either 15 g of whey protein, 6.72 g of essential amino acids (EAA), or 7.57 g of non-essential amino acids (NEAA).
| Whey Protein
(3M, 2F) |
EAA
(4M, 1F) |
NEAA
(2M, 3F) |
|
|---|---|---|---|
| Age (years) | 65.4 ± 2.3 | 67.8 ± 3.1 | 64.0 ± 1.6 |
| Weight (kg) | 79.5 ± 2.7 | 79.3 ± 5.8 | 72.4 ± 6.5 |
| Height (cm) | 166.2 ± 3.2 | 174.2 ± 3.7 | 164.0 ± 4.6 |
| Body fat (%) | 33.0 ± 4.7 | 29.0 ± 2.3 | 32.7 ± 4.2 |
| Body lean mass (kg) | 50.8 ± 2.8 | 54.0 ± 4.1 | 47.4 ± 6.2 |
| Leg lean mass (kg) | 8.0 ± 0.5 | 8.3 ± 0.7 | 7.3 ± 1.0 |
Values are presented as means ± SEM. n=5 for each group. M, male; F, female. The EAA and NEAA mixtures were prepared to contain the EAA and NEAA, respectively, found in 15 g of the whey protein. There are no significant differences between groups by ANOVA (P > .05).
All subjects underwent screening and those determined to be healthy based on medical history, physical examination, resting electrocardiogram and routine clinical blood and urine tests were admitted to the study. The screening procedure also included a determination of the blood flow to the lower extremities using the ankle/brachial index (ABI), which provides a qualitative estimation of the vascular condition in the leg. Subjects were excluded if there was a presence of vascular disease (ABI < 0.95), unstable metabolic condition, hypertension and electrocardiogram-established heart abnormalities. All subjects included in the study were asked to abstain from any type of physical exercise for at least three days prior to the study. The study protocol was approved by the Institutional Review Board and the General Clinical Research Center (GCRC) at the University of Texas Medical Branch at Galveston, and written informed consent was obtained for each subject.
2.2 Experimental procedures
This experiment was designed to evaluate the muscle protein balance in the elderly following a bolus ingestion of 15 g of whey protein (control) and compare this response with the whey protein’s constituent EAA and NEAA contents (treatments). The whey protein isolate was commercially purchased and its amino acid composition was analyzed by Amino-Science Laboratories, Ajinomoto, Co., Inc. Free amino acids were purchased from Ajinomoto AminoScience LLC, Raleigh, NC, and mixed in the appropriated amounts for the constitution of the EAA and NEAA mixtures. The amino acid composition of whey protein isolate together with the amino acid composition of the EAA and NEAA mixtures are presented in Table 2.
Table 2.
Amino acid composition of the ingested whey protein and essential amino acids (EAA) and non non-essential amino acids (NEAA) mixtures.
| Amino acids (grams) | Whey protein | EAA | NEAA |
|---|---|---|---|
| Alanine | 0.74 | - | 0.76 |
| Arginine | 0.35 | - | 0.40 |
| Asparagine/Aspartate | 1.725 | - | 1.19 |
| Cysteine | 0.45 | - | 0.40 |
| Glutamine/Glutamate | 2.46 | - | 2.52 |
| Glycine | 0.27 | - | 0.28 |
| Histidine | 0.31 | 0.30 | - |
| Isoleucine | 0.80 | 0.78 | - |
| Leucine | 1.88 | 1.72 | - |
| Lysine | 1.50 | 1.36 | - |
| Methionine | 0.33 | 0.36 | - |
| Phenylalanine | 0.51 | 0.51 | - |
| Proline | 0.66 | - | 0.76 |
| Serine | 0.57 | - | 0.78 |
| Threonine | 0.72 | 0.95 | - |
| Tryptophan | 0.30 | - | - |
| Tyrosine | 0.55 | - | 0.48 |
| Valine | 0.73 | 0.74 | - |
| Total Amino acids | 14.86 | 6.72 | 7.57 |
The EAA and NEAA mixtures were prepared to contain the EAA and NEAA, respectively, found in the whey protein. The whey protein and amino acids were dissolved in 250 mL of caffeine- and calorie-free soft drink and were ingested as a bolus.
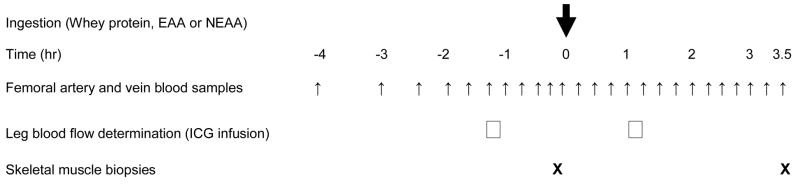
Each subject was studied on a single occasion in the postabsorptive state and following the ingestion of either whey protein, EAA or NEAA. Subjects arrived at the GCRC in the afternoon the day before the experiment of the study, and after eating dinner, did not consume anything (except water) after 10:00 PM. The next morning at ~ 4:30 AM, an 18-gauge polyethylene catheter was inserted into an antecubital vein of an arm for the collection of blood samples. Two hours later, 3-Fr, 8-cm polyethylene catheters (Cook, Bloomington, IN) were inserted in the femoral artery and vein of one of the legs under local anesthesia. These catheters were used for sampling arterial and venous blood across the leg. Fig. 1 shows the infusion protocol, together with the blood and muscle sampling time points. Blood and muscle samples were collected in the postabsorptive state (−4 to 0 hours) and following the ingestion of the whey protein or amino acids (EAA or NEAA) mixtures. The whey protein and amino acid mixtures were dissolved in 250 mL of a caffeine- and calorie-free soft drink and were ingested as a bolus at time 0.
Fig. 1.
Diagram of the blood and muscle sampling protocol. Data were collected before and after ingestion (at time 0) of 15 g of whey protein, 6.72 g of essential amino acids (EAA), or 7.57 g of non-essential amino acids (NEAA). The EAA and NEAA mixtures contain the EAA and NEAA, respectively, found in the 15 g of the whey protein. Leg arterio-venous blood samples were taken at several times before and every 15 minutes following the ingestion of either whey protein or amino acid mixtures for the determination of the leg blood phenylalanine balance. Blood flow was determined before and following ingestion of the three mixtures and by using indocyanine green (ICG) dye infusion in the femoral artery. A leg muscle biopsy was performed at the end of the sampling protocol to determine changes in the muscle free phenylalanine concentration compared to the period prior to the ingestion of the whey protein or amino acids mixtures.
Blood samples for the determination of blood phenylalanine concentration were drawn simultaneously from the femoral artery and femoral vein at given times as shown in Fig. 1. Insulin and glucose concentrations were determined from blood samples collected from the femoral artery. Muscle biopsies (~60 mg of muscle each) were taken from 2 incisions (approximately 7 mm each) in the lateral portion of vastus lateralis after anesthetizing the skin and the subcutaneous tissue with 1% lidocaine. The muscle was rinsed with ice-cold saline and after all visible blood, fat and connective tissue was removed, the muscle was blotted dry and immediately frozen in liquid nitrogen and stored at −80°C.
We determined blood flow to the study leg using the indocyanine green dye technique. Briefly, dye was infused into the femoral artery at a constant rate (0.5 mg min−1) for 20 minutes during two different time periods in the experiment (Fig. 1). The blood flow was determined using procedures that have previously been described [15, 16].
2.3 Analysis of samples
Approximately 1 mL of blood from the femoral artery and femoral vein was transferred in glass tubes containing 15% sulfosalicylic acid and a phenylalanine internal standard (~100 ul/mL blood of L-[U-13C9-15N]phenylalanine), and mixed well. The weight of the tubes was determined before and after the addition of blood and the difference was recorded as the amount of blood added in the tubes. After the tubes were centrifuged, the supernatant was collected and frozen, and processed at a later time as previously described [17]. The isotopic enrichment of blood phenylalanine, resulting from the addition of the internal standard, was determined on its t-butyldimethylsilyl derivative by gas chromatography-mass spectrometry using selected ion monitoring for phenylalanine mass to charge ratio (m/z) 336 and 346. Appropriate corrections for overlapping spectra and the natural distribution of stable isotopes were performed [18, 19]. The blood for the determination of plasma insulin concentration was collected in tubes containing EDTA and was assayed using a commercially available insulin ELISA kit (ALPCO Diagnostics, Windham, NH). Blood glucose was measured using an automatic analyzer (YSI, Yellow Springs, OH).
The muscle biopsy samples were analyzed for free intracellular phenylalanine concentration. Free intracellular phenylalanine concentration was determined as follows: 20–25 mg of the muscle biopsy sample was weighed, and an internal standard solution (2 ul/mg tissue of L-[U-13C9-15N]phenylalanine) was added; after 0.8 mLof 10% perchloroacetic acid was added to precipitate muscle proteins, the tissue was homogenized and centrifuged, and the supernatant was collected; this procedure was repeated one more time and the pooled supernatant was processed similarly to the blood supernatant.
2.4 Calculations
Blood phenylalanine concentration was determined using the internal standard approach based on the volumes of the blood and the L-[U-13C9-15N]phenylalanine added in the tubes and the resulting t/T of the L-[U-13C9-15N]phenylalanine. The concentration of free phenylalanine in the muscle was determined using the same methodology and then adjusted using the chloride method [20] to obtain the concentration of phenylalanine in the muscle intracellular water.
The phenylalanine balance (PB) across the leg was calculated at each arterio-venous blood sampling time point. PB was calculated as the product of the difference in the phenylalanine concentrations between arterial and femoral venous blood and the leg blood flow. Postabsorptive and postprandial values were averaged to obtain an overall response during the respective periods.
2.5 Statistical analyses
Statistical analyses were performed with the SPSS software, version 14.0 for Windows (SPSS Inc.). A paired-samples t test was used to compare postabsorptive and postprandial responses within a group. One-way analysis of variance (ANOVA) was used to test for significant differences between groups or across time within a group, followed by a Dunnett’s post-hoc multiple comparisons test to determine statistically significant differences between the WY (control) and EAA and NEAA groups or between postabsorptive and postprandial responses within a group. Data are expressed as means ± SEM, and a P value < .05 was considered statistically significant.
3. Results
3.1 Leg blood flow
There were no differences in the leg blood flow between the postabsorptive and postprandial states within each group (P > 0.05). For each subject, an average value for the blood flow measured in the postabsorptive and postprandial states was calculated and used for the determination of the leg muscle phenylalanine kinetics. The leg blood flow was in the whey group 308 ± 28 ml·min−1, the EAA group 282 ± 37 ml·min−1, and the NEAA group 310 ± 79 ml·min−1, with no differences between groups (P > 0.05).
3.2 Blood and muscle free phenylalanine concentrations
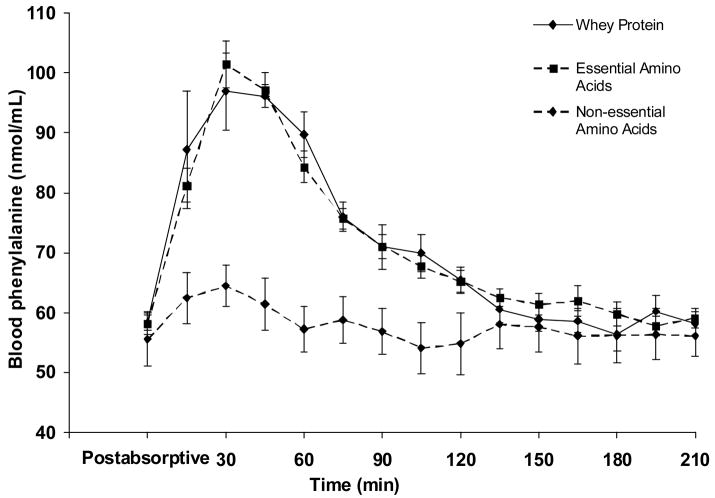
There was no statistically significant difference between groups in the postabsorptive arterial blood phenylalanine concentration (P > 0.05). As expected, arterial blood phenylalanine concentration increased over time only in the WY and EAA groups (Fig. 2), and in both groups remained significantly higher than the postabsorptive values for 75 minutes following the respective mixture ingestion (P < .05).
Fig. 2.
Blood phenylalanine concentration in the femoral artery in the postabsorptive period (average value) and during a 210-minute period following the ingestion of either whey protein or one of the amino acid (either essential or non-essential) mixtures. n=5 for each group. Values are presented as means ± SEM.
Postabsorptive muscle free phenylalanine concentration (in nmol·ml−1 intracellular water) was not different between groups (WY = 90 ± 7, EAA = 84 ± 4, NEAA = 75 ± 7; P > 0.05). At 3.5 hours postprandially (end of the study) the concentration of muscle free phenylalanine was not different from its postabsorptive value in either group (P > 0.05) and this value was also not different between groups (WY = 89 ± 5, EAA = 86 ± 6, NEAA = 69 ± 9; P > 0.05).
3.3 Plasma insulin and blood glucose
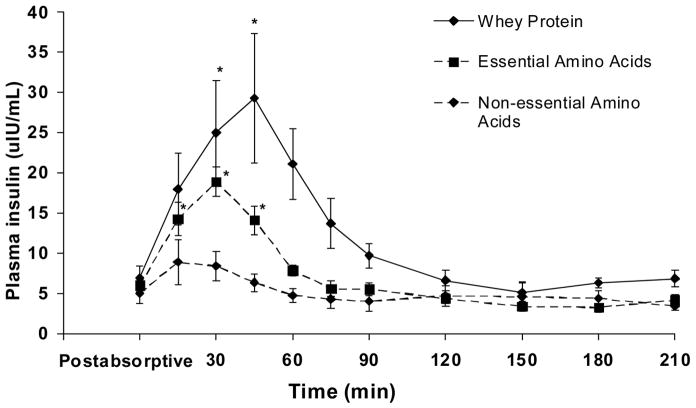
The homeostasis model assessment (HOMA), an index of insulin resistance, was calculated from the postabsorptive plasma insulin and glucose values. The HOMA values were not different between groups (WY = 1.6 ± 0.4, EAA = 1.4 ± 0.3, NEAA = 1.2 ± 0.3; P > 0.05). Fig. 3 shows the insulin response following the ingestion of whey protein or the amino acid mixtures, with the postabsorptive value representing the average plasma insulin concentration determined in two different times in the postabsorptive period. There were no differences in the postabsorptive plasma insulin concentrations between groups (P > 0.05). Plasma insulin concentration increased significantly in the WY and EAA groups and remained higher than the postabsorptive values for 45 minutes (P < .05). The overall insulin response was quantified by calculating the area under the insulin curve during the 3.5-hour period following the ingestion of the whey protein or amino acid mixtures. The value for the insulin area under the curve in the WY group (1072 ± 228 ulU·ml−1·3.5 hours−1) was significantly greater (P < .05) than the corresponding values for the EAA (213 ± 127 ulU·ml−1·3.5 hours−1) and NEAA (48 ± 40 ulU·ml−1·3.5 hours−1) groups. The postabsorptive blood glucose concentration (mg dl−1) was not different between groups (WY = 94 ± 2, EAA = 93 ± 2, NEAA = 97 ± 3; P > 0.05), and did not change significantly in either group over time after the ingestion of the whey protein or amino acid mixtures (data not shown).
Fig. 3.
Plasma insulin concentration in the femoral artery in the postabsorptive period (average value) and during a 210-minute period following the ingestion of either whey protein or one of the amino acid (either essential or non-essential) mixtures. n=5 for each group. Values are presented as means ± SEM. *Values over time within each group are significantly different from postabsorptive by ANOVA coupled with Dunnett’s post-hoc multiple comparisons tests at P < .05.
3.4 Leg phenylalanine balance
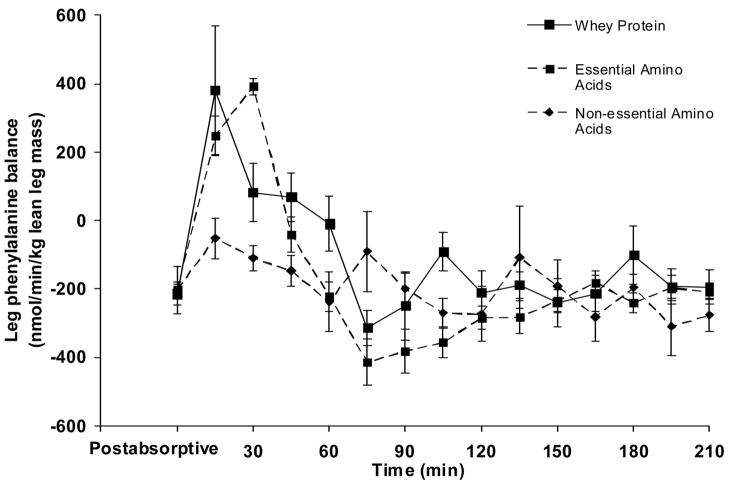
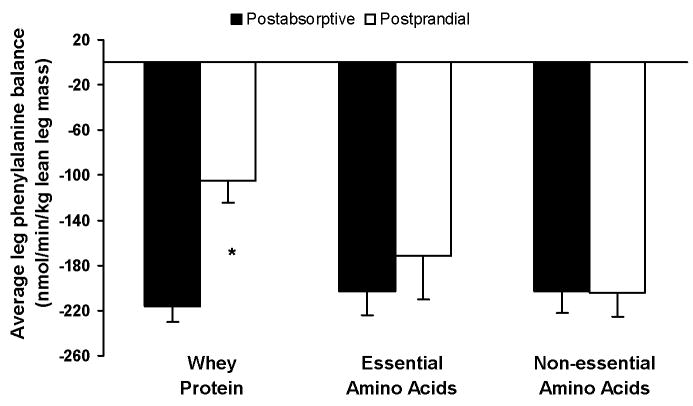
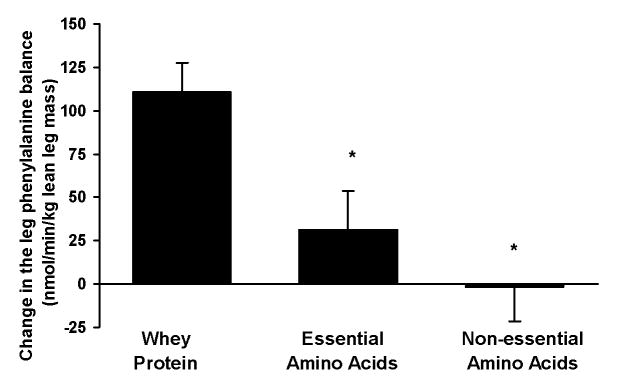
There was no difference in the average postabsorptive leg PB (nmol·min−1·kg lean leg mass−1) between groups (WY = −216 ± 14, EAA = −202 ± 21, NEAA = −203 ± 19; P > 0.05). Fig. 4 depicts the time course of the leg PB in the postabsorptive (average value) and postprandial periods. Since it is known that under a postprandial physiological circumstance, as in the present study, time specific measures of leg phenylalanine kinetics may not directly correspond to leg protein kinetics [17], we calculated the average response of the PB in both the postabsorptive and postprandial periods for each subject. The 3.5-hour duration in the postprandial period was chosen to allow sufficient time for the muscle amino acid concentrations to return to basal values, while any shorter time period (i.e 2 hours) under these circumstances would simply evaluate muscle amino acid kinetics that do not necessarily correspond to muscle protein kinetics [17]. As shown in Fig. 5, postprandial leg PB improved in the WY group (P < .05) but not in the EAA or NEAA groups (P > 0.05). The leg accrual of phenylalanine, as represented by the change in leg PB (postprandial leg PB – postabsorptive leg PB), was lower in both EAA and NEAA groups when compared to that in the WY group (P < .05; Fig. 6).
Fig. 4.
Blood phenylalanine balance in the leg in the postabsorptive period (average value) and during a 210-minute period following the ingestion of either whey protein or one of the amino acid (either essential or non-essential) mixtures. n=5 for each group. Values are presented as means ± SEM.
Fig. 5.
Average responses for the leg blood phenylalanine balance in the postabsorptive period and during a 210-minute period following the ingestion (postprandial) of either whey protein or one of the amino acid (either essential or non-essential) mixtures. n=5 for each group. Values are presented as means ± SEM. *Values between postabsorptive and postprandial responses within group are significantly different by paired-samples t test at P < .05.
Fig. 6.
Change in the leg blood phenylalanine balance (difference between the average postprandial period value and the corresponding postabsorptive period value) as a result of the ingestion of either whey protein or one of the amino acid (either essential or non-essential) mixtures. n=5 for each group. Values are presented as means ± SEM. *Values are significantly different from the whey protein group by ANOVA coupled with Dunnett’s post-hoc multiple comparisons tests at P < .05.
4. Discussion
The effects of protein ingestion on muscle protein accretion have been largely attributed to the EAA found in the ingested protein. The most important finding of this study is that whey protein, at least in the amount ingested in this study, results in greater anabolic effect in the elderly than its EAA. Therefore, this suggests that whey protein ingestion improves muscle protein accretion in the elderly through mechanisms that are beyond those associated with its EAA content.
These findings may appear to differ from our previous findings in the elderly that ingestion of 15g of EAA promotes muscle protein accrual [8], and that the response following ingestion of 15 g of EAA is greater than that following ingestion of 15g of whey protein [14]. These apparent discrepancies are likely explained by the total EAA content of the mixtures (15 g vs 6.72 in the present study). It is known that there is a dose-response effect of EAA ingestion on muscle protein synthesis [21]. Also ingestion of 15g of EAA appears to overcome an impaired responsiveness (or decreased efficiency) in the elderly, possibly related to the plasma availability of the amino acid leucine [6], and results in muscle protein accrual similar to that in young individuals [8]. However, ingestion by the elderly of half of that amount (i.e., ~ 7 g EAA), which is comparable to the EAA found in 15 g of whey protein, results in less than optimal muscle protein accrual relative to that in young individuals [7]. Therefore, our current results extent these previous findings in the elderly, by showing that muscle protein accrual is greater after ingestion of 15 g of whey protein than after ingestion of its constituent EAA content (~7 g), and also indicate that the whey protein’s EAA content is not solely responsible for its anabolic properties.
The specific role of amino acids, per se, in the observed responses of muscle protein accrual, as opposed to other concurrent metabolic processes (e.g. changes in insulin concentration) is difficult to distinguish. It is well-established that among the amino acids only the EAA are necessary to stimulate muscle protein synthesis [1, 22]. Therefore, the lack of a response in muscle anabolism following the NEAA is not surprising. Blood phenylalanine reached a peak concentration at 30 minutes following ingestion of either whey protein or EAA, pointing to the direction of a comparable blood availability of ingested EAA in both the WY and EAA groups. Such observation confirms the classification of whey protein as a “fast” protein with respect to the rate of digestion [23]. Assuming that the change in the postprandial phenylalanine concentration, which was comparable between the WY and EAA groups, is representative of changes in the other plasma EAA, plasma EAA changes may not explain the greater muscle protein accrual in the WY group compared to the EAA group. However, the lack of information on individual concentrations of all the plasma amino acids may be considered a limitation in the present study in explaining the observed responses. Despite this lack of information, ingestion of whey protein, but not EAA, is expected to increase the plasma concentration of the amino acid cysteine, which has been described to have a particular role in augmenting muscle protein anabolism [24].
The observed postprandial insulin response in the present study was greater in the WY group when compared to that in either EAA or NEAA group, and in parallel with a greater improvement in muscle protein balance. The differences in the plasma insulin response in the present study in the WY versus the EAA, or the NEAA, may relate, at least in part, to the expected postprandial presence of greater overall concentration of plasma amino acids (approximately twice as large in the WY group compared to the EAA or NEAA). Since amino acids found in both the whey protein and the EAA mixture (e.g. leucine, isoleucine, phenylalanine, threonine, methionine) are considered among the most potent amino acids in stimulating insulin secretion [25], the lower postprandial insulin response in the NEAA was expected. However, the magnitude of the postprandial insulin response in the WY group was much greater than that in the EAA group. The greater insulin response in the WY group is in accordance with previous observations where a balanced amino acid mixture increased the plasma insulin when compared to a mixture composed of only the EAA [26]. This likely reflects the presence in the whey protein of NEAA such as aspartate [25] and arginine [27], that have been shown to be potent secretagogues of insulin. The greater insulin response in the WY group when compared to that predicted by the addition of the respective responses in the EAA and the NEAA groups may indicate a possible synergestic effect of individuals amino acids on insulin secretion. Alternatively, activation of the incretin system and stimulation of insulin secretion by the glucose-dependent insulinotropic polypeptide (GIP) [28] may explain the greater insulin response in the WY group. Relative to that it is known that whey protein is a strong GIP secretagogue [29], possibly through bioactive peptides present in whey protein or formed during its digestion, and that the plasma GIP concentration is greater following ingestion of intact protein than a similar amount of protein in the form of free amino acids [30].
Data evaluating the role of insulin on muscle protein metabolism come from studies that induced changes in plasma insulin concentration that were greater than those observed in the present study and generally under non-physiological circumstances. To date these data do not appear to be in agreement. For example, in young healthy subjects, insulin has been reported to both increase protein synthesis without any change in protein breakdown [31] and reduce muscle protein breakdown without any effect on protein synthesis [32]. The improved anabolic response in the WY group, in the presence of increase in plasma insulin concentration in the present study, is in line with previously published data on the effects of increased plasma amino acids combined with a glucose-induced increase in plasma insulin on muscle protein accrual in elderly [33]. In the latter study [33] the improved muscle protein balance could be explained by a suppression of the protein breakdown similar to the reported effects of the insulin on suppressing whole body protein breakdown [34]. Based on these reports, and since determination of muscle protein breakdown under the non-steady state circumstances of the present study does not provide reliable results, we cannot exclude an effect of the plasma insulin on inhibiting protein breakdown in the muscle, and resulting in the improved postprandial muscle protein accrual in the WY group. Contrary to previous findings [33], hyperinsulinemia in the elderly and in the presence of increased plasma amino acids availability has been shown to stimulate muscle protein synthesis, albeit lower than in young [35], and it is possible to have contributed, at least in part, to the greater anabolic response in the WY group in the present study.
We have previously shown that an attenuated response of muscle protein anabolism to low amino acid availability with aging can be enhanced by ingestion of extra leucine [6]. However, recent evidence suggests that leucine does not provide any additional benefit on muscle protein anabolism when sufficient amount of protein is ingested [13]. The latter finding [13] would support the notion of ingestion of an adequate amount of protein, without emphasizing individual amino acids, as an approach to improve muscle protein anabolism in the elderly, which is in line with the findings of the present study. The findings of the present study appear to also be in agreement with findings from animal studies showing greater nitrogen retention with intact protein diet versus free amino acids diet [36, 37]. Therefore, given that, when compared to free amino acids supplements, ingestion of whey protein is an inexpensive approach to improve muscle protein metabolism, whey protein supplementation, especially if combined with resistance exercise [24], may help to prevent muscle wasting with aging. Further, supplementation with whey protein instead of EAA may provide additional health benefits, which are discussed elsewhere [38–41]. For example, cysteine-supported glutathione synthesis is implicated in protection against oxidative stress, while β-lactoglobulin and α-lactalbumin are major whey proteins modulating immune function.
In conclusion, muscle protein accrual in elderly is greater following ingestion of 15 g of whey protein than ingestion of its constituent EAA content, and this may be explained, at least in part, by a greater insulin response. Our findings suggest that stimulation of skeletal muscle protein accrual in the elderly by whey protein, at least in the amount ingested in the present study, is mediated by mechanisms that are beyond those attributed to the essential amino acids in whey protein.
Acknowledgments
The authors thank the nurses and the staff at the GCRC at UTMB in Galveston, TX, as well as Dan Creson, RN, Susan Minello, RN, MSN, ANP and Roxana Hirst, MS. We gratefully acknowledge Stephaine J. Blasé, Christopher Danesi, Gaurang K. Jariwala, and Ming-Qian Zheng for skillful technical assistance. The work was sponsored by National Institutes of Health Grants R01 AR49038 and P30 AG02483, and Shriners Hospital Grant 8490. Studies were conducted on the General Clinical Research Center (GCRC) at the University of Texas Medical Branch at Galveston, funded by grant M01 RR 00073 from the National Center for Research Resources, NIH, USPHS. The authors do not have any personal or financial conflict of interest with respect to this research.
Abbrevations
- EAA
essential amino acids
- GCRC
General Clinical Research Center
- GIP
glucose-dependent insulinotropic polypeptide
- NEAA
non-essential amino acids
- PB
phenylalanine balance
- WY
whey protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wolfe RR. Regulation of muscle protein by amino acids. J Nutr. 2002;132:3219S–24S. doi: 10.1093/jn/131.10.3219S. [DOI] [PubMed] [Google Scholar]
- 2.Evans WJ. What is sarcopenia? J Gerontol A Biol Sci Med Sci. 1995;50(Spec No):5–8. doi: 10.1093/gerona/50a.special_issue.5. [DOI] [PubMed] [Google Scholar]
- 3.Roubenoff R. Sarcopenia and its implications for the elderly. Eur J Clin Nutr. 2000;54 Suppl 3:S40–7. doi: 10.1038/sj.ejcn.1601024. [DOI] [PubMed] [Google Scholar]
- 4.Boirie Y, Gachon P, Beaufrere B. Splanchnic and whole-body leucine kinetics in young and elderly men. Am J Clin Nutr. 1997;65:489–95. doi: 10.1093/ajcn/65.2.489. [DOI] [PubMed] [Google Scholar]
- 5.Dangin M, Guillet C, Garcia-Rodenas C, Gachon P, Bouteloup-Demange C, Reiffers-Magnani K, Fauquant J, Ballevre O, Beaufrere B. The rate of protein digestion affects protein gain differently during aging in humans. J Physiol. 2003;549:635–44. doi: 10.1113/jphysiol.2002.036897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab. 2006;291:E381–7. doi: 10.1152/ajpendo.00488.2005. [DOI] [PubMed] [Google Scholar]
- 7.Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am J Clin Nutr. 2005;82:1065–73. doi: 10.1093/ajcn/82.5.1065. [DOI] [PubMed] [Google Scholar]
- 8.Paddon-Jones D, Sheffield-Moore M, Zhang XJ, Volpi E, Wolf SE, Aarsland A, Ferrando AA, Wolfe RR. Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am J Physiol Endocrinol Metab. 2004;286:E321–8. doi: 10.1152/ajpendo.00368.2003. [DOI] [PubMed] [Google Scholar]
- 9.Volpi E, Mittendorfer B, Wolf SE, Wolfe RR. Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first-pass splanchnic extraction. Am J Physiol. 1999;277:E513–20. doi: 10.1152/ajpendo.1999.277.3.E513. [DOI] [PubMed] [Google Scholar]
- 10.Volpi E, Kobayashi H, Sheffield-Moore M, Mittendorfer B, Wolfe RR. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr. 2003;78:250–8. doi: 10.1093/ajcn/78.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borsheim E, Tipton KD, Wolf SE, Wolfe RR. Essential amino acids and muscle protein recovery from resistance exercise. Am J Physiol Endocrinol Metab. 2002;283:E648–57. doi: 10.1152/ajpendo.00466.2001. [DOI] [PubMed] [Google Scholar]
- 12.Koopman R, Verdijk L, Manders RJ, Gijsen AP, Gorselink M, Pijpers E, Wagenmakers AJ, van Loon LJ. Co-ingestion of protein and leucine stimulates muscle protein synthesis rates to the same extent in young and elderly lean men. Am J Clin Nutr. 2006;84:623–32. doi: 10.1093/ajcn/84.3.623. [DOI] [PubMed] [Google Scholar]
- 13.Koopman R, Verdijk LB, Beelen M, Gorselink M, Kruseman AN, Wagenmakers AJ, Kuipers H, van Loon LJ. Co-ingestion of leucine with protein does not further augment post-exercise muscle protein synthesis rates in elderly men. Br J Nutr. 2008;99:571–80. doi: 10.1017/S0007114507812013. [DOI] [PubMed] [Google Scholar]
- 14.Paddon-Jones D, Sheffield-Moore M, Katsanos CS, Zhang XJ, Wolfe RR. Differential stimulation of muscle protein synthesis in elderly humans following isocaloric ingestion of amino acids or whey protein. Exp Gerontol. 2006;41:215–9. doi: 10.1016/j.exger.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 15.Jorfeldt L, Juhlin-Dannfelt A. The influence of ethanol on splanchnic and skeletal muscle metabolism in man. Metabolism. 1978;27:97–106. doi: 10.1016/0026-0495(78)90128-2. [DOI] [PubMed] [Google Scholar]
- 16.Jorfeldt L, Wahren J. Leg blood flow during exercise in man. Clin Sci. 1971;41:459–73. doi: 10.1042/cs0410459. [DOI] [PubMed] [Google Scholar]
- 17.Katsanos CS, Chinkes DL, Sheffield-Moore M, Aarsland A, Kobayashi H, Wolfe RR. Method for the determination of the arteriovenous muscle protein balance during non-steady-state blood and muscle amino acid concentrations. Am J Physiol Endocrinol Metab. 2005;289:E1064–70. doi: 10.1152/ajpendo.00141.2005. [DOI] [PubMed] [Google Scholar]
- 18.Rosenblatt J, Chinkes D, Wolfe M, Wolfe RR. Stable isotope tracer analysis by GC-MS, including quantification of isotopomer effects. Am J Physiol. 1992;263:E584–96. doi: 10.1152/ajpendo.1992.263.3.E584. [DOI] [PubMed] [Google Scholar]
- 19.Wolfe RR, Chinkes DL. Isotope tracers in metabolic research: principles and practice of kinetic analysis. New York: Wiley-Liss; 2004. [Google Scholar]
- 20.Bergstrom J, Furst P, Noree LO, Vinnars E. Intracellular free amino acid concentration in human muscle tissue. J Appl Physiol. 1974;36:693–7. doi: 10.1152/jappl.1974.36.6.693. [DOI] [PubMed] [Google Scholar]
- 21.Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. Faseb J. 2005;19:422–4. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- 22.Smith K, Reynolds N, Downie S, Patel A, Rennie MJ. Effects of flooding amino acids on incorporation of labeled amino acids into human muscle protein. Am J Physiol. 1998;275:E73–8. doi: 10.1152/ajpendo.1998.275.1.E73. [DOI] [PubMed] [Google Scholar]
- 23.Boirie Y, Dangin M, Gachon P, Vasson MP, Maubois JL, Beaufrere B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc Natl Acad Sci U S A. 1997;94:14930–5. doi: 10.1073/pnas.94.26.14930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayes A, Cribb PJ. Effect of whey protein isolate on strength, body composition and muscle hypertrophy during resistance training. Curr Opin Clin Nutr Metab Care. 2008;11:40–4. doi: 10.1097/MCO.0b013e3282f2a57d. [DOI] [PubMed] [Google Scholar]
- 25.Rocha DM, Faloona GR, Unger RH. Glucagon-stimulating activity of 20 amino acids in dogs. J Clin Invest. 1972;51:2346–51. doi: 10.1172/JCI107046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Volpi E, Ferrando AA, Yeckel CW, Tipton KD, Wolfe RR. Exogenous amino acids stimulate net muscle protein synthesis in the elderly. J Clin Invest. 1998;101:2000–7. doi: 10.1172/JCI939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidt HH, Warner TD, Ishii K, Sheng H, Murad F. Insulin secretion from pancreatic B cells caused by L-arginine-derived nitrogen oxides. Science. 1992;255:721–3. doi: 10.1126/science.1371193. [DOI] [PubMed] [Google Scholar]
- 28.Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153–65. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 29.Nilsson M, Stenberg M, Frid AH, Holst JJ, Bjorck IM. Glycemia and insulinemia in healthy subjects after lactose-equivalent meals of milk and other food proteins: the role of plasma amino acids and incretins. Am J Clin Nutr. 2004;80:1246–53. doi: 10.1093/ajcn/80.5.1246. [DOI] [PubMed] [Google Scholar]
- 30.Tessari P, Kiwanuka E, Cristini M, Zaramella M, Enslen M, Zurlo C, Garcia-Rodenas C. Slow versus fast proteins in the stimulation of beta-cell response and the activation of the entero-insular axis in type 2 diabetes. Diabetes Metab Res Rev. 2006;23:378–85. doi: 10.1002/dmrr.698. [DOI] [PubMed] [Google Scholar]
- 31.Fujita S, Rasmussen BB, Cadenas JG, Grady JJ, Volpi E. Effect of insulin on human skeletal muscle protein synthesis is modulated by insulin-induced changes in muscle blood flow and amino acid availability. Am J Physiol Endocrinol Metab. 2006;291:E745–54. doi: 10.1152/ajpendo.00271.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chow LS, Albright RC, Bigelow ML, Toffolo G, Cobelli C, Nair KS. Mechanism of insulin’s anabolic effect on muscle: measurements of muscle protein synthesis and breakdown using aminoacyl-tRNA and other surrogate measures. Am J Physiol Endocrinol Metab. 2006;291:E729–36. doi: 10.1152/ajpendo.00003.2006. [DOI] [PubMed] [Google Scholar]
- 33.Volpi E, Mittendorfer B, Rasmussen BB, Wolfe RR. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J Clin Endocrinol Metab. 2000;85:4481–90. doi: 10.1210/jcem.85.12.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guillet C, Zangarelli A, Gachon P, Morio B, Giraudet C, Rousset P, Boirie Y. Whole body protein breakdown is less inhibited by insulin, but still responsive to amino acid, in nondiabetic elderly subjects. J Clin Endocrinol Metab. 2004;89:6017–24. doi: 10.1210/jc.2003-031323. [DOI] [PubMed] [Google Scholar]
- 35.Guillet C, Prod’homme M, Balage M, Gachon P, Giraudet C, Morin L, Grizard J, Boirie Y. Impaired anabolic response of muscle protein synthesis is associated with S6K1 dysregulation in elderly humans. Faseb J. 2004;18:1586–7. doi: 10.1096/fj.03-1341fje. [DOI] [PubMed] [Google Scholar]
- 36.Daenzer M, Petzke KJ, Bequette BJ, Metges CC. Whole-body nitrogen and splanchnic amino acid metabolism differ in rats fed mixed diets containing casein or its corresponding amino acid mixture. J Nutr. 2001;131:1965–72. doi: 10.1093/jn/131.7.1965. [DOI] [PubMed] [Google Scholar]
- 37.Trocki O, Mochizuki H, Dominioni L, Alexander JW. Intact protein versus free amino acids in the nutritional support of thermally injured animals. JPEN J Parenter Enteral Nutr. 1986;10:139–45. doi: 10.1177/0148607186010002139. [DOI] [PubMed] [Google Scholar]
- 38.Ha E, Zemel MB. Functional properties of whey, whey components, and essential amino acids: mechanisms underlying health benefits for active people (review) J Nutr Biochem. 2003;14:251–8. doi: 10.1016/s0955-2863(03)00030-5. [DOI] [PubMed] [Google Scholar]
- 39.Krissansen GW. Emerging health properties of whey proteins and their clinical implications. J Am Coll Nutr. 2007;26:713S–23S. doi: 10.1080/07315724.2007.10719652. [DOI] [PubMed] [Google Scholar]
- 40.Walzem RL, Dillard CJ, German JB. Whey components: millennia of evolution create functionalities for mammalian nutrition: what we know and what we may be overlooking. Crit Rev Food Sci Nutr. 2002;42:353–75. doi: 10.1080/10408690290825574. [DOI] [PubMed] [Google Scholar]
- 41.Yalcin AS. Emerging therapeutic potential of whey proteins and peptides. Curr Pharm Des. 2006;12:1637–43. doi: 10.2174/138161206776843296. [DOI] [PubMed] [Google Scholar]