Abstract
Variance in expression of receptors for immunoglobulin G (FcγRs), complement (CR3) and lipopolysaccharide (mCD14) on polymorphonuclear neutrophils (PMNs) and monocytes might affect susceptibility for infection with certain pathogens in periodontitis, a chronic infectious disease of tooth-supportive tissues. Levels of FcγRI, IIa, III, CR3 and mCD14 on PMNs and monocytes were measured in 19 periodontitis patients and 18 healthy controls. Subgingival infection with Aggregatibacter actinomycetemcomitans (Aa) and Porphyromonas gingivalis (Pg) was determined. Activation of PMNs and monocytes in response to stimulation with Aa and Pg was assessed by means of change in mCD14 expression. Periodontitis is associated with an enrichment of the FcγRIII+ monocytes (P = 0·015) with concomitant low mCD14 (P = 0·001). Unadjusted data showed that the subjects culture-positive for Aa (Aa+) had significantly lower expression of monocytic FcγRI (P = 0·005) and FcγRIIa (P = 0·015) than Pg+ subjects. The FcγRI was still lower on monocytes from Aa+ subjects after adjusting for the background factors (P = 0·037). PMNs from Aa+ subjects responded in a hyper-reactive manner, in particular when stimulated with Aa (P = 0·011). Lower FcγRs expression by monocytes is related to a higher susceptibility of a subject to become infected with Aa. The higher proportion of FcγRIII+ monocytes may be involved in the chronicity of this condition. Hyper-reactive PMNs in Aa+ subjects may contribute to accelerated breakdown of tooth-supportive tissues.
Keywords: A. actinomycetemcomitans, FcγRs, mCD14, P. gingivalis, periodontitis
Introduction
Periodontitis is a chronic infectious disease of the supportive tissues of the teeth characterized by gradual loss of periodontal attachment and alveolar bone. Approximately 10% of the population suffers from the severe form and, if untreated, it may result in tooth loss. The major pathogens associated with periodontitis are Aggregatibacter actinomycetemcomitans (Aa) and Porphyromonas gingivalis (Pg) [1,2]. In conjunction with the bacterial challenge, an important role in the onset and progression of periodontitis is played by the host immune response [3]. A phagocytic response represents the first barrier to the penetration of bacteria into periodontal tissue [4]. However, in addition to their defensive role, the phagocytes [polymorphonuclear neutrophils (PMNs) and monocytes] may also be responsible for collateral damage to the periodontal tissues; thus the host defence may be at the cost of periodontal attachment and alveolar bone [5,6].
One aspect of the host-derived breakdown of periodontal tissues is related to a hyper-reactive trait of PMNs in response to immunoglobulin G (IgG)-opsonized antigens [7–9]. The nature of this hyper-reactivity might be related either to the genetically-determined increased binding capacity of the receptors for IgG (FcγR) on PMNs or to the increased expression levels of Fcγ-receptors.
There are several types of FcγRs identified on phagocytes: FcγRI (CD64), FcγRIIa (CD32) and FcγRIII (CD16) [10]. Previous studies have shown that single-nucleotide polymorphisms, affecting the IgG-binding domain of the genes of FcγRIIa and IIIb, have functional consequences. PMNs from periodontitis patients bearing the more reactive genotype (i.e. FcγRIIa-131H/H and FcγRIIIb-NA1/NA1) show hyper-reactivity in response to stimulation with Aa or Pg[9,11]. Furthermore, periodontitis patients with FcγRIIa-131H/H genotype have more severe periodontal breakdown than the patients with FcγRIIa-131H/R or 131R/R genotype [9,12–14]. There are several studies that reported the expression of FcγRs on peripheral PMNs in periodontitis [15,16], but a difference of expression levels between patients and controls could not be demonstrated.
In contrast to the PMN, little data exist on the expression of FcγR by monocytes in periodontitis. Nagasawa et al.[17] described an increased percentage of FcγRIII+ monocytes in chronic periodontitis, but did not analyse expression of other FcγRs on monocytes. Percentages of FcγRIII+ monocytes are also increased in other chronic inflammatory conditions, such as rheumatoid arthritis [18]. Moreover, the monocytes and macrophages of rheumatoid arthritis patients express increased levels of FcγRI and FcγRIIa. It is conceivable that similar changes in the FcγR expression would occur in periodontitis, as periodontitis and rheumatoid arthritis appear to share many pathologic features [19].
In addition to the FcγRs, there are other classes of immune receptors important for the antibacterial functions of PMNs and monocytes. CD14 is a lipopolysaccharide (LPS) co-receptor and involved in the recognition of gram-negative bacteria and clearance of circulating LPS. Membrane-bound CD14 (mCD14) is mainly expressed on mature monocytes, macrophages and activated neutrophils [20]. The β2-integrins are heterodimeric receptors consisting of a common β-subunit (CD18) associated with a unique α-subunit (CD11a, b, c, or d). These receptors are involved in infection and inflammation by mediating cell-cell, cell-extracellular matrix and cell-pathogen interactions [21].
The first aim of the present study was to assess the expression of different FcγRs by PMNs and monocytes from untreated periodontitis patients and healthy controls. In addition we analysed the expression levels of two other molecules important for phagocyte-bacterial interactions: the complement receptor CR3 (CD11b/CD18) and membrane-bound CD14. The second aim of the study was to explore the activation of PMNs and monocytes in response to stimulation with two important periodontal pathogens, Aa and Pg.
Materials and methods
Study population
We recruited 19 periodontitis patients who were referred to the Department of Periodontology of the Academic Centre for Dentistry Amsterdam (ACTA) for diagnosis and treatment of periodontitis. All patients had ≥ eight teeth with radiographic bone loss beyond 30% of the root length. Age-, gender-, race- and smoking status-matched controls were selected among subjects registered for restorative dental procedures or who visited the dental school for regular dental check-ups. Control subjects were selected if they were not missing more than one tooth per quadrant (3rd molar excluded) and if they showed on dental bitewing radiographs ≤ 1 year old a distance between the cemento-enamel junction and the alveolar bone crest of ≤ 3 mm. Besides their periodontal condition, all participants were otherwise healthy and had not taken any antibiotics during the last 6 months and no medication that could influence the immune response during the last 2 weeks.
All patients were initially screened in the departmental clinic and had agreed to accept the proposed treatment plan. All samples were taken before the start of the periodontal therapy. All subjects were informed verbally and in writing about the purposes of the study and signed an informed consent. The Medical Ethical Committee of the Academic Medical Center of the University of Amsterdam approved the study.
Blood sampling
All participants were asked to fast for at least 12 h before the clinical visit. Fasting blood samples were obtained between 8·30 and 10·30 AM by venipuncture in the antecubital fossa. The venous blood was collected into evacuated tubes (BD Vacutainer System, Plymouth, UK) with anti-coagulant (one 5 ml EDTA tube for automated leukocyte counting and leukocyte differential count and one 5 ml Na-citrate tube for flow cytometric analysis).
Bacterial sampling
Subgingival microbiological samples were taken from all subjects to determine the presence of the periodontal pathogens Aa and Pg. Samples were taken after venous blood collection to avoid possible phagocyte activation. Sampling, laboratory procedures, and identification of Aa and Pg were performed as described before [2].
Flow cytometric analysis
For cell surface staining, citrated whole blood (40 µl) was incubated for 30 min on ice with saturated concentrations (1–10 µg/ml) of the fluorochrome-conjugated antibodies. CD14-PE (8G3), CD16-FITC (5D2) and CD11b-FITC (B2) were purchased from Sanquin (Amsterdam, The Netherlands). CD32-FITC (AT10) and CD64-FITC (10·1) were obtained from Serotec (Marseille, France). Appropriate isotype controls were used: PE-IgG2a, FITC-IgG1, FITC-IgG2a from Sanquin and FITC-IgM from Beckman-Coulter (Fullerton, CA, USA). After incubation, 3 ml of ice-cold NH4Cl buffer (155 mM NH4Cl, 10 mM KHCO3, 0·1 mM EDTA at pH 7·4) was added to lyse the erythrocytes, samples placed on ice for 15 min and then they were centrifuged at 500 g, 4°C. The cell pellet was washed two times (500 g, 4°C) in HEPES-buffer (137 mM NaCl, 2·7 mM KCl, 1·0 mM MgCl2, 5·6 mM glucose, 20 mM HEPES, 1 mg/ml bovine serum albumin, 3·3 mM NaH2PO4, pH 7·4) and resuspended in HEPES-buffer containing 0·3% formaldehyde (final concentration). Flow cytometric analysis was performed within 2 h of sample preparation. Expression of CD11b, CD14, CD16, CD32 and CD64 is indicated as the geometric mean fluorescence intensity (MFI).
Flow cytometric analysis was performed in a FACScan flow cytometer with CellQuest software (Becton Dickinson, San Jose, CA, USA). The PMNs and the monocytes were identified based on their forward and sideward scatter light patterns (Fig. 1a). Based on the binding of the corresponding isotype control antibody, a fluorescence threshold was set (≤ 1% of cells being positive for the isotype control antibody) [22]; monocytes were considered to be FcγRIII+ when fluorescence was above this threshold (Fig. 1b and c). The fraction (%) of FcγRIII+ was calculated from the total monocyte population.
Fig. 1.
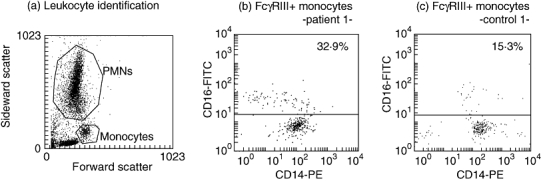
(a) Identification of PMNs and monocytes by whole blood flow cytometry. (b, c) Representative dot plots of monocytes in whole blood from (b) one periodontitis patient and (c) one control subject. The fraction of FcγRIII+ monocytes (above fluorescence threshold on the y-axis) from the total monocyte population was 32·9% in the periodontitis patient and 15·3% in the control subject.
Bacterial stimulation assays
Brain-heart infusion broth enriched with hemin (5 mg/l) and menadione (1 mg/l) was used for the bacterial culturing. Aa Y4 was grown aerobically for 18 h at 37°C in humidified 5% CO2, whereas Pg W83 was grown anaerobically (80% N2, 10% H2, 10% CO2) for 48 h at 37°C. The bacterial suspensions were washed, reduced to an optical density of 1 at 600 nm in HEPES buffer, and stored in aliquots at −20°C.
Fresh citrated blood (25 µl) was added to a mix containing 55 µl of Aa- or Pg- suspension, 4 µg/ml CD14-PE. The mix was incubated for 60 min at room temperature. In the control tubes, whole blood was incubated with HEPES containing no bacteria. After the incubation period, 1 ml of lysis solution was added to the samples, thoroughly mixed and placed on ice for 15 min. The cells were mildly fixed by addition of 1 ml of HEPES containing 0·3% PFA.
The same FACS-settings were used to determine the expression of mCD14 on PMNs and monocytes (see above: Flow cytometric analysis). As the mCD14 baseline values were different among subjects within our study group, the effect of stimulation was expressed as the percentage change of mCD14-MFI (the % change in MFI = (the MFI of stimulated cells – MFI of untreated cells)/MFI of untreated cells × 100); the value of untreated cells was taken as 0% change [23].
Statistical analyses
Data analyses were performed with the SPSS package, version 14·0 (SPSS Inc., Chicago, IL, USA). Means ± standard deviations and frequency distributions were calculated. Prior to the analyses normal distributions of data were confirmed by Kolmogorov-Smirnov goodness-of-fit test. Differences in background characteristics between patients and controls were statistically analysed with the Student's t-test or the χ2-test (or Fisher exact test), where appropriate. Differences in the receptor expression levels between patients and controls were compared using t-tests. Furthermore, differences in the analysed parameters were explored in a general linear model (GLM) using periodontal condition and colonization with Aa or Pg as fixed factors, and age, gender, race and smoking status as co-variates. Colonization with Aa and Pg appeared to significantly influence the expression of some receptors. Therefore the study population was grouped according to the subgingival presence of Aa or Pg (i.e. Pg-Aa- (n = 18), Aa+ (n = 9), Pg+ (n = 7) and Pg+Aa+ (n = 3) donors) and receptor expression patterns and cell activation after bacterial stimulation were compared in a one-way anova with the Tukey-Kramer post-test for uneven groups (after confirming equal variances between subgroups using the Levene's test). A Pearson's correlation coefficient and a partial correlation coefficient correcting for periodontal condition, colonization with Aa or Pg, age, gender, race, smoking status, between mCD14 on monocytes and % FcγRIII+ monocytes were calculated. The effects of Aa- versus Pg- stimulation were compared with paired t-tests; P-values < 0·05 were considered statistically significant.
Results
Characteristics of the study population (Table 1)
Table 1.
Summary of the characteristics of the study population.
| Parameter† | Control n = 18 | Periodontitis n = 19 |
|---|---|---|
| Age | 40·8 ± 10·1 | 42·0 ± 9·9 |
| Gender | ||
| Male | 6 (33%) | 7 (37%) |
| Female | 12 (67%) | 12 (63%) |
| Ethnicity | ||
| Non-Caucasian | 4 (22%) | 4 (21%) |
| Caucasian | 14 (78%) | 15 (79%) |
| Smoking | ||
| Non-smoker | 13 (72%) | 13 (68%) |
| Smoker | 5 (28%) | 6 (32%) |
| C-reactive protein (mg/l) | 2·2 ± 3·9 | 3·6 ± 5·1 |
| Leukocytes (×109/l) | 5·8 ± 1·6 | 6·5 ± 2·1 |
| Neutrophils (×109/l) | 3·3 ± 1·1 | 3·8 ± 1·5 |
| Monocytes (×109/l) | 0·4 ± 0·1 | 0·5 ± 0·2 |
| Lymphocytes (×109/l) | 2·0 ± 0·6 | 2·1 ± 0·5 |
| Subgingival colonization* | ||
| Aa-Pg- | 14 (78%) | 4 (21%) |
| Aa+Pg- | 3 (17%) | 6 (32%) |
| Aa-Pg+ | 1 (5%) | 6 (32%) |
| Aa+Pg+ | 0 | 3 (15%) |
| Number of teeth | ||
| Total | 28·7 ± 1·6 | 27·2 ± 2·5 |
| With bone loss | ||
| ≥ 30% | 0 | 17·1 ± 5·1 |
| ≥ 50% | 0 | 6·7 ± 4·2 |
P = 0·004 (χ2-test).
Values are means ± standard deviations or numbers (%) of subjects. Aa, A. actinomycetemcomitans; Pg, P. gingivalis.
On the basis of the radiographic bone loss criterion (less than 3 mm between the alveolar bone crest and the cemento-enamel junction on all teeth) one control subject was excluded from the analysis. The culturing results revealed that 12 subjects were Aa+, of whom nine were periodontitis patients and three controls. Nine patients were Pg+, whereas only one control tested positive for Pg (P = 0·004, Table 1). The Aa+ subjects were younger (mean age 34·8 ± 11·2) than the Pg+ individuals (mean 48·3 ± 6·4, P = 0·033).
Receptors expression by PMNs and monocytes (Figs 2 and 3, Tables 2 and 3)
Fig. 2.
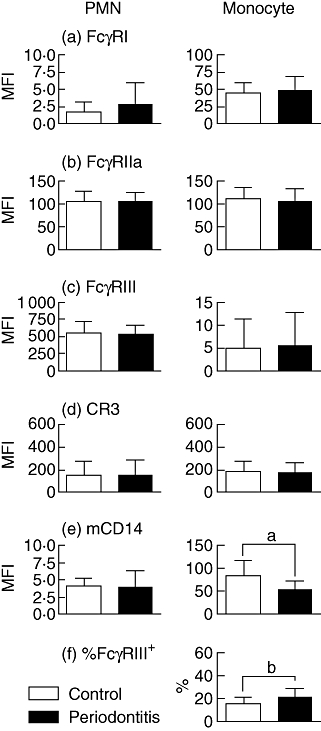
PMN and monocyte expression of (a) FcγRI, (b) FcγRIIa, (c) FcγRIII, (d) CR3, (e) mCD14 and (f) %s of FcγRIII+ monocytes for controls (open bars, n = 18) and periodontitis patients (closed bars, n = 19). Values are means ± standard deviation. aPatients have lower monocytic mCD14 than controls (P = 0·001). bPatients have higher % of FcγRIII+ monocytes (P = 0·015).
Fig. 3.
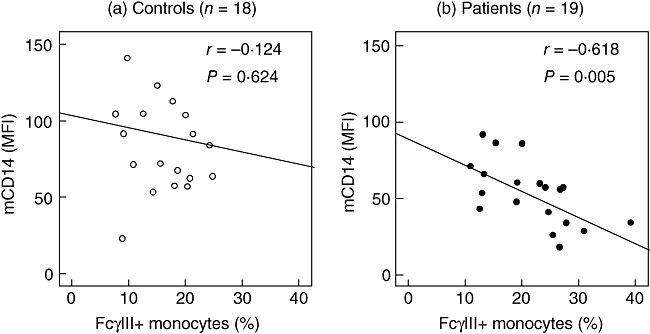
mCD14 and % FcγRIII+ monocytes in (a) controls (open symbols) and (b) periodontitis patients (closed symbols). Each symbol represents one subject. There was an overall correlation between mCD14 and % FcγRIII+ monocytes (Pearson's correlation coefficient r = −0·473, P = 0·003), mainly because of a strong correlation in patients (r = −0·618, P = 0·005), while controls showed no correlation (r = −0·124, P = 0·624).
Table 2.
Receptor expression by PMNs.
| PMNs† | Control n = 18 | Periodontitis n = 19 | Padj | Aa-Pg-n = 18 | Aa+n = 9 | Pg+n = 7 | Aa+Pg+n = 3 | Padj |
|---|---|---|---|---|---|---|---|---|
| FcγRI | 2·4 (0·3–4·4) | 2·5 (1·1–3·8) | 0·960 | 2·1 (0·6–3·6) | 2·1 (0–4·2) | 4·9 (2·4–7·3) | 0·7 (0–4·4) | 0·157 |
| FcγRIIa | 116·9 (103·1–130·7) | 105·4 (96·2–114·6) | 0·159 | 102·7 (92·4–112·9) | 108·1 (93·8–122·4) | 115·6 (99·2–132·0) | 118·2 (93·1–143·2) | 0·538 |
| FcγRIII | 508·9 (398·2–619·6) | 522·9 (449·1–596·8) | 0·828 | 578·7 (496·2–661·2) | 505·9 (391·1–620·6) | 502·8 (371·5–634·2) | 476·3 (275·7–677·0) | 0·668 |
| CR3 | 96·9 (2·9–191·0) | 153·1 (90·4–215·8) | 0·308 | 195·9 (125·8–266·0) | 88·8 (0–186·3) | 147·3 (35·76–258·9) | 68·0 (0–238·4) | 0·352 |
| mCD14 | 3·1 (1·8–4·4) | 3·9 (3·0–4·8) | 0·301 | 4·7 (3·7–5·7) | 4·1 (2·7–5·5) | 3·5 (1·9–5·1) | 1·8 (0–4·2) | 0·204 |
Values are adjusted means (confidence intervals) for MFIs and corresponding adjusted P-values obtained from a general linear model using periodontal condition and colonization with Aa or Pg as fixed factors and age, gender, race and smoking status as covariates. Aa, A. actinomycetemcomitans; Pg, P. gingivalis.
Table 3.
Receptor expression by monocytes.
| Monocytes† | Control n = 18 | Periodontitis n = 19 | Padj | Aa-Pg-n = 18 | Aa+n = 9 | Pg+n = 7 | Aa+Pg+n = 3 | Padj |
|---|---|---|---|---|---|---|---|---|
| FcγRI | 42·5 (30·6–54·3) | 47·8 (39·9–55·7) | 0·441 | 50·9 (42·0–59·7) | 32·3‡ (20·0–44·7) | 59·6 (45·5–73·7) | 37·7 (16·2–59·2) | 0·037 |
| FcγRIIa | 113·7 (96·0–131·5) | 107·0 (95·2–118·8) | 0·515 | 107·6 (94·4–120·8) | 91·5 (73·4–109·9) | 126·8 (105·7–147·8) | 115·6 (83·4–147·7) | 0·105 |
| FcγRIII | 2·4 (0–7·4) | 5·6 (2·2–8·9) | 0·285 | 7·6 (3·8–11·3) | 1·3 (0–6·5) | 6·4 (0·4–12·3) | 0·7 (0–9·8) | 0·269 |
| CR3 | 136·3 (69·8–202·7) | 175·9 (131·6–220·2) | 0·309 | 216·8 (167·3–266·3) | 118·3 (49·4–187·1) | 166·9 (88·1–245·8) | 122·3 (1·9–242·7) | 0·177 |
| mCD14 | 83·9 (62·6–105·2) | 52·1 (37·9–66·4) | 0·015 | 66·2 (50·3–82·0) | 79·5 (57·4–101·6) | 62·9 (37·6–88·2) | 63·6 (25·0–102·2) | 0·707 |
| % FcγRIII+§ | 16·3 (11·4–21·2) | 21·7 (18·4–25·0) | 0·068 | 19·4 (15·7–23·0) | 15·7 (10·6–20·8) | 21·8 (15·9–27·6) | 19·2 (10·3–28·2) | 0·470 |
Values are adjusted means (confidence intervals) for MFIs or proportion of FcγRIII+ cells and corresponding Padj-values obtained from a general linear model using periodontal condition and colonization with Aa or Pg as fixed factors and age, gender, race and smoking status as covariates.
Aa+ subjects had lower levels of monocytic FcγRI than Aa-Pg- (Padj = 0·026) and Pg+ subjects (Padj = 0·007) in post-hoc testing.
% FcγRIII+ is inversely correlated with mCD14 on monocytes; partial correlation coefficient (corrected for periodontal condition, colonization with Aa/Pg, age, gender, race, smoking status) r = −0·382, P = 0·034. Aa, A. actinomycetemcomitans; Pg, P. gingivalis.
The expression of all tested receptors by PMNs was comparable in patients and controls (Fig. 2a–e). Periodontitis patients had a lower monocytic mCD14 expression and a higher percentage of FcγRIII+ monocytes than controls (Fig. 2e and f). Overall, the level of mCD14 was inversely correlated with the percentage of FcγRIII+ monocytes (r = −0·473, P = 0·003). This correlation was not found in control subjects (r = −0·124, P = 0·624; Fig. 3a), but was strong within periodontitis patients (r = −0·618, P = 0·005; Fig. 3b).
In Tables 2 and 3 we present adjusted data from a GLM, for which periodontal condition and colonization with Aa/Pg were entered as fixed factors and age, gender, race and smoking status as co-variates. Neither colonization with Aa/Pg nor other potential confounding factors were associated with expression levels by the PMNs of any of the receptors tested (Table 2). Further, in the GLM we observed that a lower expression of FcγRI by monocytes was present in the Aa+ subjects (Table 3). After correcting for periodontal condition, colonization with Aa/Pg, age, gender, race and smoking status, the correlation between mCD14 and % FcγRIII+ monocytes was still apparent (radj = −0·382, Padj = 0·034).
Receptor expression on PMNs and monocytes in Aa-Pg-, Aa+, Pg+ and Aa+Pg+ subjects (4)
Fig. 4.

PMN and monocyte expression of (a) FcγRI, (b) FcγRIIa, (c) FcγRIII, (d) CR3 and (e) mCD14 for Aa-Pg- subjects (open bars, n = 18), Aa+ subjects (bright grey bars, n = 9), Pg+ subjects (dark grey bars, n = 7), and Aa+Pg+ subjects (closed bars, n = 3). Values are means ± standard deviation; Aa (A. actinomycetemcomitans), Pg (P. gingivalis). aOn panel (a), monocytes: P = 0·005 for the overall anova; Aa+ subjects have lower monocytic FcγRI than Pg+ (post hoc P = 0·004). bOn panel (b), monocytes: P = 0·015 for the overall anova; Aa+ subjects have lower monocytic FcγRIIa than Pg+ (post hoc P = 0·009).
The significance of colonization with Aa or Pg for some of the receptor expression levels prompted us to further explore expression of receptors in subgroups of subjects (patients and controls) based on their colonization with Aa or Pg.
Receptor expression levels on PMNs did not significantly differ between Aa-Pg-, Aa+, Pg+ and Aa+Pg+ subjects (Fig. 4a–e). On monocytes we observed that FcγRI and FcγRIIa were present at lower levels in Aa+ subjects than in the other three subgroups (overall anovaP = 0·005 and P = 0·015 respectively). In particular, the FcγRI and FcγRIIa expression levels on monocytes from Aa+ subjects were lower than on monocytes from Pg+ subjects (P = 0·004 and P = 0·009 respectively; Fig. 4a and b).
Expression levels of FcγRIII, CR3 and mCD14 were not significantly different between the monocytes from Aa-Pg-, Aa+, Pg+ and Aa+Pg+ donors (Fig. 4c–e), even though the same trend of lower monocytic expression in Aa+ subjects was visible for FcγRIII and CR3 (overall anovaP = 0·168 and P = 0·149 respectively). The expression profile in the subjects colonized with both Aa and Pg (Aa+Pg+ subjects) seemed to be the result of a combined effect of the two bacterial species; the levels of FcγRI, FcγRIIa, FcγRIII and CR3 resembled the levels of Aa+ subjects, whereas the mCD14 in Aa+Pg+ subjects seemed more similar to the Pg+ subjects.
The PMN and monocyte activation in response to Aa or Pg (5)
Fig. 5.
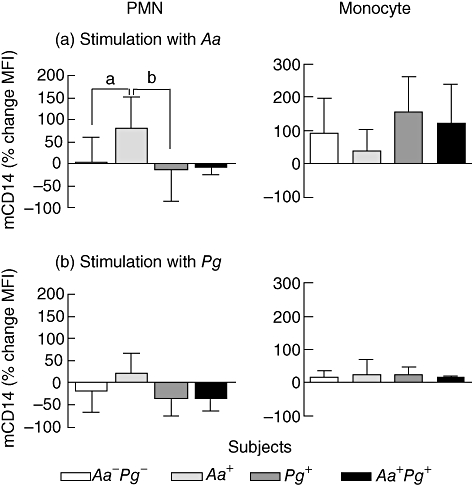
Percentage change of mCD14 expression on PMNs and monocytes in response to (a) A. actinomycetemcomitans (Aa) or (b) P. gingivalis (Pg). Whole blood was incubated for 60 min with Aa or Pg. Data are means ± standard deviation for Aa-Pg- subjects (open bars, n = 18), Aa+ subjects (bright grey bars, n = 9), Pg+ subjects (dark grey bars, n = 7), and Aa+Pg+ subjects (closed bars, n = 3). a,bOn panel (a), PMN activation: P = 0·011 for the overall anova; Aa+ subjects have higher levels of mCD14 after Aa stimulation than Aa-Pg- subjects (post hoc P = 0·021) and Pg+ subjects (post hoc P = 0·022).
The above results indicated that the subjects having different periodontal infection patterns show variability in the numbers of phagocytic receptors. The question arose of whether Aa and Pg can activate PMNs and monocytes differentially. Thus, we tested the reactivity of PMNs and monocytes from all donors to these periodontal pathogens. The change in mCD14 expression was used as a measure of cell activation. Both stimulation with Aa and Pg resulted in activation of PMNs and monocytes. Important to note was that the % change in mCD14-MFI on PMNs and monocytes after Pg or Aa stimulation did not differ significantly between patients and controls (data not shown).
The % change in mCD14 expression by PMNs induced by Aa was of a higher magnitude than after Pg stimulation (P < 0·0001). PMNs from Aa-culture positive subjects, showed an enhancement of mCD14 after Aa or Pg stimulation, in contrast to PMNs from Pg-Aa-, Pg+ and Aa+Pg+ subjects, who showed down-regulation or less up-regulation of mCD14 (P = 0·011 and P = 0·053 respectively; Fig. 5a and b). In particular, Aa stimulation resulted in increased mCD14 on PMNs from Aa+ subjects, thus higher than on PMNs from Pg+ and Pg-Aa- subjects (P = 0·022 and P = 0·021 respectively).
On monocytes, Aa induced also a higher % change in mCD14 than Pg (P < 0·0001). The activation of monocytes by Aa was strong, as evidenced by the up-regulation of mCD14 up to 200%, whereas Pg induced hardly any increase in mCD14 on monocytes (<25%). Monocytes from Pg-Aa-, Aa+, Pg+ or Aa+Pg+ subjects had comparable response to Aa and Pg (Fig. 5a and b).
Discussion
This study was undertaken to investigate expression patterns of some major receptors by phagocytic cells in periodontitis and health to further elucidate susceptibility, infection patterns and biological pathways in this inflammatory condition. On PMNs we found no differences between patients and controls in the expression patterns of the tested receptors. On monocytes we found also a comparable expression of FcγRI, II and CR3 in patients and controls. However, an intriguing finding of this study is the heterogeneity of receptor expression levels between individuals. In the GLM, bacterial infection patterns appeared as a major determinant for expression levels by monocytes. When we subdivided the study population into Pg-Aa-, Aa+, Pg+ and Aa+Pg+ subjects, the expression of FcγRI and IIa by monocytes was significantly lower in Aa+ subjects than in Pg+subjects (Fig. 4); after correcting for periodontal condition and potential confounding factors, the expression of FcγRI monocytes was still decreased in Aa+ subjects (Table 3).
One possible explanation for the lower levels of FcγRs on monocytes from Aa+ subjects might be due to genotypic differences, possibly making these individuals more susceptible to become infected with Aa. Similarly, genetically-defined deficiencies in various components of the innate immune system (mannose-binding lectin, vitamin D receptor, mannose-associated serine-protease-2, Toll-like receptors, etc.) have been associated with a greater risk of infection with Mycobacterium tuberculosis, meningococci or gram-negative bacteria [24–28]. In periodontitis it has been shown that polymorphisms in the FcγRs and IL-6 genes are associated with increased odds of detecting Aa, Pg and Tannerella forsythia after adjustment for age, ethnicity, smoking and periodontitis extent [29]. However, in our study neither the FcγRIIa 131H+ (131 H/R or H/H) nor the CD14 − 260T+ (−260 C/T or T/T) genotypes showed increased odds of detecting Aa or Pg in subgingival plaque samples (data not shown). Alternatively, the lower expression of FcγRs on monocytes in Aa+ subjects might be a consequence of the infection with Aa. In order to elucidate this, future studies are needed evaluating the effects of therapeutic elimination of Aa from the subgingival flora on the expression of FcγRs. In line with this possibility there are studies demonstrating the effects of medication on FcγRs expression levels in rheumatoid arthritis. Expression levels of FcγRs with cell activation potential on peripheral monocytes in patients suffering from rheumatoid arthritis were increased before therapy in comparison with healthy subjects [30–33]. The levels of FcγRs are changed to ‘healthy’ levels because of treatment [33–35]. This change in expression levels of FcγRs correlates with clinical improvement, thus restoring expression levels of FcγRs can have beneficial effects. Similarly, it is conceivable that periodontal therapy may have an effect on expression levels of FcγRs.
Normally, the majority of monocytes (90%) do not express FcγRIII and show high levels of mCD14 [36]. However, we found an increased % of FcγRIII+ monocytes and a lower level of mCD14 on monocytes from periodontitis patients compared with healthy controls (Fig. 2). These results are in line with previous studies showing an increased % of FcγRIII+ monocytes in chronic periodontitis and lower mCD14 on peripheral monocytes from early-onset periodontitis patients [17,37]. Furthermore, we demonstrated a correlation between the mCD14 on monocytes and the % of FcγRIII+ monocytes, suggesting that periodontal infection affects the monocyte phenotype, leading to differentiation of the CD14lowFcγRIII+ cells [36]. The CD14lowFcγRIII+ monocytes account for about 10% of monocytes in healthy adults and are expanding up to 40% in inflammatory conditions such as rheumatoid arthritis [18,31], sepsis [38] or Kawasaki disease [39]. In our study mCD14 was lowest on monocytes from Pg+ subjects (51·8 ± 13·8 MFI, Fig. 4e; adjusted mean 62·9 MFI, Table 3) and not significantly different to the overall value noted for the whole periodontitis patient group (53·1 ± 20·9 MFI, Fig. 2e; adjusted mean 52·1 MFI, Table 3). This suggests that individuals infected with Pg show the most marked changes in the monocyte phenotype and they account for the difference in mCD14 expression noted between patients and controls.
The association between Pg and a lower mCD14 expression on monocytes is supported by in vitro findings where Pg LPS was able to induce maturation of CD14lowFcγRIII+ dendritic cells [40]. Compared with the CD14bright-monocytes, the CD14lowFcγRIII+ monocyte subset can differentiate into dendritic cells that produce lower amounts of IL-1β, IL-6, IL-12, TNF-α and IL-8, but have higher phagocytic and oxidative activity [41]. These dendritic cells are capable of antigen presentation, but fail to efficiently stimulate T-cells, possibly because of lack of co-stimulatory molecules [40]. These characteristics of the dendritic cells originating from the CD14lowFcγRIII+ monocytes are possibly contributing to the perpetuation of chronic inflammation in periodontitis, as they might be unable to efficiently coordinate the antibacterial defence in the inflamed periodontium. We hypothesize that during periodontal infection frequent bacteraemic episodes, especially of Pg origin, lead to the early selection of the CD14lowFcγRIII+ precursors of dendritic cells, which are ultimately unable to clear the periodontal infection.
The PMN receptor expression was essentially not different between patients and controls and a possible relation to the infection pattern was not found. Thus, in contrast to monocytes for which we suggest a role in the susceptibility to certain infection patterns, the PMNs may be solely involved in bacterial clearance. It is commonly accepted that during their antibacterial functions PMNs induce collateral damage in inflamed periodontal tissues, which is largely attributable to the production of proteolytic enzymes (i.e. matrix-metalloproteinases, elastase) and reactive oxygen species [42–45]. In particular, this process is relevant, because hyper-reactive PMNs in periodontitis have been demonstrated by several research groups and several mechanisms have been proposed [7–9,46]. The PMNs from Aa+ subjects showed a stronger response to both Pg and Aa (measured by the change in mCD14), further providing evidence for a hyper-reactive trait of the PMNs from Aa-infected subjects, possibly aggravating the periodontal inflammatory reactions in these individuals [7–9]. This hypothesis may be strengthened by the observations that Aa induced stronger PMN activation than Pg (Fig. 5a and b). Aa might be in general a stronger stimulator of phagocytes than Pg[47–50]. Aa LPS is inducing higher amounts of IL-1β, TNF-α and IL-8 in PMNs than Pg LPS [51], evoking a more acute, E. coli-like inflammatory immune response. This might help to explain the severe and relative early onset of periodontal breakdown associated with Aa infection, especially in patients with aggressive periodontitis [52]. An interesting aspect of the periodontal pathogen Pg is its capability to produce bacterial cysteine proteinases (gingipains) that are able to proteolyse human monocyte mCD14 [53,54]. However, protease inhibitors present in serum inhibit the gingipain activity [54], because in our bacterial stimulation assays Aa and Pg were similarly able to induce mCD14 up-regulation on monocytes, although of different amplitude.
In conclusion, we suggest that receptor expression patterns by monocytes may be related to the susceptibility of a subject to become infected with certain periodontal pathogens. The enrichment of the FcγRIII+ monocytes in periodontitis, in particular in patients that are culture-positive for Pg, may result in formation of a dendritic cell type that can stimulate T cells less efficiently. As monocytes and their progeny, the dendritic cells, are expected to orchestrate the immune response in periodontitis, the FcγRIII+ monocytes may be involved in the chronicity of the infection. PMNs from Aa-culture positive subjects respond in a hyper-reactive fashion to the infection, and in particular seem to get strongly activated when stimulated with Aa. In this way PMNs may contribute to advanced breakdown of tooth-supportive tissues through an enhanced release of a variety of proteolytic enzymes and reactive oxygen species.
Acknowledgments
This study was supported by the Netherlands Institute for Dental Sciences (IOT), the Department of Periodontology, ACTA and Philips Oral Healthcare EMEA.
We thank Dimitris Papapanagiotou and Denise Duijsters for their help in recruiting the participants of this study.
References
- 1.Slots J. Update on Actinobacillus Actinomycetemcomitans and Porphyromonas gingivalis in human periodontal disease. J Int Acad Periodontol. 1999;1:121–6. [PubMed] [Google Scholar]
- 2.van Winkelhoff AJ, Loos BG, van der Reijden WA, van der Velden U. Porphyromonas gingivalis, Bacteroides forsythus and other putative periodontal pathogens in subjects with and without periodontal destruction. J Clin Periodontol. 2002;29:1023–8. doi: 10.1034/j.1600-051x.2002.291107.x. [DOI] [PubMed] [Google Scholar]
- 3.Ebersole JL, Taubman MA, Smith DJ, Frey DE, Haffajee AD, Socransky SS. Human serum antibody responses to oral microorganisms. IV. Correlation with homologous infection. Oral Microbiol Immunol. 1987;2:53–9. doi: 10.1111/j.1399-302x.1987.tb00290.x. [DOI] [PubMed] [Google Scholar]
- 4.Van Dyke TE, Levine MJ, Genco RJ. Neutrophil function and oral disease. J Oral Pathol. 1985;14:95–120. doi: 10.1111/j.1600-0714.1985.tb00474.x. [DOI] [PubMed] [Google Scholar]
- 5.Page RC, Schroeder HE. Pathogenesis of inflammatory periodontal disease. A summary of current work. Lab Invest. 1976;34:235–49. [PubMed] [Google Scholar]
- 6.Madianos PN, Bobetsis YA, Kinane DF. Generation of inflammatory stimuli: how bacteria set up inflammatory responses in the gingiva. J Clin Periodontol. 2005;32(Suppl 6):57–71. doi: 10.1111/j.1600-051X.2005.00821.x. [DOI] [PubMed] [Google Scholar]
- 7.Fredriksson M, Gustafsson A, Asman B, Bergstrom K. Hyper-reactive peripheral neutrophils in adult periodontitis: generation of chemiluminescence and intracellular hydrogen peroxide after in vitro priming and FcgammaR-stimulation. J Clin Periodontol. 1998;25:394–8. doi: 10.1111/j.1600-051x.1998.tb02461.x. [DOI] [PubMed] [Google Scholar]
- 8.Matthews JB, Wright HJ, Roberts A, Cooper PR, Chapple IL. Hyperactivity and reactivity of peripheral blood neutrophils in chronic periodontitis. Clin Exp Immunol. 2007;147:255–64. doi: 10.1111/j.1365-2249.2006.03276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nicu EA, van der Velden U, Everts V, van Winkelhoff AJ, Roos D, Loos BG. Hyper-reactive PMNs in FcgammaRIIa 131 H/H genotype periodontitis patients. J Clin Periodontol. 2007;34:938–45. doi: 10.1111/j.1600-051X.2007.01136.x. [DOI] [PubMed] [Google Scholar]
- 10.Deo YM, Graziano RF, Repp R, Van de Winkel JG. Clinical significance of IgG Fc receptors and Fc gamma R-directed immunotherapies. Immunol Today. 1997;18:127–35. doi: 10.1016/s0167-5699(97)01007-4. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi T, van der Pol WL, Van de Winkel JG, et al. Relevance of IgG receptor IIIb (CD16) polymorphism to handling of Porphyromonas gingivalis: implications for the pathogenesis of adult periodontitis. J Periodontal Res. 2000;35:65–73. doi: 10.1034/j.1600-0765.2000.035002065.x. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto K, Kobayashi T, Grossi S, et al. Association of Fcgamma receptor IIa genotype with chronic periodontitis in Caucasians. J Periodontol. 2004;75:517–22. doi: 10.1902/jop.2004.75.4.517. [DOI] [PubMed] [Google Scholar]
- 13.Wolf DL, Neiderud AM, Hinckley K, Dahlen G, van de Winkel JGJ, Papapanou PN. Fcgamma receptor polymorphisms and periodontal status: a prospective follow-up study. J Clin Periodontol. 2006;33:691–8. doi: 10.1111/j.1600-051X.2006.00973.x. [DOI] [PubMed] [Google Scholar]
- 14.Loos BG, Leppers-Van de Straat FG, Van de Winkel JG, van der Velden U. Fcgamma receptor polymorphisms in relation to periodontitis. J Clin Periodontol. 2003;30:595–602. doi: 10.1034/j.1600-051x.2003.00355.x. [DOI] [PubMed] [Google Scholar]
- 15.Leino L, Hurttia HM, Sorvajarvi K, Sewon LA. Increased respiratory burst activity is associated with normal expression of IgG-Fc-receptors and complement receptors in peripheral neutrophils from patients with juvenile periodontitis. J Periodontal Res. 1994;29:179–84. doi: 10.1111/j.1600-0765.1994.tb01211.x. [DOI] [PubMed] [Google Scholar]
- 16.Gustafsson A, Asman B. Increased release of free oxygen radicals from peripheral neutrophils in adult periodontitis after Fc delta-receptor stimulation. J Clin Periodontol. 1996;23:38–44. doi: 10.1111/j.1600-051x.1996.tb00502.x. [DOI] [PubMed] [Google Scholar]
- 17.Nagasawa T, Kobayashi H, Aramaki M, Kiji M, Oda S, Izumi Y. Expression of CD14, CD16 and CD45RA on monocytes from periodontitis patients. J Periodontal Res. 2004;39:72–8. doi: 10.1111/j.1600-0765.2004.00713.x. [DOI] [PubMed] [Google Scholar]
- 18.Kawanaka N, Yamamura M, Aita T, et al. CD14+,CD16+ blood monocytes and joint inflammation in rheumatoid arthritis. Arthritis Rheum. 2002;46:2578–86. doi: 10.1002/art.10545. [DOI] [PubMed] [Google Scholar]
- 19.Mercado FB, Marshall RI, Bartold PM. Inter-relationships between rheumatoid arthritis and periodontal disease. A review. J Clin Periodontol. 2003;30:761–72. doi: 10.1034/j.1600-051x.2003.00371.x. [DOI] [PubMed] [Google Scholar]
- 20.Ziegler-Heitbrock HW, Ulevitch RJ. CD14: cell surface receptor and differentiation marker. Immunol Today. 1993;14:121–5. doi: 10.1016/0167-5699(93)90212-4. [DOI] [PubMed] [Google Scholar]
- 21.Luo BH, Carman CV, Springer TA. Structural basis of integrin regulation and signaling. Annu Rev Immunol. 2007;25:619–47. doi: 10.1146/annurev.immunol.25.022106.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ziegler-Heitbrock HW, Strobel M, Kieper D, et al. Differential expression of cytokines in human blood monocyte subpopulations. Blood. 1992;79:503–11. [PubMed] [Google Scholar]
- 23.Inoue T, Sakai Y, Morooka S, Hayashi T, Takayanagi K, Takabatake Y. Expression of polymorphonuclear leukocyte adhesion molecules and its clinical significance in patients treated with percutaneous transluminal coronary angioplasty. J Am Coll Cardiol. 1996;28:1127–33. doi: 10.1016/S0735-1097(96)00308-7. [DOI] [PubMed] [Google Scholar]
- 24.Garred P, Madsen HO, Svejgaard A, Michaelsen TE. Mannose-binding lectin and meningococcal disease. Lancet. 1999;354:336. doi: 10.1016/S0140-6736(05)75241-7. [DOI] [PubMed] [Google Scholar]
- 25.Stengaard-Pedersen K, Thiel S, Gadjeva M, et al. Inherited deficiency of mannan-binding lectin-associated serine protease 2. N Engl J Med. 2003;349:554–60. doi: 10.1056/NEJMoa022836. [DOI] [PubMed] [Google Scholar]
- 26.Agnese DM, Calvano JE, Hahm SJ, et al. Human toll-like receptor 4 mutations but not CD14 polymorphisms are associated with an increased risk of gram-negative infections. J Infect Dis. 2002;186:1522–5. doi: 10.1086/344893. [DOI] [PubMed] [Google Scholar]
- 27.Bellamy R, Ruwende C, Corrah T, et al. Tuberculosis and chronic hepatitis B virus infection in Africans and variation in the vitamin D receptor gene. J Infect Dis. 1999;179:721–4. doi: 10.1086/314614. [DOI] [PubMed] [Google Scholar]
- 28.Garred P, Harboe M, Oettinger T, Koch C, Svejgaard A. Dual role of mannan-binding protein in infections: another case of heterosis? Eur J Immunogenet. 1994;21:125–31. doi: 10.1111/j.1744-313x.1994.tb00183.x. [DOI] [PubMed] [Google Scholar]
- 29.Nibali L, Ready DR, Parkar M, et al. Gene polymorphisms and the prevalence of key periodontal pathogens. J Dent Res. 2007;86:416–20. doi: 10.1177/154405910708600505. [DOI] [PubMed] [Google Scholar]
- 30.Hepburn AL, Mason JC, Davies KA. Expression of Fc{gamma} and complement receptors on peripheral blood monocytes in systemic lupus erythematosus and rheumatoid arthritis. Rheumatology (Oxford) 2004;43:547–54. doi: 10.1093/rheumatology/keh112. [DOI] [PubMed] [Google Scholar]
- 31.Wijngaarden S, van Roon JAG, Bijlsma JWJ, van de Winkel JGJ, Lafeber FPJG. Fc{gamma} receptor expression levels on monocytes are elevated in rheumatoid arthritis patients with high erythrocyte sedimentation rate who do not use anti-rheumatic drugs. Rheumatology (Oxford) 2003;42:681–8. doi: 10.1093/rheumatology/keg174. [DOI] [PubMed] [Google Scholar]
- 32.Shinohara S, Hirohata S, Inoue T, Ito K. Phenotypic analysis of peripheral blood monocytes isolated from patients with rheumatoid arthritis. J Rheumatol. 1992;19:211–5. [PubMed] [Google Scholar]
- 33.Torsteinsdottir I, Arvidson NG, Hallgren R, Hakansson L. Monocyte activation in rheumatoid arthritis (RA): increased integrin, Fc gamma and complement receptor expression and the effect of glucocorticoids. Clin Exp Immunol. 1999;115:554–60. doi: 10.1046/j.1365-2249.1999.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Magnusson SE, Engstrom M, Jacob U, Ulfgren AK, Kleinau S. High synovial expression of the inhibitory FcgammaRIIb in rheumatoid arthritis. Arthritis Res Ther. 2007;9:R51. doi: 10.1186/ar2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wijngaarden S, van Roon JA, Van de Winkel JG, Bijlsma JW, Lafeber FP. Down-regulation of activating Fcgamma receptors on monocytes of patients with rheumatoid arthritis upon methotrexate treatment. Rheumatology (Oxford) 2005;44:729–34. doi: 10.1093/rheumatology/keh583. [DOI] [PubMed] [Google Scholar]
- 36.Ziegler-Heitbrock HW. Heterogeneity of human blood monocytes: the CD14+ CD16+ subpopulation. Immunol Today. 1996;17:424–8. doi: 10.1016/0167-5699(96)10029-3. [DOI] [PubMed] [Google Scholar]
- 37.Buduneli N, Bicakci N, Keskinoglu A. Flow-cytometric analysis of lymphocyte subsets and mCD14 expression in patients with various periodontitis categories. J Clin Periodontol. 2001;28:419–24. doi: 10.1034/j.1600-051x.2001.028005419.x. [DOI] [PubMed] [Google Scholar]
- 38.Fingerle G, Pforte A, Passlick B, Blumenstein M, Strobel M, Ziegler-Heitbrock HW. The novel subset of CD14+/CD16+ blood monocytes is expanded in sepsis patients. Blood. 1993;82:3170–6. [PubMed] [Google Scholar]
- 39.Katayama K, Matsubara T, Fujiwara M, Koga M, Furukawa S. CD14+CD16+ monocyte subpopulation in Kawasaki disease. Clin Exp Immunol. 2000;121:566–70. doi: 10.1046/j.1365-2249.2000.01321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kanaya S, Nemoto E, Ogawa T, Shimauchi H. Porphyromonas gingivalis lipopolysaccharides induce maturation of dendritic cells with CD14+CD16+ phenotype. Eur J Immunol. 2004;34:1451–60. doi: 10.1002/eji.200324549. [DOI] [PubMed] [Google Scholar]
- 41.Almeida J, Bueno C, Alguero MC, et al. Comparative analysis of the morphological, cytochemical, immunophenotypical, and functional characteristics of normal human peripheral blood lineage-/CD16+/HLA-DR+/CD14-/lo cells, CD14+ monocytes, and CD16- dendritic cells. Clin Immunol. 2001;100:325–38. doi: 10.1006/clim.2001.5072. [DOI] [PubMed] [Google Scholar]
- 42.Woessner JF., Jr Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991;5:2145–54. [PubMed] [Google Scholar]
- 43.Ries C, Petrides PE. Cytokine regulation of matrix metalloproteinase activity and its regulatory dysfunction in disease. Biol Chem Hoppe Seyler. 1995;376:345–55. [PubMed] [Google Scholar]
- 44.Morel F, Doussiere J, Vignais PV. The superoxide-generating oxidase of phagocytic cells. Physiological, molecular and pathological aspects. Eur J Biochem. 1991;201:523–46. doi: 10.1111/j.1432-1033.1991.tb16312.x. [DOI] [PubMed] [Google Scholar]
- 45.Noguera A, Batle S, Miralles C, et al. Enhanced neutrophil response in chronic obstructive pulmonary disease. Thorax. 2001;56:432–7. doi: 10.1136/thorax.56.6.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gustafsson A, Ito H, Asman B, Bergstrom K. Hyper-reactive mononuclear cells and neutrophils in chronic periodontitis. J Clin Periodontol. 2006;33:126–9. doi: 10.1111/j.1600-051X.2005.00883.x. [DOI] [PubMed] [Google Scholar]
- 47.Nonnenmacher C, Dalpke A, Zimmermann S, et al. DNA from periodontopathogenic bacteria is immunostimulatory for mouse and human immune cells. Infect Immun. 2003;71:850–6. doi: 10.1128/IAI.71.2.850-856.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harokopakis E, Albzreh MH, Haase EM, Scannapieco FA, Hajishengallis G. Inhibition of proinflammatory activities of major periodontal pathogens by aqueous extracts from elder flower (Sambucus nigra) J Periodontol. 2006;77:271–9. doi: 10.1902/jop.2006.050232. [DOI] [PubMed] [Google Scholar]
- 49.Kobayashi H, Nagasawa T, Aramaki M, Mahanonda R, Ishikawa I. Individual diversities in interferon gamma production by human peripheral blood mononuclear cells stimulated with periodontopathic bacteria. J Periodontal Res. 2000;35:319–28. doi: 10.1034/j.1600-0765.2000.035006319.x. [DOI] [PubMed] [Google Scholar]
- 50.Reddi K, Wilson M, Nair S, Poole S, Henderson B. Comparison of the pro-inflammatory cytokine-stimulating activity of the surface-associated proteins of periodontopathic bacteria. J Periodontal Res. 1996;31:120–30. doi: 10.1111/j.1600-0765.1996.tb00473.x. [DOI] [PubMed] [Google Scholar]
- 51.Yoshimura A, Hara Y, Kaneko T, Kato I. Secretion of IL-1 beta, TNF-alpha, IL-8 and IL-1ra by human polymorphonuclear leukocytes in response to lipopolysaccharides from periodontopathic bacteria. J Periodontal Res. 1997;32:279–86. doi: 10.1111/j.1600-0765.1997.tb00535.x. [DOI] [PubMed] [Google Scholar]
- 52.van der Velden U, Abbas F, Van Steenbergen TJ, et al. Prevalence of periodontal breakdown in adolescents and presence of Actinobacillus actinomycetemcomitans in subjects with attachment loss. J Periodontol. 1989;60:604–10. doi: 10.1902/jop.1989.60.11.604. [DOI] [PubMed] [Google Scholar]
- 53.Duncan L, Yoshioka M, Chandad F, Grenier D. Loss of lipopolysaccharide receptor CD14 from the surface of human macrophage-like cells mediated by Porphyromonas gingivalis outer membrane vesicles. Microb Pathog. 2004;36:319–25. doi: 10.1016/j.micpath.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 54.Sugawara S, Nemoto E, Tada H, Miyake K, Imamura T, Takada H. Proteolysis of human monocyte CD14 by cysteine proteinases (Gingipains) from Porphyromonas gingivalis leading to lipopolysaccharide hyporesponsiveness. J Immunol. 2000;165:411–8. doi: 10.4049/jimmunol.165.1.411. [DOI] [PubMed] [Google Scholar]


