Abstract
The chemokine receptor CXCR6 has been described on lymphoid cells and is thought to participate in the homing of activated T-cells to non-lymphoid tissue. We now provide evidence that the chemokine receptor CXCR6 is also expressed by activated polymorphonuclear neutrophils (PMN) in vivo: Examination of biopsies derived from patients with pancreatic carcinoma by confocal laser scan microscopy revealed a massive infiltration of PMN that expressed CXCR6, while PMN of the peripheral blood of these patients did not. To answer the question whether CXCR6 expression is a property of infiltrated and activated PMN, leucocytes were collected from patients with localized soft tissue infections in the course of the wound debridement. By cytofluorometry, the majority of these cells were identified as PMN. Up to 50% of these PMN were also positive for CXCR6. Again, PMN from the peripheral blood of these patients were nearly negative for CXCR6, as were PMN of healthy donors. In a series of in vitro experiments, up-regulation of CXCR6 on PMN of healthy donors by a variety of cytokines was tested. So far, a minor, although reproducible, effect of tumour necrosis factor (TNFα) was seen: brief exposure with low-dose TNFα induced expression of CXCR6 on the surface of PMN. Furthermore, we could show an increased migration of PMN induced by the axis CXCL16 and CXCR6. In summary, our data provide evidence that CXCR6 is not constitutively expressed on PMN, but is up-regulated under inflammatory conditions and mediates migration of CXCR6-positive PMN.
Keywords: acute bacterial infections, CXCR6, pancreatic carcinoma, polymorphonuclear neutrophils
Introduction
Chemokines and their receptors are crucial for the directed migration, the infiltration, and the homing of leucocytes. Not to the exclusion of other receptors, CXCR6, also known as Bonzo, is critically involved in the infiltration of T-lymphocytes to non-lymphatic tissue. CXCR6-positive T-cells are found at the sites of chronic inflammation, for example in patients with chronic inflammatory liver disease [1–4] or interstitial lung disease [5]. Moreover, in the affected joints of patients with rheumatoid arthritis CXCR6-positive T-cells are found, as well as in a variety of tumours [6]. Taken together, there is unequivocal evidence for the relevance of CXCR6 and of its ligand CXCL16 as well in the trafficking of T-lymphocytes.
Aside from T-lymphocytes, also polymorphonuclear neutrophils (PMN) are found in chronically inflamed tissues and in tumours as well. Although more appreciated for their role as ‘first line defence’ in acute bacterial infection, there is increasing evidence for the participation of PMN in destructive, chronic inflammatory disease, including rheumatoid arthritis [7,8], primary vasculitis [8], or chronic inflammatory bowel disease [9]. Also in tumours, infiltration of PMN is seen as reviewed by di Carlo et al.[10]. While trafficking of PMN to the site of primary infection is well studied [11–13], it is unclear how they are attracted to the site of a chronic inflammation or to a tumour. To this end, we now tested whether or not the chemokine receptor CXCR6 is expressed by PMN, and whether it is subject to regulation. To assess expression of CXCR6 in vivo, we analysed two different diseases: pancreatic carcinoma as an example of a tumour with a prominent PMN infiltration [14,15], and patients with implant-associated post-traumatic osteomyelitis as an example of a localized, persistent bacterial infection, where again a prominent PMN infiltrate was described [16]. We found expression of CXCR6 on the infiltrated PMN, but not or to a much lesser extent on peripheral blood PMN of the patients; moreover, we found evidence for up-regulation of CXCR6 on PMN of healthy donors when cultivated under infection-analogue conditions. Finally, we described an increased migration of PMN in a direction towards a CXCL16 gradient or supernatants from CXCL16 liberating tumour cells.
Materials and methods
Tissue and blood collection in patients with pancreatic carcinoma
Tissue samples of pancreatic carcinoma patients (n = 6) were obtained from patients undergoing resection because of pancreatic carcinoma at the Department of Surgery, University of Heidelberg, Germany. For immunohistochemistry procedures, tissue samples were fixed in formalin and subsequently embedded in paraffin as described previously [17]. In parallel, venous blood of patients, suffering from pancreatic carcinoma (n = 12), was drawn preoperatively into heparin-coated tubes (Sarstedt, Nübrecht, Germany). Healthy age-matched donors served as controls. The study was approved by the Ethics Committee of the University of Heidelberg, and a written informed consent was obtained from all the patients.
Sample collection in patients with implant-associated osteomyelitis
Patients with implant-associated osteomyelitis due to bacterial biofilm infection (n = 10) were included in the study. The patients attended the BG Trauma Centre Ludwigshafen, Germany. The diagnosis was based on the medical history, the clinical examination, the leucocytes count, the C-reactive protein serum concentrations, and the identification of the bacteria. Venous blood from all patients was drawn into heparin-coated tubes (Sarstedt) at the time of the surgical intervention, and from healthy age-matched donors as well (n = 10). Implant-associated osteomyelitis requires the removal of the infected implant. During surgery the infiltrated cells were recovered as described previously [18]. The cytofluorometric studies described below were carried out within 3 h after blood withdrawal, or collection of the local samples. The study was approved by the Ethics Committee of the University of Heidelberg, and a written informed consent was obtained from all the patients.
Confocal laser scan microscopy
Paraffin-embedded pancreatic carcinoma tissues were used. After cutting into 3-µm thick sections, deparaffinization and dehydration, slides were washed for 10 min with TBS/0·1% BSA. Sections were incubated for 1 h at room temperature with Beriglobin™ (ZLB Behring, Marburg, Germany) prior to 1 h incubation at room temperature with an APC-coupled monoclonal antibody to CXCR6 (R & D Systems GmbH, Wiesbaden-Nordenstadt, Germany), diluted 1:100 in Tris-buffered saline (TBS/3% BSA). After washing two times with TBS/0·1% BSA – 0·5% Tween 20, and once with TBS/0·1% BSA, the slides were incubated for 1 h with a FITC-labelled antibody to CD66b (Immunotech, Marseille, France), diluted 1: 200. The slides were examined by confocal laser microscopy (Leica microscope DM RBE, Leica laser system TCS NT, Leica, Bensheim, Germany) and Leica TCS as software.
Cytofluorometry
Cytofluorometry was performed as described before [19]. The following antibodies were used: FITC-labelled CD66b and the respective FITC-labelled isotypic mouse IgG1 (Immunotech); antibodies to CXCR6 (clone 56 811·111, either unlabelled or directly labelled with APC or PE (R & D Systems). The antibodies were used in a final concentration of 2·5 µg per 1 ml of whole blood or 1 × 106 cells, respectively. Following incubation with the respective antibodies (20 min, room temperature), erythrocytes were lysed using FACS-lysing solution (Becton Dickinson, Heidelberg, Germany). Cells were analysed by FACSCalibur® and CellQuest® Pro software (Becton Dickinson). Results are expressed as per cent positive cells in the appropriate gate or quadrant, respectively. The markers (M1, M2) were set according to the IgG isotype controls. For the detection of the intracellular antigens, cells were solubilized using FACSPerm (Becton Dickinson) and the protocol provided by the supplier.
Activation of PMN
For activation of PMN in whole blood, 1 ml heparinized blood was incubated with tumour necrosis factor alpha (TNFα) (5 ng/ml), interleukin (IL)-6 (1–20 ng/ml), IL-8 (1–10 ng/ml) or PMA (1 µg/ml) (all obtained from Sigma-Aldrich, Steinheim, Germany) for 20 min. Then surface expression was measured as described above. Isolated PMN, prepared by centrifugation on PolymorphPrep™ (Nycomed, Oslo, Norway), and positive selection using anti-CD15 magnetic beads (Miltenyi, Bergisch-Gladbach, Germany) were suspended in AIM V (Gibco, purchased from Invitrogen, Karlsruhe, Germany) at a concentration of 1 × 106/ml and incubated with either TNFα or PMA as described above.
Chemotaxis
A modified Boyden chamber equipped with a nitrocellulose filter (5 µm pore size; 200 µm thick) was used (Schleicher & Schuell GmbH, Dassel, Germany) with yeast-activated normal human serum as a source for complement C5a as bona fide chemoattractant [20]. Random migration was assessed using Hanks balanced salt solution (HBSS). The cells (1 × 106 in 1 ml), suspended in HBSS containing 0·1% bovine serum albumin, were placed into the upper compartment, the chemoattractants to be tested in the lower. After 90 min the cells that had migrated into the filters were fixed with propanol, stained with haematoxylin, and evaluated using an Omnicon Alpha Image Analyser (Bausch & Lomb, Heidelberg, Germany). Chemotaxis was measured as ‘leading front’, defined as the distance in µm from the top of the filter to a level where at least five cells per visual field could still be seen. Two parallel filters were prepared, and on each filter, 10 different areas were evaluated. The mean ± s.d. was calculated; differences between mean values were determined using anova.
ADAM10 siRNA transfection of human pancreatic carcinoma cell lines
ADAM10 is a disintegrin-like metalloproteinase, which is responsible for the membrane-bound release (‘shedding’) of CXCL16 [21,22]. Two human pancreatic carcinoma cell-lines, BxPC-3 and COLO-357 (ATCC, purchased from LGC, Teddington, United Kingdom), were grown in RPMI-medium containing 10% fetal bovine serum, 100 U/ml penicillin and 100 µg/ml streptomycin (complete medium). Cells were kept at 37°C in and 5% CO2 and 95% air atmosphere. In this study ADAM10 silencing was performed as previously described for siRNA transfection [23]. Briefly, BxPC-3 and COLO-357 cells (2 × 105/well) were plated. After 24 h, medium was changed, and 100 µl transfection solution, containing 5 µg of two different specific CXCL16 siRNA (siRNA 1, Ambion, Austin, USA; siRNA2, Qiagen, Hilden, Germany) or 5 µg control siRNA and ECR buffer (Qiagen). In addition, 15 µl transfection reagent (Qiagen) was added to 1900 µl medium. After 24 h, medium was replaced with serum-free medium. After another 24 h supernatants were collected, centrifuged and chemotaxis assays were performed as described above. With this experimental set-up, a clear decrease of soluble CXCL16 in the supernatants of cultivated pancreatic carcinoma cells can be established by siRNA transfection [24].
Statistics
Differences between the mean values of the groups (cells from the patients versus cells from the healthy donors) were calculated using anova. Linear correlation was assessed using OriginPro 7·5 (Additive Software GmbH, Friedrichsdorf, Germany). P < 0·05 was set at the level of statistical significance.
Results
Detection of CXCR6 on infiltrated PMN of patients with pancreatic carcinoma
By confocal laser scan microscopy, a colocalization of CXCR6 with CD66b, the latter used as marker molecule for PMN, was seen, indicating that the infiltrated PMN were CXCR6 positive (Fig. 1a–e). Specimens of six patients were analysed, with similar results obtained in all evaluated samples. In a parallel set of experiments, expression of CXCR6 on PMN of the peripheral blood of patients with pancreatic carcinoma was tested and compared with healthy donors. Of the patients' PMN 1·8 ± 1·8% (mean ± s.d., n = 12) expressed CXCR6, of the donors' 3·4 ± 4·9%.
Fig. 1.
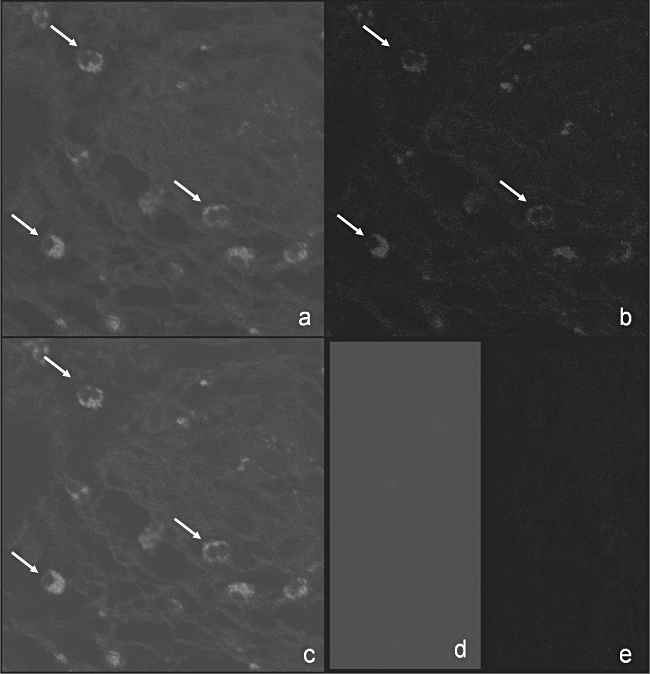
Detection of CXCR6 on infiltrated PMN of patients with pancreatic carcinoma. Expression of CXCR6 on PMN of patients (n = 6) with pancreatic carcinoma was determined with confocal laser scan microscopy by using colocalization of CD66b (a), a marker molecule for PMN, and CXCR6 (b). In (c) a representative example of the overlay of both stainings revealing CXCR6-positive PMN infiltrated in pancreatic carcinoma specimens is given. The appropriate isotype negative controls are presented in (d) and (e).
Detection of CXCR6 on infiltrated PMN of patients with implant-associated post-traumatic osteomyelitis
In another set of experiments, PMN having infiltrated sites of bacterial infection were analysed, and compared with PMN obtained from the peripheral blood of the same patient. By cytofluorometry using unseparated lavage or whole blood samples, the majority of the infiltrated PMN were found to express CXCR6 (example see Fig. 2b), but on average only 12·8 ± 14·9% of the peripheral blood PMN (example see Fig. 2a, data of 10 patients are summarized in Fig. 2e). Of four patients, we were able to test the peripheral blood PMN again as the patients recovered (7–14 days after surgery) (Fig. 2d). The CXCR6 expression was then within the normal range as determined by testing healthy donors (Fig. 2c and e).
Fig. 2.
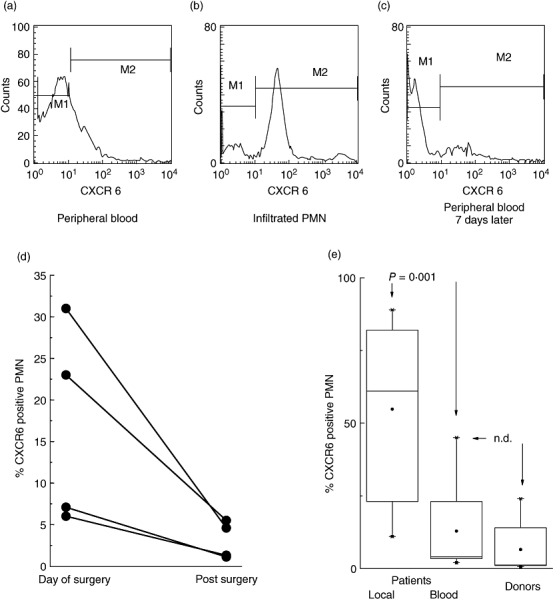
Detection of CXCR6 on PMN of patients with implant-associated osteomyelitis analysed by cytofluorometry. Expression of CXCR6 on PMN of patients (n = 10) with implant-associated osteomyelitis was measured on the day of surgery and on the 7th postoperative day and compared with healthy donors (n = 10) using cytofluorometry. Only a small amount of peripheral blood PMN expressed CXCR6, a representative example on the day of surgery is shown in (a). In contrast, nearly all PMN obtained from the infected site revealed distinct CXCR6 expression on the day of surgery; a representative example is shown in (b). After recovery, the peripheral blood PMN were negative for CXCR6 on the 7th postoperative day; a representative example is shown in (c); data of four patients are summarized in (d). In (e) the results of the CXCR6 expression from peripheral PMN of patients (n = 10) and controls (n = 10) as well as infiltrated PMN of patients (n = 10) are summarized as box plots (the box contains 50% of the values; the horizontal bar indicates the median, the dot the mean values; the differences between the mean values were evaluated by anova; n.d. = not different). The marker M1 was set for the isotype controls in all given experiments.
Up-regulation of CXCR6 on PMN in vitro
The data described so far implied an up-regulation of CXCR6 in the context of PMN activation. Indeed, exposure of whole blood to TNFα or PMA for 20 min resulted in a surface expression of CXCR6 (examples and summary of five donors are shown in Fig. 3). As further stimuli, IL-6, IL-8 and interferon-γ were tested over a wide range of concentrations. With these cytokines, we did not see an up-regulation of CXCR6 (data not shown). On purified PMN of healthy donors, which expressed CXCR6 also only weakly (5·5 ± 4·5% mean ± s.d. of n = 5), CXCR6 could also be up-regulated by brief exposure to TNFα (data not shown). In line with rapid translocation to the surface, CXCR6 could be detected intracellularly in nearly all PMN. By quantitative RT-PCR CXCR6 specific RNA was not detected (data not shown).
Fig. 3.
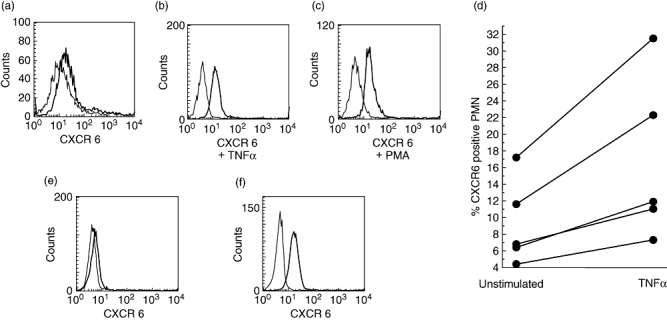
Expression of CXCR6 by PMN of healthy donors and up-regulation by TNFα. By cytofluorometry, expression of CXCR6 on the PMN population in whole blood was tested. PMN only weakly expressed CXCR6 (a), after exposure of whole blood samples to TNFα (b) or PMA (c), CXCR6 is translocated to the cell surface. In these experiments CD66b was used to identify the PMN. Data for healthy donors (n = 5) are summarized in (d) (each pair of dots connected by the line represents an individual donor before stimulation and after exposure to TNFα). Purified PMN of healthy donors express CXCR6 only weakly (e), but intracellularly nearly all PMN are positive for CXCR6 (f).
The ligand for CXCR6 induces chemotaxis of isolated PMN of healthy donors
Chemotaxis was tested in a Boyden chamber assay using activated serum as source of C5a as positive control (Fig. 4). Recombinant CXCL16, the ligand for CXCR6, induced a minor, though reproducible, migration of PMN in the concentration of 25 ng/ml; no significant effect was seen in the concentration of 50 ng/ml or higher. An induction of PMN migration was seen by stimulation with cell culture supernatants of the human pancreatic carcinoma cell lines BxPC-3 and COLO-357, which are known to produce CXCL16. By blocking the CXCL16 liberation by silencing with two different specific ADAM10 siRNA oligonucleotides, the chemotaxis was clearly reduced with both siRNA (siRNA 1 and siRNA 2) and reached statistical significance by using the siRNA 2 (COLO-357 siRNA 2 versus control, P = 0·0015; BxPC-3 siRNA 2 versus control, P = 0·012).
Fig. 4.
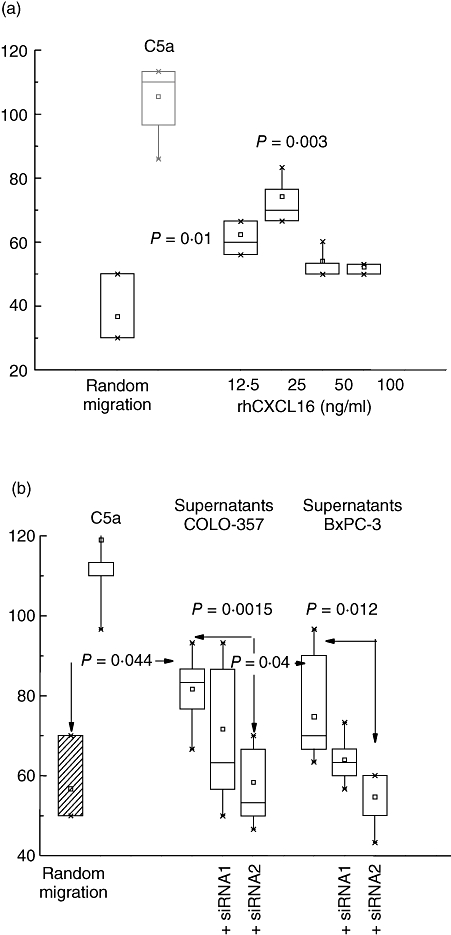
Chemotaxis of isolated PMN of healthy donors induced by recombinant CXCL16 or by supernatants of two pancreatic carcinoma cell lines. Recombinant human CXCL16 (rhCXCL16) induced chemotaxis of PMN (a), as did the supernatants of two pancreatic carcinoma cells lines (BxPC-3 and COLO-357), known to produce CXCL16 (b). When the chemokine production of these cell lines was inhibited by silencing via specific ADAM10 siRNA transfection with two different oligonucleotides (siRNA 1 and siRNA2), the chemotaxis was clearly reduced. The data are given as distance in µm covered by at least five PMN. Twenty filter areas were evaluated and the mean ± s.d. is shown. For comparison, on the very left, random migration as negative control and migration towards C5a as positive control are shown. (In (a) the P-values were calculated for the rhCXCL16-induced migration in comparison with random migration; in (b), in addition, differences between CXCL16-containing supernatants and supernatants of cells pre-treated with siRNA were calculated).
Discussion
Polymorphonuclear neutrophils, as the largest group of leucocytes infiltrating into infected sites, play a pivotal role in the host reaction to localized infections [25]. In localized infections, PMN have crucial functions in the innate immune response against bacteria by phagocytosis. Furthermore, by transdifferentiation to cells with dendritic-like characteristics, infiltrated PMN are leading to a proliferation of T-lymphocytes and a perpetuation of the local inflammatory process in acute infections as well as rheumatoid arthritis [8,26].
There is increasing evidence that an immune cell interaction is also involved in the regulation of proliferation in various solid malignancies by stimulation of tumour cell proliferation and angiogenesis [27,28]. In the complex regulation from immune surveillance to immune escape, various steps are involved, triggered by tumour cells and their interaction with the innate as well as the adaptive immune responses [29,30]. Various solid tumour malignancies, and here in particular pancreatic carcinoma as one of the most aggressive entities, reveal a distinct involvement of infiltrated inflammatory cells in carcinogenesis and tumour progression [31,32].
The large group of chemotactic cytokines called chemokines are involved in acute and chronic inflammatory processes as well as in the regulation of cancer growth and progression [33–35]. The superfamily of CXC chemokines, initially described as factors prompting the migration and infiltration of leucocytes, and their complex function in chemotaxis, migration and invasion of inflammatory and tumour cells as well as the induction of angiogenesis has been evaluated in various inflammatory and malignant diseases [36,37].
The exact link between the function of PMN and CXC chemokines and their receptors in inflammatory processes is still not fully discovered. However, in particular the two ligand receptor axis CXCL16 – CXCR6 might play an important role in the orchestration of regulating the immune response in localized or chronic infections and tumour progression mediated by an interaction with immune cells [38–40]. CXCR6 and CXCL16 have been shown to regulate chronic inflammatory processes such as sarcoidosis, rheumatoid arthritis and glomerulonephritis via T lymphocytes [41–43].
In the present study, an expression of CXCR6 on PMN as a local phenomenon has been discovered as a novel finding in the regulation of inflammatory processes guided by a crosslink of CXC chemokines and PMN. Under these two defined localized inflammatory conditions, such as the tumour-inflammation in pancreatic cancer specimens and acute localized bacterial wound infections, the expression of the CXC chemokine receptor CXCR6 on infiltrated PMN was found, but not on PMN of the peripheral blood. Thus, CXCR6 surface expression on PMN seems to be a temporary event involved in the local regulation of the immune response in vivo. This can be underlined by the finding that CXCR6 expression on the surface of PMN can be up-regulated using TNFα and phorbolester PMA as classical immunomodulatory factors.
Migration of PMN was determined in a standardized chemotaxis assay. CXCL16, the only described ligand of CXCR6, induced migration in the typical dose-dependent manner, with high doses being inhibitory, as is described for all chemokines. Similarly, supernatants from cultivated human pancreatic cancer cells, releasing CXCL16, induced chemotaxis. Compared with the bona fide chemoattractant C5a, chemotaxis was lower (40–60%), which is most probably due to the fact that only a minor portion of peripheral blood PMN is expressing CXCR6. Up-regulation of CXCR6 by PMA or TNFα was precluded by the fact that in vitro the pre-incubation of PMN with either TNFα or PMA abolishes the capacity to migrate [44]. As human pancreatic cancer cell lines produce high levels of soluble CXCL16 in vitro[24], and because the infiltrated PMN express CXCR6, we hypothesize that the tumour cells attract the PMN.
In summary, these novel findings identify a potential crosstalk between CXC chemokines and CXC receptor expressing PMN and imply a role of this interaction in the attraction of PMN to sites of localized bacterial infections, or tumours, respectively. The function of the CXCR6-positive PMN at the site of infection or within a tumour has not yet been elucidated. The question remains of whether those attracted CXCR6 positive PMN participate in the host defence or whether they rather contribute to the destructive inflammatory processes known to be associated with persistent bacterial infections or with pancreatic tumours in particular.
Acknowledgments
We thank Thomas Giese, Institute of Immunology, University of Heidelberg, Germany, for performing the quantitative RT-PCR.
Parts of the given results have been presented as a poster during the 37th Annual Meeting of the German for Immunology, Heidelberg, Germany, 5–8 September 2007.
References
- 1.Boisvert J, Kunkel EJ, Campbell JJ, Keeffe EB, Butcher EC, Greenberg HB. Liver-infiltrating lymphocytes in end-stage hepatitis C virus: subsets, activation status, and chemokine receptor phenotypes. J Hepatol. 2003;38:67–75. doi: 10.1016/s0168-8278(02)00328-8. [DOI] [PubMed] [Google Scholar]
- 2.Heydtmann M, Lalor PF, Eksteen JA, Hubscher SG, Briskin M, Adams DH. CXC chemokine ligand 16 promotes integrin-mediated adhesion of liver- infiltrating lymphocytes to cholangiocytes and hepatocytes within the inflamed human liver. J Immunol. 2005;174:1055–62. doi: 10.4049/jimmunol.174.2.1055. [DOI] [PubMed] [Google Scholar]
- 3.Sato T, Thorlacius H, Johnston B, et al. Role for CXCR6 in recruitment of activated CD8+ lymphocytes to inflamed liver. J Immunol. 2005;174:277–83. doi: 10.4049/jimmunol.174.1.277. [DOI] [PubMed] [Google Scholar]
- 4.Wang J, Holmes TH, Cheung R, Greenberg HB, He XS. Expression of chemokine receptors on intrahepatic and peripheral lymphocytes in chronic hepatitis C infection: its relationship to liver inflammation. J Infect Dis. 2004;190:989–97. doi: 10.1086/423283. [DOI] [PubMed] [Google Scholar]
- 5.Morgan AJ, Guillen C, Symon FA, et al. Expression of CXCR6 and its ligand CXCL16 in the lung in health and disease. Clin Exp Allergy. 2005;35:1572–80. doi: 10.1111/j.1365-2222.2005.02383.x. [DOI] [PubMed] [Google Scholar]
- 6.Nanki T, Shimaoka T, Hayashida K, Taniguchi K, Yonehara S, Miyasaka N. Pathogenic role of the CXCL16-CXCR6 pathway in rheumatoid arthritis. Arthritis Rheum. 2005;52:3004–14. doi: 10.1002/art.21301. [DOI] [PubMed] [Google Scholar]
- 7.Aglas F, Hermann J, Egger G. Abnormal directed migration of blood polymorphonuclear leukocytes in rheumatoid arthritis. Potential role in increased susceptibility to bacterial infections. Med Inflamm. 1998;7:19–23. doi: 10.1080/09629359891333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iking-Konert C, Ostendorf B, Sander O, et al. Transdifferentiation of polymorphonuclear neutrophils to dendritic-like cells at the site of inflammation in rheumatoid arthritis: evidence for activation by T cells. Ann Rheum Dis. 2005;64:1436–42. doi: 10.1136/ard.2004.034132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silberer H, Kuppers B, Mickisch O, et al. Fecal leukocyte proteins in inflammatory bowel disease and irritable bowel syndrome. Clin Lab. 2005;51:117–26. [PubMed] [Google Scholar]
- 10.di Carlo E, Iezzi M, Pannellini T, et al. Neutrophils in anti-cancer immunological strategies: old players in new games. J Hematother Stem Cell Res. 2001;10:739–48. doi: 10.1089/152581601317210836. [DOI] [PubMed] [Google Scholar]
- 11.Chin AC, Parkos CA. Neutrophil transepithelial migration and epithelial barrier function in IBD: potential targets for inhibiting neutrophil trafficking. Ann N Y Acad Sci. 2006;1072:276–87. doi: 10.1196/annals.1326.018. [DOI] [PubMed] [Google Scholar]
- 12.Heydtmann M, Adams DH. Understanding selective trafficking of lymphocyte subsets. Gut. 2002;50:150–2. doi: 10.1136/gut.50.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wagner JG, Driscoll KE, Roth RA. Inhibition of pulmonary neutrophil trafficking during endotoxemia is dependent on the stimulus for migration. Am J Respir Cell Mol Biol. 1999;20:769–76. doi: 10.1165/ajrcmb.20.4.3481. [DOI] [PubMed] [Google Scholar]
- 14.Sordi V, Malosio ML, Marchesi F, et al. Bone marrow mesenchymal stem cells express a restricted set of functionally active chemokine receptors capable of promoting migration to pancreatic islets. Blood. 2005;106:419–27. doi: 10.1182/blood-2004-09-3507. [DOI] [PubMed] [Google Scholar]
- 15.Tamamori Y, Sawada T, Nishihara T, et al. Granulocyte-colony stimulating factor enhances chimeric antibody Nd2 dependent cytotoxicity against pancreatic cancer mediated by polymorphonuclear neutrophils. Int J Oncol. 2002;21:649–54. [PubMed] [Google Scholar]
- 16.Wagner C, Kaksa A, Muller W, et al. Polymorphonuclear neutrophils in posttraumatic osteomyelitis: cells recovered from the inflamed site lack chemotactic activity but generate superoxides. Shock. 2004;22:108–15. doi: 10.1097/01.shk.0000132488.71875.15. [DOI] [PubMed] [Google Scholar]
- 17.Wente MN, Mayer C, Gaida MM, et al. CXCL14 expression and potential function in pancreatic cancer. Cancer Lett. 2008;259:209–17. doi: 10.1016/j.canlet.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 18.Wagner C, Kondella K, Bernschneider T, Heppert V, Wentzensen A, Hansch GM. Post-traumatic osteomyelitis: analysis of inflammatory cells recruited into the site of infection. Shock. 2003;20:503–10. doi: 10.1097/01.shk.0000093542.78705.e3. [DOI] [PubMed] [Google Scholar]
- 19.Wagner C, Hansch GM. Genetic deficiency of CD16, the low-affinity receptor for immunoglobulin G, has no impact on the functional capacity of polymorphonuclear neutrophils. Eur J Clin Invest. 2004;34:149–55. doi: 10.1111/j.1365-2362.2004.01298.x. [DOI] [PubMed] [Google Scholar]
- 20.Brenneis H, Schmidt A, Blaas-Mautner P, Worner I, Ludwig R, Hansch GM. Chemotaxis of polymorphonuclear neutrophils (PMN) in patients suffering from recurrent infection. Eur J Clin Invest. 1993;23:693–8. doi: 10.1111/j.1365-2362.1993.tb01288.x. [DOI] [PubMed] [Google Scholar]
- 21.Abel S, Hundhausen C, Mentlein R, et al. The transmembrane CXC-chemokine ligand 16 is induced by IFN-gamma and TNF-alpha and shed by the activity of the disintegrin-like metalloproteinase ADAM10. J Immunol. 2004;172:6362–72. doi: 10.4049/jimmunol.172.10.6362. [DOI] [PubMed] [Google Scholar]
- 22.Gough PJ, Garton KJ, Wille PT, Rychlewski M, Dempsey PJ, Raines EW. A disintegrin and metalloproteinase 10-mediated cleavage and shedding regulates the cell surface expression of CXC chemokine ligand 16. J Immunol. 2004;172:3678–85. doi: 10.4049/jimmunol.172.6.3678. [DOI] [PubMed] [Google Scholar]
- 23.Kayed H, Jiang X, Keleg S, et al. Regulation and functional role of the Runt- related transcription factor-2 in pancreatic cancer. Br J Cancer. 2007;97:1106–15. doi: 10.1038/sj.bjc.6603984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaida MM, Haag N, Mayer C, et al. CXCL16, CXCR6 and a distintegrin and matrix metalloproteinase (ADAM10) – an interplay leading to progression of pancreatic cancer? HPB (Oxford) 2008;10:215. Abstract. [Google Scholar]
- 25.Kobayashi SD, Voyich JM, Burlak C, DeLeo FR. Neutrophils in the innate immune response. Arch Immunol Ther Exp (Warsz) 2005;53:505–17. [PubMed] [Google Scholar]
- 26.Wagner C, Iking-Konert C, Hug F, et al. Cellular inflammatory response to persistent localized Staphylococcus aureus infection: phenotypical and functional characterization of polymorphonuclear neutrophils (PMN) Clin Exp Immunol. 2006;143:70–7. doi: 10.1111/j.1365-2249.2005.02963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruegg C. Leukocytes, inflammation, and angiogenesis in cancer: fatal attractions. J Leukoc Biol. 2006;80:682–4. doi: 10.1189/jlb.0606394. [DOI] [PubMed] [Google Scholar]
- 28.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 29.Nelson D, Ganss R. Tumor growth or regression: powered by inflammation. J Leukoc Biol. 2006;80:685–90. doi: 10.1189/jlb.1105646. [DOI] [PubMed] [Google Scholar]
- 30.Kim R, Emi M, Tanabe K. Cancer immunoediting from immune surveillance to immune escape. Immunology. 2007;121:1–14. doi: 10.1111/j.1365-2567.2007.02587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Esposito I, Menicagli M, Funel N, et al. Inflammatory cells contribute to the generation of an angiogenic phenotype in pancreatic ductal adenocarcinoma. J Clin Pathol. 2004;57:630–6. doi: 10.1136/jcp.2003.014498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garcea G, Dennison AR, Steward WP, Berry DP. Role of inflammation in pancreatic carcinogenesis and the implications for future therapy. Pancreatology. 2005;5:514–29. doi: 10.1159/000087493. [DOI] [PubMed] [Google Scholar]
- 33.Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354:610–21. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- 34.Murdoch C, Finn A. Chemokine receptors and their role in inflammation and infectious diseases. Blood. 2000;95:3032–43. [PubMed] [Google Scholar]
- 35.Murphy PM. Chemokines and the molecular basis of cancer metastasis. N Engl J Med. 2001;345:833–5. doi: 10.1056/NEJM200109133451113. [DOI] [PubMed] [Google Scholar]
- 36.Romagnani P, Lasagni L, Annunziato F, Serio M, Romagnani S. CXC chemokines: the regulatory link between inflammation and angiogenesis. Trends Immunol. 2004;25:201–9. doi: 10.1016/j.it.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 37.Strieter RM, Burdick MD, Gomperts BN, Belperio JA, Keane MP. CXC chemokines in angiogenesis. Cytokine Growth Factor Rev. 2005;16:593–609. doi: 10.1016/j.cytogfr.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 38.Koizumi K, Hojo S, Akashi T, Yasumoto K, Saiki I. Chemokine receptors in cancer metastasis and cancer cell-derived chemokines in host immune response. Cancer Sci. 2007;98:1652–8. doi: 10.1111/j.1349-7006.2007.00606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lenoir M, Djerdjouri B, Perianin A. Stroma cell-derived factor 1alpha mediates desensitization of human neutrophil respiratory burst in synovial fluid from rheumatoid arthritic patients. J Immunol. 2004;172:7136–43. doi: 10.4049/jimmunol.172.11.7136. [DOI] [PubMed] [Google Scholar]
- 40.Lu Y, Wang J, Xu Y, et al. CXCL16 functions as a novel chemotactic factor for prostate cancer cells in vitro. Mol Cancer Res. 2008;6:546–54. doi: 10.1158/1541-7786.MCR-07-0277. [DOI] [PubMed] [Google Scholar]
- 41.Agostini C, Cabrelle A, Calabrese F, et al. Role for CXCR6 and its ligand CXCL16 in the pathogenesis of T-cell alveolitis in sarcoidosis. Am J Respir Crit Care Med. 2005;172:1290–8. doi: 10.1164/rccm.200501-142OC. [DOI] [PubMed] [Google Scholar]
- 42.Garcia GE, Truong LD, Li P, et al. Inhibition of CXCL16 attenuates inflammatory and progressive phases of anti- glomerular basement membrane antibody-associated glomerulonephritis. Am J Pathol. 2007;170:1485–96. doi: 10.2353/ajpath.2007.060065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koprak S, Matheravidathu S, Springer M, Gould S, Dumont FJ. Down-regulation of cell surface CXCR6 expression during T cell activation is predominantly mediated by calcineurin. Cell Immunol. 2003;223:1–12. doi: 10.1016/s0008-8749(03)00130-8. [DOI] [PubMed] [Google Scholar]
- 44.Arraes SM, Freitas MS, da Silva SV, et al. Impaired neutrophil chemotaxis in sepsis associates with GRK expression and inhibition of actin assembly and tyrosine phosphorylation. Blood. 2006;108:2906–13. doi: 10.1182/blood-2006-05-024638. [DOI] [PubMed] [Google Scholar]


