Abstract
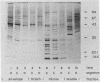
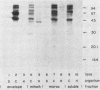
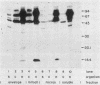
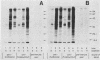
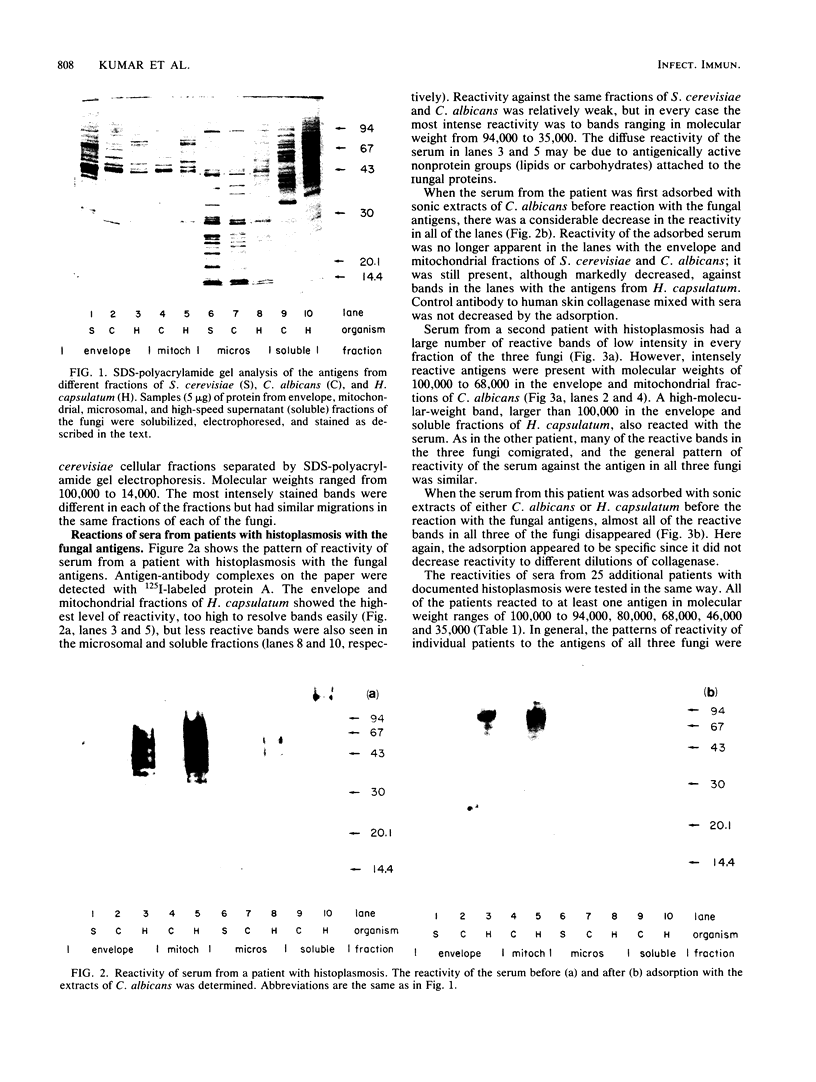
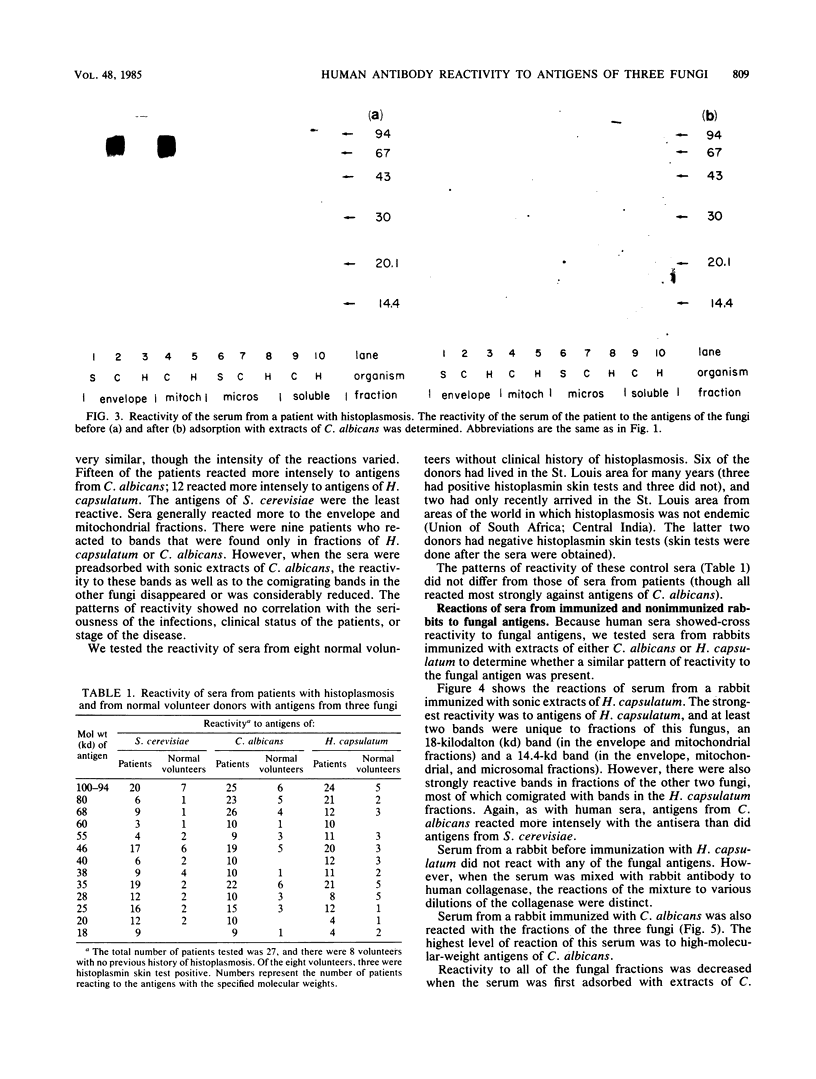
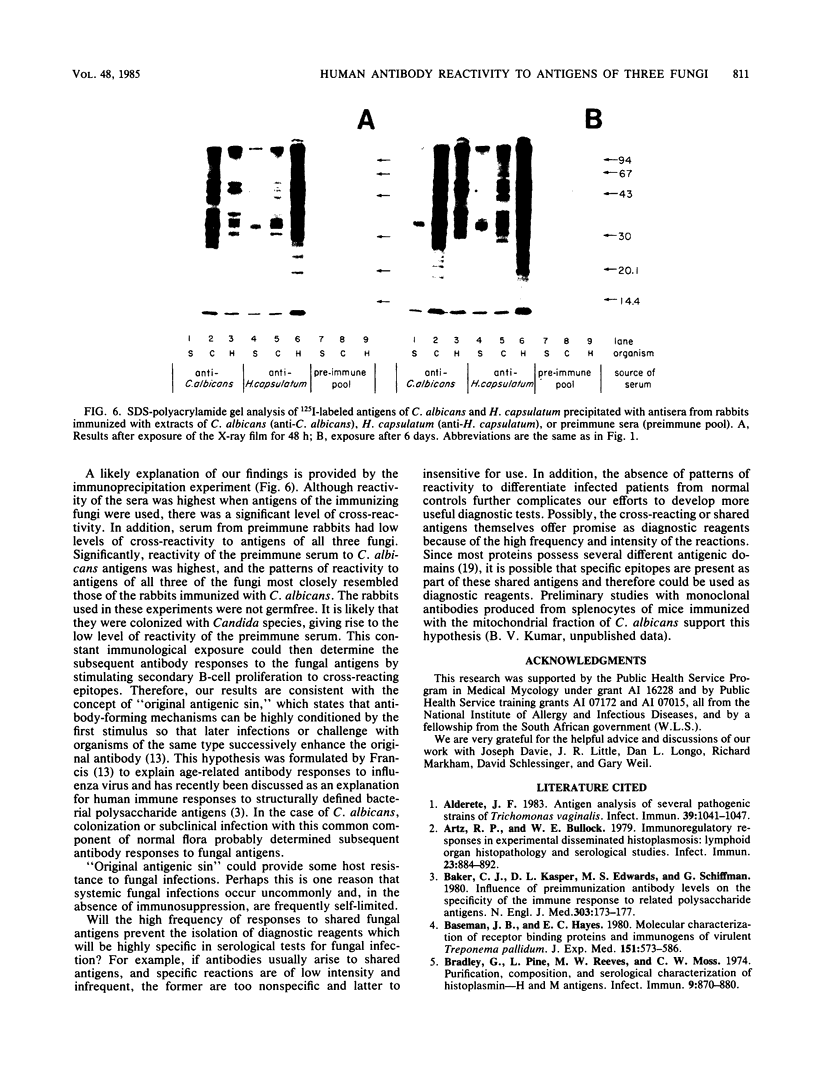
Using Western blots of electrophoretically separated antigens, we show that human antibodies react most frequently to antigens shared by three fungi (Histoplasma capsulatum, Candida albicans, and Saccharomyces cerevisiae). Reactivity to antigens specific for individual fungi was relatively uncommon. The pattern of reactivity could not distinguish infected patients from uninfected controls. Rabbits immunized with extracts of each fungus also produced antibodies to cross-reactive or shared antigens of the other two fungi. Furthermore, preimmune sera showed similar but lower reactivity with the same fungal antigens. We believe that the preimmunization antibodies, which probably resulted from earlier fungal colonization or inapparent infections, predisposed the immune responses elicited by the vaccinations. A similar mechanism likely explains the results with human sera.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderete J. F. Antigen analysis of several pathogenic strains of Trichomonas vaginalis. Infect Immun. 1983 Mar;39(3):1041–1047. doi: 10.1128/iai.39.3.1041-1047.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artz R. P., Bullock W. E. Immunoregulatory responses in experimental disseminated histoplasmosis: lymphoid organ histopathology and serological studies. Infect Immun. 1979 Mar;23(3):884–892. doi: 10.1128/iai.23.3.884-892.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker C. J., Kasper D. L., Edwards M. S., Schiffman G. Influence of preimmunization antibody levels on the specificity of the immune response to related polysaccharide antigens. N Engl J Med. 1980 Jul 24;303(4):173–178. doi: 10.1056/NEJM198007243030401. [DOI] [PubMed] [Google Scholar]
- Baseman J. B., Hayes E. C. Molecular characterization of receptor binding proteins and immunogens of virulent Treponema pallidum. J Exp Med. 1980 Mar 1;151(3):573–586. doi: 10.1084/jem.151.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley G., Pine L., Reeves M. W., Moss C. W. Purification, composition, and serological characterization of histoplasmin-H and M antigens. Infect Immun. 1974 May;9(5):870–880. doi: 10.1128/iai.9.5.870-880.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- CAMPBELL C. C. The accuracy of serologic methods in diagnosis. Ann N Y Acad Sci. 1960 Aug 27;89:163–177. doi: 10.1111/j.1749-6632.1960.tb20139.x. [DOI] [PubMed] [Google Scholar]
- Chick E. W., Baum G. L., Furcolow M. L., Huppert M., Kaufman L., Pappagianis R. Scientific Assembly statement. The use of skin tests and serologic tests in histoplasmosis, coccidioidomycosis, and blastomycosis, 1973. Am Rev Respir Dis. 1973 Jul;108(1):156–159. doi: 10.1164/arrd.1973.108.1.156. [DOI] [PubMed] [Google Scholar]
- Cohen G. H., Long D., Eisenberg R. J. Synthesis and processing of glycoproteins gD and gC of herpes simplex virus type 1. J Virol. 1980 Nov;36(2):429–439. doi: 10.1128/jvi.36.2.429-439.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. S., Isturiz R. E., Malech H. L., Root R. K., Wilfert C. M., Gutman L., Buckley R. H. Fungal infection in chronic granulomatous disease. The importance of the phagocyte in defense against fungi. Am J Med. 1981 Jul;71(1):59–66. doi: 10.1016/0002-9343(81)90259-x. [DOI] [PubMed] [Google Scholar]
- Crawford L., Leppard K., Lane D., Harlow E. Cellular proteins reactive with monoclonal antibodies directed against simian virus 40 T-antigen. J Virol. 1982 May;42(2):612–620. doi: 10.1128/jvi.42.2.612-620.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorval G., Welsh K. I., Wigzell H. A radioimmunoassay of cellular surface antigens on living cells using iodinated soluble protein A from Staphylococcus aureus. J Immunol Methods. 1975 Jun;7(2-3):237–250. doi: 10.1016/0022-1759(75)90021-6. [DOI] [PubMed] [Google Scholar]
- Greenfield R. A., Jones J. M. Comparison of cytoplasmic extracts of eight Candida species and Saccharomyces cerevisiae. Infect Immun. 1982 Mar;35(3):1157–1161. doi: 10.1128/iai.35.3.1157-1161.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handman E., Mitchell G. F., Goding J. W. Identification and characterization of protein antigens of Leishmania tropica isolates. J Immunol. 1981 Feb;126(2):508–512. [PubMed] [Google Scholar]
- Handman E., Remington J. S. Antibody responses to toxoplasma antigens in mice infected with strains of different virulence. Infect Immun. 1980 Jul;29(1):215–220. doi: 10.1128/iai.29.1.215-220.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen E. J., Frisch C. F., McDade R. L., Jr, Johnston K. H. Identification of immunogenic outer membrane proteins of Haemophilus influenzae type b in the infant rat model system. Infect Immun. 1981 Jun;32(3):1084–1092. doi: 10.1128/iai.32.3.1084-1092.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilman C. J., Jr, Zweig M., Hampar B. Herpes simplex virus type 1 and 2 intracellular p40: type-specific and cross-reactive antigenic determinants on peptides generated by partial proteolysis. J Virol. 1981 Nov;40(2):508–515. doi: 10.1128/jvi.40.2.508-515.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katiyar S. S., Porter J. W. Antibodies specific for NADPH-binding region of enzymes possessing dehydrogenase activities. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1221–1223. doi: 10.1073/pnas.80.5.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lane D. P., Hoeffler W. K. SV40 large T shares an antigenic determinant with a cellular protein of molecular weight 68,000. Nature. 1980 Nov 13;288(5787):167–170. doi: 10.1038/288167a0. [DOI] [PubMed] [Google Scholar]
- McDade R. L., Jr, Johnston K. H. Characterization of serologically dominant outer membrane proteins of Neisseria gonorrhoeae. J Bacteriol. 1980 Mar;141(3):1183–1191. doi: 10.1128/jb.141.3.1183-1191.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molday R. S., MacKenzie D. Monoclonal antibodies to rhodopsin: characterization, cross-reactivity, and application as structural probes. Biochemistry. 1983 Feb 1;22(3):653–660. doi: 10.1021/bi00272a020. [DOI] [PubMed] [Google Scholar]
- Papageorge A. G., Defeo-Jones D., Robinson P., Temeles G., Scolnick E. M. Saccharomyces cerevisiae synthesizes proteins related to the p21 gene product of ras genes found in mammals. Mol Cell Biol. 1984 Jan;4(1):23–29. doi: 10.1128/mcb.4.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillemer E., Weissman I. L. A monoclonal antibody that detects a V kappa-TEPC15 idiotypic determinant cross-reactive with a Thy-1 determinant. J Exp Med. 1981 May 1;153(5):1068–1079. doi: 10.1084/jem.153.5.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine L., Gross H., Malcolm G. B., Green J. H., Barbaree J. M., Harrell W. K., Suggs M. T., Blumer S. O., Kaufman L., Ajello L. Evaluation of candidate international reference reagents and a microimmunodiffusion test for the identification of precipitins to the H and M antigens of histoplasmin. J Biol Stand. 1981 Oct;9(4):513–530. doi: 10.1016/s0092-1157(81)80044-3. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Shen V., King T. C., Kumar V., Daugherty B. Monoclonal antibodies to Escherichia coli 50S ribosomes. Nucleic Acids Res. 1980 Oct 24;8(20):4639–4649. doi: 10.1093/nar/8.20.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strockbine N. A., Largen M. T., Zweibel S. M., Buckley H. R. Identification and molecular weight characterization of antigens from Candida albicans that are recognized by human sera. Infect Immun. 1984 Feb;43(2):715–721. doi: 10.1128/iai.43.2.715-721.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston D. J., Gale R. P., Meyer D. V., Young L. S. Infectious complications of human bone marrow transplantation. Medicine (Baltimore) 1979 Jan;58(1):1–31. doi: 10.1097/00005792-197901000-00001. [DOI] [PubMed] [Google Scholar]
- Zweig M., Heilman C. J., Jr, Rabin H., Hampar B. Shared antigenic determinants between two distinct classes of proteins in cells infected with herpes simplex virus. J Virol. 1980 Sep;35(3):644–652. doi: 10.1128/jvi.35.3.644-652.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]