Abstract
Meningococcal disease is a leading infectious cause of death in children in industrialized countries. The induction of high levels of proinflammatory cytokines has been implicated in the pathogenesis of Neisseria meningitidis-related multi-organ failure. Here, we demonstrate that N. meningitidis serotypes A and B induce significantly higher levels of tumour necrosis factor (TNF)-α positive cells in vitro in infants and young children compared with adults (serotype A/B; infants: 64·9%/63·9%; children: 77·8%/64·3% versus adults: 27·7%/32%; P < 0·005). Serotype A induces also higher levels of interleukin (IL)-6 positive cells in neonates and infants compared with adults (serotype A; newborn 55·4%; infants 58·8% versus adults 49%; P < 0·05). Treatment with human recombinant erythropoietin in vitro resulted in significant attenuation of the N. meningitidis-induced proinflammatory response in all age groups (reduction rate of erythropoietin for IL-6 after stimulation with serotype B: newborn 28%, infants 15%, children 23% and adults 28% and for TNF-α after stimulation with serotype B: newborn 27%, infants 22%, children 20% and adults 28%; P < 0·05). We conclude that (i) Neisseria meningitidis induces a higher TNF-α response in infants and children compared with adults and (ii) erythropoietin was able to attenuate IL-6 and TNF-α production in all investigated age groups. These data may explain the high incidence of meningococcal infection in infants and makes erythropoietin a potentially attractive candidate for interventional strategies in an otherwise devastating course of the disease.
Keywords: erythropoietin, IL-6, N. meningitides, TNF-α
Introduction
Neisseria meningitidis remains one of the leading causes of bacterial meningitis and sepsis worldwide. The mortality and morbidity is still high, due principally to the inability to manage effectively the endotoxin-induced vascular collapse caused frequently by this organism. Infants and young children are particularly prone to N. meningitidis infections, with an incidence almost 10 times higher in children of less than 2 years of age compared with the general population [1]. Poor responses to N. meningitidis at the humoral and cellular level [2] in this age group are contributing factors for this vulnerability. Very high serum levels of proinflammatory cytokines have been implicated to be involved essentially in the pathogenesis of N. meningitidis-induced septic shock and multiple organ failure [3]. Previously, we could demonstrate an imbalance between pro- and anti-inflammatory cytokines in neonates and infants with an enhanced production of proinflammatory cytokines after endotoxin challenge [4,5]. In this study, we tested the hypothesis that proinflammatory cytokine production to N. meningitidis is age-dependent and can be influenced by erythropoietin, for which immunoregulatory properties have been described [6].
Materials and methods
Study population
Blood samples were taken after informed consent from healthy adult blood donors (n = 21), cord blood from healthy term newborns (n = 20) and infants and young children (aged between 1 month and 34 months, n = 29). For ethical reasons, we restricted our study population to those infants and children who underwent a medically indicated peripheral venous blood sampling before elective surgical intervention or diagnostic procedures. Age groups were defined as infants below 12 months (n = 12) and children above 12 months (n = 17). Exclusion criteria were history of intra-amniotic infection, clinical or laboratory signs of infection and medication with known influence on immunological factors (e.g. corticosteroids). The study was approved by the ethical review board of the University of Lübeck Medical School.
The N. meningitidis culture
Experiments were performed with the N. meningitidis serogroup A American Type Culture Collection-13077 strain (LGC Promochem, Wesel, Germany) isolated from spinal fluid from a patient with fatal meningitis and a clinical N. meningitidis serogroup B isolate from cerebrospinal fluid of a 6-month-old infant with meningitis treated in our hospital. Infectious bacteria were adjusted to a total number of 1·2 × 109/ml, as determined by densitometry corresponding to McFarland 4. For safety reasons, both strains were heat-inactivated by exposition to 56°C for 1 h, as described recently, [7] and aliquots were stored at −70°C.
Intracytoplasmic detection of cytokines
Heparinized whole blood was suspended in RPMI-1640 supplemented with 1% penicillin/streptomycin, 2 mM glutamine, 1 mM pyruvate and non-essential amino acids at a concentration of 2 × 106 leucocytes/ml in multi-well plates at 37°C and 5% CO2. Because non-haematopoietic effects of erythropoietin require higher concentrations [6] we employed human recombinant erythropoietin beta (NeoRecormon, Firma Roche, Grenzen-Wylach, Germany), diluted in RPMI-1640 in final concentrations of 100 and 500 U/ml respectively, which was added to the cultures 1 h prior to stimulation with heat-inactivated meningococcal strains at a final concentration of 1·2 × 108 per ml for 5 h. As a positive control, lipopolysaccharide (LPS) obtained from Escherichia coli (E. coli 0127: B8; no. L3129; Sigma, Deisenhofen, Germany) was added at a concentration of 30 ng/ml for 5 h. Cells were washed in Hanks's balanced salt solution (HBSS) and resuspended in a buffer consisting of HBSS, 0·1% saponin (Riedel de Haen, Hanover, Germany) and 0·01 M HEPES buffer (Seromed Biochrome, Berlin, Germany); 200 µl aliquots of cells were added to tubes containing 0·5 µg/10 µl of monoclonal antibodies (mAbs) against CD14, tumour necrosis factor (TNF)-α and interleukin (IL)-6 (Becton Dickinson, Heidelberg, Germany). Preincubation with a surplus of unconjugated anti-cytokine mAbs (5 µg/10 µl; Pharmingen, Heidelberg, Germany) served as a negative control for intracellular staining to each sample. Two-colour flow cytometric analysis was performed on a fluorescence activated cell sorter Canto flow cytometer (Becton Dickinson). A total of 2000 CD14 positive monocytes were acquired from each sample. Dead cells were excluded by forward- and side-scatter gating. Thresholds were set according to the purified antibody-blocking control. Positive cells <2% were allowed beyond the statistical marker. Data were expressed as percentage of CD14 positive monocytes producing each cytokine. In formerly published articles we could demonstrate a good correlation between soluble and intracytoplasmic IL-6 [8] and TNF-α[9] in our laboratory.
Statistical analysis
Statistical differences were tested for paired data by the non-parametric Wilcoxon rank test and non-paired data with the Mann–Whitney U-test. Both test were used as two-tailed tests. The level of significance was defined as P < 0·05. Statistical analyses were performed using SPSS® version 13·0 statistical software (SPSS, Inc., Chicago, IL, USA).
Results
Cytokine production in relation to meningococcal serogroup
There was no relevant difference in cytokine production between serogroup A and serogroup B meningococci. However, in comparison with LPS stimulation at the concentration used, N. meningitidis induced preferentially higher TNF-α and lower IL-6 responses, especially in infants and children (Table 1).
Table 1.
Cytokine-positive monocytes in neonates (n = 20), infants (n = 12), children (n = 17) and adults (n = 21) after stimulation with Neisseria meningitidis serogroupA (N.m. A), serogroup B (N.m. B) and lipopolysaccharide (LPS). Data are shown as median and interquartile range in parentheses.
| TNF-α (postive cells) | IL-6 (% postive cells) | |||||
|---|---|---|---|---|---|---|
| N.m. A | N.m. B | LPS | N.m. A | N.m. B | LPS | |
| Newborn | 53·1 (17–69)* | 38·5 (15–67)* | 33·1 (12–47) | 55·4 (44–70) | 52·3 (48–63)* | 58·8 (49–67) |
| Infants | 64·9 (44–70)* | 63·9 (49–76)* | 36·2 (22–54) | 58·8 (53–67)* | 56·6 (52–60)* | 67·6 (63–72) |
| Children | 77·8 (66–79)* | 64·3 (52–71)* | 47·8 (42–57) | 54·0 (44–65)* | 49·9 (41–61)* | 69·6 (60–79) |
| Adults | 27·7 (14–53) | 32·0 (20–59) | 32·4 (12–61) | 49·0 (33–54)** | 54·0 (37–64)* | 65·2 (54–73) |
Differences are significant at a level of
P ≤ 0·05 or
P ≤ 0·001 (**) compared with LPS stimulation.
IL, interleukin; TNF, tumour necrosis factor.
Cytokine production in relation to age group
The percentage of TNF-α-positive cells was highest in infants and children compared with neonates or adults after stimulation with both meningococcal serogroups (serogroups A and B: infants and children versus adults P < 0·005) (Table 1, Fig. 1a, b). The percentage of IL-6-positive cells was comparable in neonates and infants and higher than in adults after stimulation with serogroup A (serogroup A: neonates and infants versus adults P < 0·05). For serogroup B there was no statistical significant difference for IL-6 between age groups.
Fig. 1.
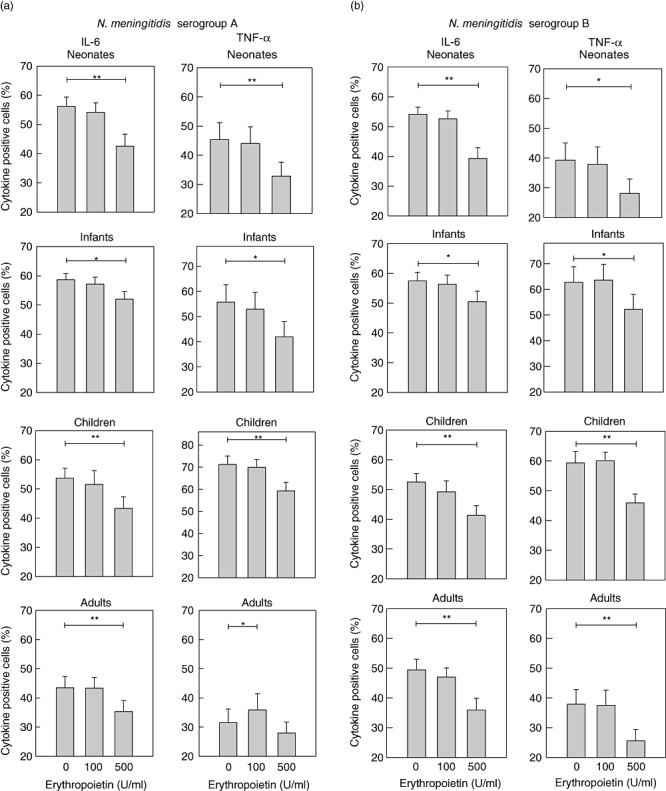
Influence of erythropoietin on meningococcal-induced cytokine production. Interleukin (IL)-6 and tumour necrosis factor (TNF)-α-positive monocytes are shown in neonates (n = 20), infants (defined as < 12 months; n = 12), children (defined as > 12 months; n = 17) and adults (n = 21) after preincubation with erythropoietin and stimulation with heat-inactivated Neisseria meningitidis serogroup A (a) and serogroup B (b). At the highest concentration of erythropoietin (500 U/ml) a clear inhibition of both cytokines is demonstrated in all age groups. The lower concentration of erythropoietin (100 U/ml) shows only a slight inhibition of IL-6 in children. Differences are significant at a level of P < 0·05 (*) or P ≤ 0·001 (**). Data are represented as vertical bars showing mean and standard error of the mean.
Influence of erythropoietin on N. meningitidis-induced cytokine production
Preincubation with erythropoietin at the higher concentration attenuated the production of IL-6 and TNF-α in all investigated age groups. The effect was seen in both serogroups (Fig. 1a and b). In children, there was also a significant reduction of IL-6 in both serogroups (Fig. 1a, b) and for TNF-α in serogroup A (P < 0·05) using the lower concentration of erythropoietin.
Discussion
The N. meningitidis is the leading cause of bacterial meningitis in children and young adults, with the highest mortality during early infancy. The majority of disease in industrialized countries is caused by serogoup B, whereas in sub-Saharan Africa serogroup A is most prevalent. Here, we characterize the proinflammatory cytokine responses of neonates, infants, children and adults to N. meningitidis serogroups A and B using an in vitro sepsis model. Although some studies have assessed in vivo cytokine production during meningococcal infection in children, interpretation of these data can be difficult because of frequent variation in important confounders, including the time between onset of clinical disease and blood collection as well as bacterial load. Furthermore, cytokine production in severe sepsis can be impaired by a phenomenon called immunoparalysis [10]. Therefore, we investigated the N. meningitidis-induced cytokine responses and the therapeutic effect of human recombinant erythropoietin in a standardized ex vivo model of sepsis [4].
In infants and children, we could demonstrate a significantly higher number of TNF-α-positive cells after stimulation with both serogroups A and B and a higher number IL-6-positive cells after stimulation with serogroup A compared with adults (Table 1). This is of special interest because both cytokines are involved directly in the pathogenesis of N. meningitidis septicaemia. TNF-α has been implicated as the main cytokine responsible for the devastating cascade fulminating in septic shock and multiple organ failure [11], and recent data also indicate a central role for IL-6 in myocardial dysfunction during meningococcal septic shock [12]. Therefore, we hypothesize that the enhanced production of these cytokines in response to N. meningitidis in infants and children demonstrated in our study might be involved in the severity of clinical meningococcal disease in infancy and early childhood.
Recent studies have shown that erythropoietin is a crucial mediator of an evolutionarily conserved, ancient immune response that limits damage to several organs following hypoxic–ischaemic, traumatic, excitotoxic and inflammatory injuries [6]. Different mechanisms of erythropoietin on cells have been demonstrated, especially anti-apoptotic effects [6]. In our model we found no toxic effect on cells demonstrated by a stable amount of dead cells in forward and side-scatter gating. Previously, we could demonstrate a hitherto unknown cytokine inhibition of IL-2, IFN-γ, TNF-α, IL-4, IL-5, IL-6, and IL-10 in children and adults by erythropoietin after stimulation with LPS [13]. The results of this study show that erythropoietin attenuates both TNF-α and IL-6 production after stimulation with heat-inactivated N. meningitidis. These suppressive effects are even more remarkable, taking into consideration that in vitro stimulation represents a maximum challenge. Therefore, it is conceivable that lower concentrations of therapeutic erythropoietin have an anti-inflammatory action in vivo, where the stimulation of leucocytes is probably to be less intense. The cytokine-inhibitory properties of erythropoietin represent an important mechanism by which some of its tissue protective effects are mediated. Thus, erythropoietin may be considered not only in hypoxia-mediated tissue injuries, but also in inflammation-based diseases such as severe sepsis with end organ damage. Indeed, treatment of preterm infants to prevent anaemia [14] and a first human trial in acute stroke found erythropoietin to be a safe, well-tolerated and beneficial drug [15]. Although an increased incidence of thrombotic events was reported, overall mortality was reduced in critically ill adults with trauma treated with erythropoietin [16].
The broad therapeutic range and wide spectrum of an evolutionarily emerged tissue-protective mechanism make erythropoietin a potentially attractive candidate for interventional strategies in an otherwise devastating course of meningococcal infection.
Acknowledgments
The study was supported in part by Possehl-Stiftung, Verein Lübeck-Hilfe für krebskranke Kinder and the Friedrich Bluhme and Else Jebsen Stiftung.
References
- 1.Kaplan SL, Schutze GE, Leake JA, et al. Multicenter surveillance of invasive meningococcal infections in children. Pediatrics. 2006;118:e979. doi: 10.1542/peds.2006-0281. [DOI] [PubMed] [Google Scholar]
- 2.Pollard AJ, Galassini R, Rouppe van der Voort EM, et al. Cellular immune responses to Neisseria meningitidis in children. Infect Immun. 1999;67:2452–63. doi: 10.1128/iai.67.5.2452-2463.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woods CR. Neisseria meningitidis (meningococcus) In: Kliegmann RM, Behrmann RE, Jenson HB, Stanton BF, editors. Nelson textbook of pediatrics. Philadelphia: Saunders Elsevier; 2007. pp. 1164–9. [Google Scholar]
- 4.Schultz C, Rott C, Temming P, Schlenke P, Möller JC, Bucsky P. Enhanced interleukin-6 and interleukin-8 synthesis in term and preterm infants. Pediatr Res. 2002;51:317–22. doi: 10.1203/00006450-200203000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Schultz C, Temming P, Bucsky P, Göpel W, Strunk T, Härtel C. Immature antiinflammatory response in neonates. Clin Exp Immunol. 2004;135:130–6. doi: 10.1111/j.1365-2249.2004.02313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strunk T, Härtel C, Schultz C. Does erythropoietin protect the preterm brain? Arch Dis Child Fetal Neonatal Ed. 2004;89:F364–6. doi: 10.1136/adc.2003.041533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robinson K, Neal KR, Howard C, et al. Characterization of humoral and cellular immune response elicited by meningococcal carriage. Infect Immun. 2002;70:1301–9. doi: 10.1128/IAI.70.3.1301-1309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schultz C. Correspondence responding to correspondence relating to Dembinski et al. Pediatr Res. 2003;53:866–8. [Google Scholar]
- 9.Härtel C, Adam N, Strunk T, Temming P, Müller-Steinhardt M, Schultz C. Cytokine responses correlate differentially with age in infancy and early childhood. Clin Exp Immunol. 2005;142:446–53. doi: 10.1111/j.1365-2249.2005.02928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ertel W, Kremer JP, Kenney J, et al. Downregulation of proinflammatory cytokine release in whole blood from septic patients. Blood. 1995;85:1341–7. [PubMed] [Google Scholar]
- 11.Westendorp RG, Langermans JA, de Bel CE, et al. Release of tumor necrosis factor: an innate host characteristic that may contribute to the outcome of meningococcal disease. J Infect Dis. 1995;171:1057–60. doi: 10.1093/infdis/171.4.1057. [DOI] [PubMed] [Google Scholar]
- 12.Pathan N, Hemingway CA, Alizadeh AA, et al. Role of interleukin-6 in myocardial dysfunction of meningococcal septic schock. Lancet. 2004;363:203–9. doi: 10.1016/S0140-6736(03)15326-3. [DOI] [PubMed] [Google Scholar]
- 13.Strunk T, Härtel C, Temming P, Matzke N, Zimmer J, Schultz C. Erythropoietin inhibits cytokine production of human leukocytes. Acta Paediatr. 2008;97:16–20. doi: 10.1111/j.1651-2227.2007.00560.x. [DOI] [PubMed] [Google Scholar]
- 14.Maier RF, Obladen M, Müller-Hansen I, et al. Early treatment with erythropoietin β ameliorates anemia and reduces transfusion requirements in infants with birth weights below 1000 g. J Pediatr. 2002;141:8–15. doi: 10.1067/mpd.2002.124309. [DOI] [PubMed] [Google Scholar]
- 15.Ehrenreich H, Hasselblatt M, Dembowski C, et al. Erythropoietin therapy for acute stroke is both safe and beneficial. Mol Med. 2002;8:495–505. [PMC free article] [PubMed] [Google Scholar]
- 16.Corwin HL, Gettinger A, Fabian TC, et al. Efficacy and safety of Epoetin alfa in critically ill patients. N Engl J Med. 2007;357:965–76. doi: 10.1056/NEJMoa071533. [DOI] [PubMed] [Google Scholar]


