Abstract
Enzymes play an important role in inducing airway inflammation, but knowledge is limited to few proteins. This study was carried out to assess the role of Epi p 1, a serine protease of Epicoccum purpurascens, in inducing allergy and inflammation in a murine model. Balb/c mice were sensitized with Epi p 1 active protease (EAP) or Epicoccum extract. Subsequently, Epi p 1 sensitized mice were boosted on day 14 with EAP or inactivated protease (EIAP). Three intranasal challenges were given and mice were killed to obtain blood, bronchoalveolar lavage fluid (BALF), spleen and lung tissues. Cellular airways infiltration, immunoglobulin E (Ig)E titres and cytokine levels in BALF and splenocyte culture supernatant were compared. Mice immunized with EAP had higher Epi p 1-specific serum IgE and IgG1 than EIAP immunized mice (P < 0·01). There was a twofold difference in the number of eosinophils in BALF of EAP mice and EIAP mice (P < 0·01). A similar trend was recorded for eosinophil peroxidase activity (P < 0·05), indicating the role of proteolytic activity in inducing inflammation. Further, lung histology revealed increased leucocyte infiltration and airway narrowing, with higher inflammation scores in the EAP group than in the EIAP group. The lungs of EAP mice showed increased mucus and goblet cell metaplasia. Interleukin (IL)-4 and IL-5 levels were higher in BALF and splenocyte culture supernatant of EAP mice than in EIAP mice (P < 0·05), indicating a T helper 2 response. Proteolytic activity of Epi p 1 plays an important role in inducing allergic inflammation. The enzymatically inactive form may be investigated for immunotherapy.
Keywords: allergen, allergic inflammation, Epicoccum purpurascens, mice model, serine protease
Introduction
Exposure to inhalant allergens can induce airway inflammation in predisposed individuals and lead to respiratory diseases such as allergic rhinitis, asthma or both. Allergenic molecules responsible for these disorders are isolated and have been characterized from various sources [1–4]. A number of clinically relevant allergens from sources such as cockroach, dust mites, pollens and fungi show proteolytic activity. Studies with dust mite (Dermatophagoides species) cysteine protease have demonstrated that the enzymatic activity contributes significantly towards mounting the immunological response [4–7]. These findings are corroborated by studies that demonstrate the proinflammatory role of fungal proteases [8–13].
Saprophytic fungi have efficient hydrolytic machinery to utilize organic matter as food. Previously, it has been demonstrated that the active protease content of fungal extracts can influence the induction and severity of allergic airway disease in mice [12]. It is possible that the biological activity of such proteases allows them to bypass the airway tolerogenic mechanisms that usually forbid allergic responses to inhaled antigens. Such proteolytically active molecules can facilitate the presentation of other (non-proteolytic) allergens to the immune system, thereby augmenting sensitization to such allergens [12]. The role of fungal proteases in facilitating an immune response was investigated by comparing the effect of fungal extracts on the morphology of cultured epithelial cells and secretion of proinflammatory cytokines, in the presence or absence of protease inhibitors [13]. Thus, allergens with enzymatic activity have a greater impact on the pathogenesis of respiratory allergies. However, a large number of moulds are known to harbour proteases; the knowledge about biological activity of these molecules in mounting immune response is limited to few fungi [3,8,10,11].
Epicoccum purpurascens is a potent fungal allergen source inducing respiratory allergic disorders worldwide [14–17]. A recent study identified 16 allergens of this mould, showing immunoglobulin E (IgE) binding with 50–87% of individual patients' sera tested by two-dimensional immunoblot [17]. Six of these allergens were identified as phosphoglycerate kinase (43 kDa), RNA-dependent RNA polymerase (67 kDa), pyrroline-5-carboxylate dehydrogenase (32·5 kDa) and a 40S ribosomal protein (36 kDa) and two proteins of unknown function [17]. Previously, a 33 kDa protein isolated from E. purpurascens was detected as a major allergen reacting with >80% of patients' sera tested by one-dimensional immunoblot [18]. The protein was designated as Epi p 1 and constitutes ∼0·9% of Epicoccum extract (EE). Epi p 1 showed homology to subtilisin-like serine protease from Pneumocystis carinii sp. ratti[16]. Epi p 1 activates the mannose binding lectin-mediated complement pathway and may play a role in the initiation of fungal infection [19]. The present study was aimed to assess the role of proteolytic activity of Epi p 1 in inducing allergic inflammation in mice.
Materials and methods
Purification of Epi p 1
The E. purpurascens extract (EE) was prepared as described previously [14]. Epi p 1 (33 kDa) was purified from spore-mycelial extract by concanavalin A Sepharose chromatography, gel filtration and electroelution and tested for proteolytic activity [18].
Enzymatic activity of Epi p 1
Epi p 1 was inactivated (>90%) by incubation with phenyl methyl sulphonyl fluoride (50 µg PMSF/µg purified protein), an irreversible serine protease-specific inhibitor, for 30 min at 37°C. The inhibitor was removed by dialysis with seven changes of saline over a period of 24 h at 4°C and enzymatic inactivation was confirmed qualitatively by agarose plate assay and quantitatively using Nα-benzoyl-L-arginine-ethyl ester hydrochloride (BAEE; Sigma, St Louis, MO, USA) as a substrate. For agarose plate assay, EE, EAP (Epi p 1 active protease) and PMSF-treated EIAP (Epi p 1 inactivated protease) were incubated overnight at 37°C in wells [2 µg protein/20 µl phosphate-buffered saline (PBS)] perforated on 1% agarose gel containing 0·1% protein substrate (gelatin). PBS and bovine serum albumin (BSA) were used as negative controls. The gel was washed with PBS and stained with Coomassie brilliant blue. A clear unstained zone around the wells indicated the proteolytic activity of the protein.
For quantitative assay, 3 µg/200 µl of Epi p1 was added to 2·3 ml of 0·1 M Tris-HCl buffer, pH 7·0 and 0·5 ml of 0·06 M BAEE. A rise in absorbance at 253 nm [Δ optical density (OD)253]/min/µg protein was taken as a measure of enzymatic activity.
Animals
Female Balb/c mice (4–6 weeks old) were procured from Vallabhbhai Patel Chest Institute, Delhi. The mice were housed in the animal care facility of Institute of Genomics and Integrative Biology, Delhi under standard laboratory conditions. Mice were bled from the tail vein prior to the start of the experiment to obtain preimmune sera. The animal ethical committee of the institute approved the protocol for the study.
Groups
Mice were divided randomly into five groups of six mice each. Group 1 mice were given saline and used as negative control. Group 2 mice were given ovalbumin (OVA) to be used as positive control. Groups 3, 4 and 5 were administered with EE, EAP and EIAP respectively.
Immunization protocol
Immunization with antigens was carried out following the protocol of Gough et al., with slight modification [5]. Briefly, groups of mice were sensitized intraperitoneally (i.p.) and subcutaneously on day 0 with 10 µg of OVA (Sigma), EE or EAP in 100 µl saline adsorbed with 0·2 mg of aluminium hydroxide (Sigma). Saline (100 µl) with alum was given to the negative control group. On day 14, mice were immunized again both i.p. and intranasally with 5 µg of OVA, EE, EAP or EIAP. Intranasal challenges were carried out with 2 µg of respective proteins on days 17, 19 and 21 on mild anaesthetized mice. The control group was challenged with 100 µl of saline alone. The mice were killed after 24 h of the last challenge using sodium pentobarbital (100 mg/kg i.p.). Blood, bronchoalveolar lavage fluid (BALF), spleen and lung tissue were collected. Lung tissue from the right lobe was fixed for histological analysis and that from the left lobe was freeze-dried to quantitate eosinophil peroxidase (EPO) activity.
Sera separation and Ig analysis
Blood was collected, serum separated and used for analysis of IgE, IgG1 and IgG2a by indirect enzyme-linked immunosorbent assay (ELISA). Briefly, microtitre plates (Nunc, Roskilde, Denmark) were coated with 250 ng/100 µl/well of respective protein in 0·1 M carbonate buffer (pH 9·6) in separate wells and incubated overnight at 4°C. The plates were washed with PBS and blocked with 3% defatted milk in PBS for 3 h at 37°C. Mice sera (IgE, 1:10; IgG1 and IgG2a, 1:50) were added (100 µl/well) and incubated overnight at 4°C. Preimmune sera were also added and processed simultaneously. The plates were washed with PBST (PBS containing 0·05% Tween-20) and incubated for 3 h at 37°C with peroxidase-conjugated anti-IgG1 and anti-IgG2a (1:1000; BD Pharmingen, San Diego, CA, USA). For detection of IgE, biotinylated anti-mouse IgE (2 µg/ml; BD Pharmingen) was incubated at 25°C for 90 min. Following washing, the plates were washed and incubated with streptavidin–peroxidase (1:1000; BD Pharmingen) for 30 min. The plates were washed, colour was developed using o-phenylenediamine (OPD) and absorbance was read at 492 nm. ELISA for Ig levels was carried out on the same day.
Collection of BALF
The BALF was collected by cannulating the lungs with 0·5 ml of chilled 0·1 M PBS (pH 7·4) containing 5 mM ethylenediamine tetraacetic acid (EDTA). The procedure was repeated three times and 1·5 ml of BALF was collected. It was centrifuged at 400 g for 15 min at 4°C and the cell pellets were resuspended in 100 µl PBS. Cell counts in pellets were determined using a haemocytometer. BALF cell smears were prepared and stained with Leishman's stain and analysed under a light microscope (Nikon, Melville, NY, USA). At least 200 cells were counted per mouse and the eosinophils were calculated. The supernatant was used for determination of total protein content, cytokine levels and EPO activity.
Total protein in BALF
Protein content in BALF was measured by Bradford assay following the manufacturer's instructions (BioRad, Hercules, CA, USA). BSA (Sigma) was used for calibration.
Lung homogenate preparation
Lung homogenate was prepared using a slight modification of the protocol followed by Schneider et al.[20]. The lung tissue was homogenized (1:10 w/v) in HEPES buffer (50 mM, pH 8·0) using a pestle homogenizer (Werke, Staufer, Germany). It was centrifuged and the resulting pellet was resuspended in the same volume of 0·5% hexadecyl trimethyl ammonium bromide containing 5 mM EDTA. The suspension was rehomogenized and centrifuged. EPO activity was measured in supernatant.
Histopathology
Lungs from the killed mice were fixed immediately in 10% neutral-buffered formalin (v/v) and processed for histology. The lung tissue was embedded in paraffin, sliced to 4-µm sections and stained either with haematoxylin and eosin (H&E) for analysis of lung inflammation or with periodic acid-Schiff (PAS) stain for the detection of mucus-producing cells. Sections were scanned for antigen-induced peribronchial and perivascular inflammation under a light microscope. Images were captured by a light microscope with an in-line camera (Nikon TE 2000-S) and assembled into multi-panel figures using Photoshop software (version 7·0, Adobe version). The degree of leucocyte infiltration around airways was graded as: 0, not present; 1, very few; 2, few; 3, moderate; 4, moderate to marked; and 5, marked. Goblet cell metaplasia in bronchial epithelium was scored based on the number of PAS-stained cells as grade 1: <20% cells stained; 2: 20–40% stained cells; 3: 40–60%; 4: 60–80% and 5: >80% airways affected [21].
Eosinophil peroxidase activity
The EPO activity was evaluated in BALF and lung homogenate following Cui et al.[22]. The activity assay was based on the oxidation of OPD (Sigma) in the presence of H2O2 by EPO. A volume of 50 µl BALF supernatant or lung homogenate (1:10) was transferred to microplate wells (Tarsons, Kolkata, India) in duplicate. One hundred µl of substrate solution containing 3 mM OPD, 0·05 M Tris pH 8·0, 0·1% Triton X-100 and 8·8 mM H2O2, was added to the wells. To differentiate the EPO activity from that of neutrophil and macrophage myeloperoxidases, activity was measured in the absence or presence of EPO-specific inhibitor, resorcinol (1·5 mg/ml; Sigma). The change in absorbance (492 nm) per minute was recorded using a spectramax ELISA reader (Molecular Devices, Sunny Vales, CA, USA). The activity per microgram BALF protein or per gram dry weight of lung in each group was calculated as the slope of linear rise in absorbance with time.
Splenocyte culture
Spleens from the killed mice (naive and sensitized mice) were collected aseptically. Single-cell suspensions were prepared in complete RPMI-1640 medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal calf serum, 1 mM sodium pyruvate, 2 mM L-glutamine, 0·05 mM 2-mercaptoethanol, 100 U/ml penicillin and 100 µg/ml streptomycin. Spleen cells (1 × 106) from different groups were added at 100 µl per well of culture plate (Corning, New York, NY, USA). The cells were challenged with 5 µg/ml of EE, EAP or EIAP (100 µl/well in RPMI-1640). Splenocytes of OVA-sensitized mice were stimulated with OVA. The cells were allowed to proliferate for 72 h at 37°C in a CO2 incubator (Jouan, St-Herblain Cedex, France). The plates were centrifuged at 1000 g, the supernatant collected and used for cytokine analysis [23]. The cell pellet was washed with RPMI-1640 and incubated for 2 h with 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT; 100 µg/ml). The cells were lysed in acidic isopropanol and absorbance was read at 570 nm. OD more than or equal to three times of the negative control was considered as positive stimulation index. Wells without antigen or with phytohaemagglutinin (PHA; 5 µg/ml) were taken as negative and positive controls respectively.
Determination of cytokine levels
Interleukin (IL)-4, IL-5 and interferon (IFN)-γ were determined in BALF and spleen cell culture supernatants (CS) by sandwich ELISA following the manufacturer's instructions (BD Pharmingen). The detection range was 7·8–500 pg/ml, 15·6–1000 pg/ml and 31·3–2000 pg/ml for IL-4, IL-5 and IFN-γ respectively.
Statistical analysis
Student's t-test was employed to compare the hydrolytic activity of EAP and PMSF treated Epi p 1 (inactivated protease). One-way analysis of variance (anova) with Bonferroni's multiple comparison test was performed using GraphPad Prism software to examine differences in various parameters in groups of mice immunized with different antigens (GraphPad Prism, San Diego, CA, USA; http://www.graphpad.com). P < 0·05 was considered statistically significant.
Results
Purification of protease and enzyme inhibition
Purified Epi p 1 (protease) appeared as a single band at ∼33 kDa on Coomassie-stained gel. In the agarose plate assay only EAP showed hydrolytic activity, as demonstrated by a clear zone on the gel. The clear zone was also visible around the well containing EE, indicating the presence of active proteases in the extract. In contrast, there was no such zone around the well containing EIAP, indicating a lack of proteolytic activity (Fig. 1a). In a quantitative assay, EAP hydrolyzed BAEE causing a change of 0·046 absorbance units over 5 min. EIAP, however, caused a small change of 0·005 absorbance units in the same period, showing >90% inhibition of proteolytic activity (Fig. 1b). The hydrolytic ability of EAP was significantly higher than that of EIAP (P < 0·0001).
Fig. 1.
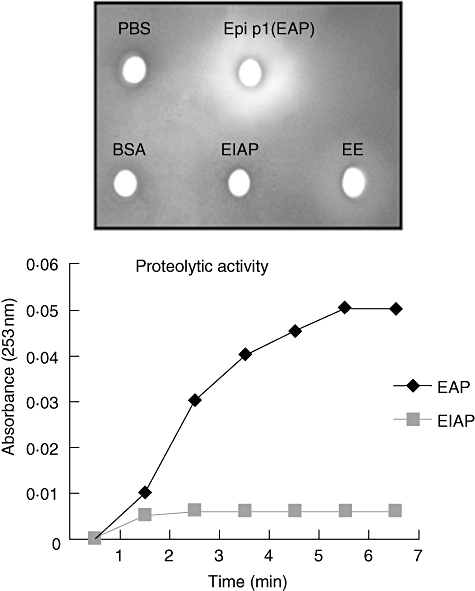
Enzymatic activity of Epi p 1. (a) Hydrolytic activity of Epi p 1 active protease (EAP) or phenyl methyl sulphonyl fluoride (PMSF) treated, i.e. inactivated, protease and Epicoccum extract (EE) was checked by agarose plate assay. Phosphate-buffered saline (PBS) and bovine serum albumin (BSA) were used as negative controls. Each antigen was incubated in wells of 0·1% agarose gel containing 1% gelatin. Proteolytic activity was recorded as clear zone on Coomassie brilliant blue-stained gel. (b) Active protease (EAP) and PMSF-treated, i.e. inactivated protease, were assayed by Nα-benzoyl-L-arginine-ethyl ester hydrochloride hydrolysis as an increase in absorbance per unit time. There was a statistically significant difference in hydrolytic activity of EAP and EIAP.
Serum antibodies
Mice immunized with EE showed the highest specific IgE levels (average OD = 0·59 ± 0·04) followed by EAP (average OD = 0·54 ± 0·05) and EIAP (average OD = 0·33 ± 0·03). Specific IgE levels in mice exposed with EAP and EIAP were significantly different (P < 0·005) (Fig. 2). The OVA mice group showed high OVA-specific IgE levels (average OD = 0·37 ± 0·06). A similar trend was observed for specific IgG1 with these antigens. IgG1 levels differed significantly in the EAP and EIAP immunized groups (P < 0·01) (Fig. 2). Mice immunized with EE showed the highest IgG1 levels, followed by the EAP group. IgG2a levels did not differ between the EAP and EIAP immunized groups. The saline control group showed low levels of specific IgE and IgG1 to all the antigens tested.
Fig. 2.
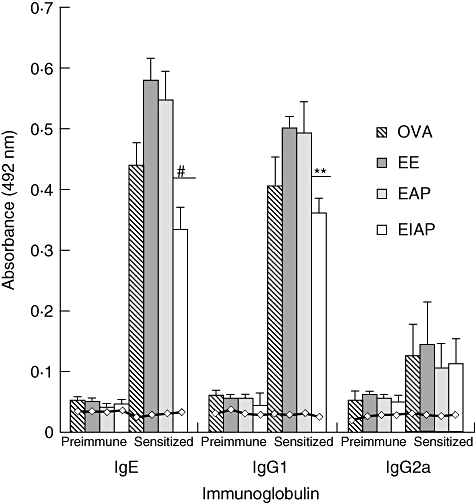
Serum antibody responses to ovalbumin (OVA), Epicoccum extract (EE), Epi p 1 active protease (EAP) and Epi p 1 inactivated protease (EIAP), prior to and after sensitization with respective antigens. The immunoglobulin E (IgE), IgG1 levels and IgG2a were detected using appropriate secondary antibodies in enzyme-linked immunosorbent assay (ELISA). Average absorbance value of six mice in each group is plotted with standard deviation (s.d.). Horizontal line ( ) shows the values of saline control in each group. Statistical significance between specific IgE/IgG1 of EAP and EIAP sensitized mice is mentioned as P-value (at a confidence level of 0·05); #P < 0·005; **P < 0·01. Data are presented as ± s.d.
) shows the values of saline control in each group. Statistical significance between specific IgE/IgG1 of EAP and EIAP sensitized mice is mentioned as P-value (at a confidence level of 0·05); #P < 0·005; **P < 0·01. Data are presented as ± s.d.
The BALF analysis
Protein content. The protein level in BALF was significantly high in the antigen groups compared with the control group (P < 0·05). The protein content was highest in the mice challenged with EE, followed by the EAP and EIAP groups. There was a significant difference (P < 0·05) in the BALF protein content between mice administered with EAP and EIAP (Fig. 3).
Fig. 3.
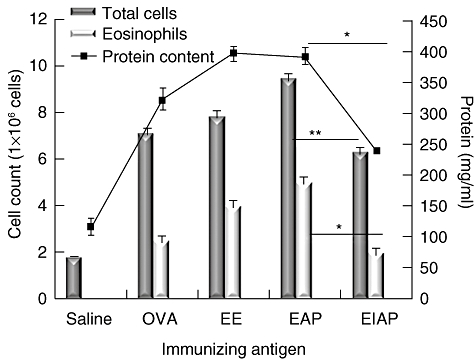
Effects of administration of various antigens on total cell and eosinophil number (primary axis) and protein content (secondary axis) in BALF. Saline, ovalbumin (OVA), Epicoccum extract (EE), Epi p 1 active protease (EAP) and Epi p 1 inactivated protease (EIAP) were administered to the mice in different groups. The protein concentration (shown as line plotted) was measured by Bradford assay. The total number of cells was counted using a haemocytometer. The eosinophils were counted on cell smears stained with Leishman's stain. Statistical difference in each parameter of EAP and EIAP sensitized mice is mentioned as P-value (at a confidence level of 0·05); *P < 0·05; **P < 0·01.
Total cell and eosinophil count. A significant increase was recorded in the total number of cells in the antigen-administered groups compared with the saline control mice. The total number of cells was highest in BALF of the EAP-immunized mice followed by the EE and OVA groups (Fig. 3). The EIAP mice group also showed increased numbers of infiltrating cell, while the saline (control) group showed occasional infiltrates. The eosinophil count was highest in the EAP group followed by the EE and EIAP immunized mice. The eosinophil counts were high in the OVA group, whereas levels were low in the saline control mice. There was a significant difference in total cell count (P < 0·01) and eosinophil count (P < 0·05) in EAP and EIAP administered mice.
Mitogenic activity towards splenocytes
Spleen cells from all antigen-administered mice showed a positive stimulation index with PHA. EAP induced a high degree of proliferation in splenocytes from mice immunized with EE or EAP, while stimulation with EIAP induced a low proliferative response in mice (Fig. 4a). Splenocytes from OVA-immunized mice showed a high proliferative response upon stimulation with OVA (Fig. 4a). The proliferation of splenocytes from OVA immunized mice in response to EE, EAP and EIAP was similar to that in the saline control.
Fig. 4.
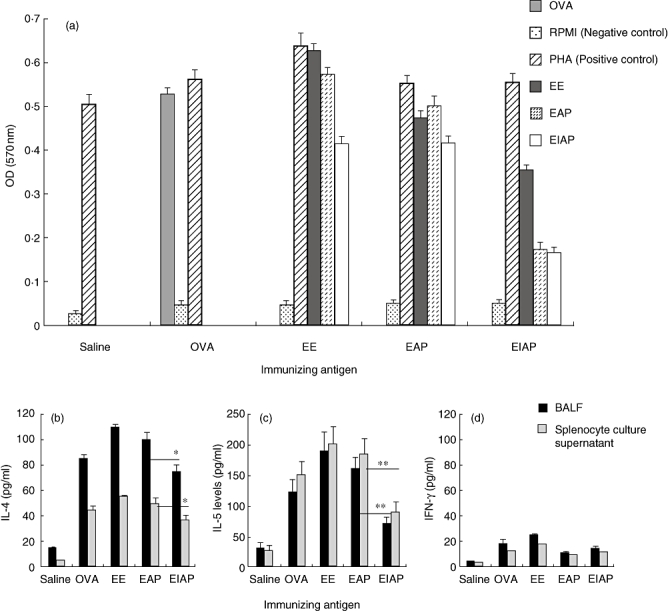
Effect of various antigens on (a) splenocyte proliferation, (b) interleukin (IL)-4, (c) IL-5 and (d) interferon (IFN)-γ release. Spleen cells of mice from different groups were stimulated with Epicoccum extract (EE), Epi p 1 active protease (EAP) and/or Epi p 1 inactivated protease (EIAP). The cells from the ovalbumin (OVA) immunized group were stimulated with OVA. Phytohaemagglutinin and RPMI-1640 were used as positive and negative controls respectively. The cell proliferation was measured by 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide assay after 72 h of incubation at 37°C in a CO2 incubator. Cytokines were measured by enzyme-linked immunosorbent assay. Statistical significance between cytokine levels of EAP and EIAP sensitized mice is mentioned as P-value (at a confidence level of 0·05); *P < 0·05; **P < 0·01.
Cytokine analysis
The IL-4 levels in BALF and splenocyte CS were highest in the EE group mice followed by the EAP and EIAP groups (Fig. 4b). The OVA group mice demonstrated high IL-4 levels, while the saline group mice showed low IL-4 levels. IL-4 levels in mice immunized with active protease were significantly higher in BALF (P < 0·05) and CS (P < 0·05) than in mice immunized with inactivated protease. The mean levels of IL-5 were twofold higher in mice immunized with EAP than mice immunized with EIAP (P < 0·01) in BALF (161 pg/ml versus 73 pg/ml) and splenocytes CS (183 pg/ml versus 90 pg/ml). Immunization with EE also induced significantly higher IL-5 levels than immunization with EIAP (P < 0·01) or saline (P < 0·01) (Fig. 4c). IFN-γ levels were, however, low in BALF and CS of the antigen and control groups (saline) (Fig. 4d).
Lung histology
The H&E-stained lungs of EE and EAP immunized mice showed peribronchial inflammatory cell infiltrates and airway narrowing, with marked disruption of alveolar structure (average infiltration score: 4). A moderate level of inflammation was seen in the EIAP immunized mice (lung inflammation score: 2). The OVA immunized mice also showed increased cellular infiltration and loss of normal lung structure. The lungs of control group (saline) mice showed a normal structure, with occasional inflammatory cells (Fig. 5a).
Fig. 5.

Light photomicrographs of lungs of mice treated with various antigens (20 × resolution). (a) Haematoxylin and eosin-stained paraffin section of lung tissue; (b) periodic acid-Schiff (PAS)-stained paraffin section of lung tissue. The mice were exposed to saline and antigens, e.g. OVA, Epicoccum extract (EE), Epi p 1 active protease (EAP) and Epi p 1 inactivated protease (EIAP). Mice immunized with OVA, EE and EAP showed increased cellular infiltration and the obstruction of airways with mucus. Inset on the top left of each figure shows area marked by arrow at higher resolution (40×).The infiltration score and goblet cell metaplasia score is shown with each staining. Statistical difference in each parameter of EAP and EIAP sensitized mice is mentioned as P-value (at a confidence level of 0·05); *P < 0·05.
The mice immunized with EE showed the highest number of mucus-secreting cells in the bronchi by PAS staining. The number of goblet cells was substantially higher in the EAP and OVA groups than in the saline control group (Fig. 5b). Relatively few mucus-secreting cells were recorded in lung sections from the EIAP group mice. There was a significant difference in goblet cell metaplasia score as well as cellular infiltration score in the lungs of EAP and EIAP mice (P < 0·05).
The EPO activity
Eosinophil peroxidase activity was measured as a marker for activation of eosinophils. The EPO activity was increased markedly in the BALF and lung homogenate of the EE group. The EAP-challenged group demonstrated significantly higher EPO activity than the EIAP mice in both BALF (P < 0·05) and lung homogenate (P < 0·01), indicating greater activation of eosinophils by active protease (Fig. 6). EPO activity in the EAP and EIAP groups differed significantly (P < 0·01) from the control group (saline). OVA immunized mice also demonstrated a significant enhancement in EPO activity compared with the control group (P < 0·001).
Fig. 6.
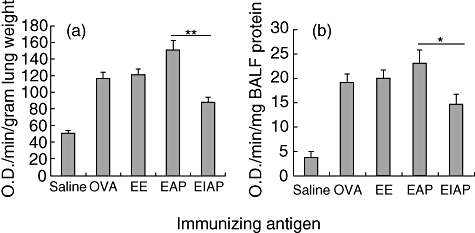
Eosinophil peroxidase (EPO) activity in (a) lung homogenate and (b) bronchoalveolar lavage fluid (BALF) of saline, ovalbumin (OVA), Epicoccum extract (EE), Epi p 1 active protease (EAP) and Epi p 1 inactivated protease (EIAP) treated mice. Change in absorbance of substrate in wells containing BALF or lung homogenate from the above-mentioned groups was recorded as EPO activity. Statistical difference in each parameter of EAP and EIAP sensitized mice is mentioned as P-value (at a confidence level of 0·05); *P < 0·05; **P < 0·01.
Discussion
Allergens with enzymatic activity have been identified from a variety of sources. Hydrolytic enzymes such as trypsin, chymotrypsin, lipases, pepsin, bromelain, papain and cellulase are implicated in occupational asthma [24]. Apart from these, cysteine and serine proteases from house dust mite (Der p 1, Der p 3 and Der p 9), serine proteases of Aspergillus (Asp f 13) and Penicillium species (Pen c 1, Pen ch 13) and phospholipase A 2 of bee venom are clinically important domestic allergens [5–7,10,11,25–28]. The proinflammatory effects of proteases have been attributed to their ability to augment mucosal permeability, to interact with protease activated receptors, to degrade tight junction proteins, to induce cytokine secretion and to cleave regulatory molecules present on various cells [2,3,28–30]. In the present study, the role of Epi p 1 protease was investigated in allergic inflammation.
Studies indicate that proteolytically active molecules have the ability to induce IgE synthesis and consequent allergic response [5–8,13]. It is speculated that interaction of external proteases with cell surface molecules such as CD40 and CD86 or cleavage of some other cell molecules such as CD23 and CD25 creates a milieu that favours the hyperproduction of IgE [4,28]. In the present study, the administration of EAP or EE induced significantly higher specific IgE and IgG1 levels than EIAP. The allergenic activity observed in EIAP immunized mice may be attributed to its residual proteolytic activity or to its structural epitopes. Further, spleen cells of the EAP group mice showed a higher mitogenic response compared with EIAP immunized mice, indicating that intrinsic protease activity of Epi p 1 influences its ability to induce T cell proliferation. The administration of EE to mice also led to enhanced specific IgE and IgG1 levels. The saline immunized mice (control) showed low levels of specific IgE and IgG1 to all the antigens tested.
In vitro studies with Der p 1 allergen suggest that it can cleave CD40 from the dendritic cell surface causing reduced IL-12 production [4]. This signals an increase in IL-4 levels and a reduction in IFN-γ production. In the present study, EAP and EE immunized mice showed a bias towards the T helper 2 (Th2) milieu. IL-4 and IL-5 levels were more highly elevated in the BALF and splenocytes CS of mice challenged with EAP than that from mice immunized with EIAP. The inactivated Epi p 1 retains allergenicity, but the potency of EIAP was attenuated because of blocking of enzymatic activity. The high IL-4 and IL-5 levels with EE can be attributed to active proteases and other allergens present in the extract. IFN-γ, a representative of Th1 response, was low in all the experimental groups.
Proteases from mites and moulds react with cell surface receptors in the airways to generate leucocyte infiltration and amplify the immune response to allergens. Such protease-activated receptors are distributed widely on the cells of airways, e.g. epithelial cells, mast cells, eosinophils, neutrophils, lymphocyte, smooth muscle, endothelium and fibroblasts [30]. Studies suggest that proteases cause epithelial cell disruption by proteolysis [9,31,32]. Exposure to proteases by inhalation may thus induce plasma exudation and cellular infiltration. Raised protein levels in BALF of the EAP immunized group in the present study may be the result of increased bronchoalveolar permeability caused by the active protease. There was increased infiltration of proinflammatory cells and mucus-secreting cells in the lung tissue of EAP immunized mice. In contrast, EIAP immunized mice showed moderate infiltration and few mucus-secreting goblet cells. The increased EPO activity in EAP challenged mice further supports the influence of proteolytic activity on mediator release. The EIAP immunized group showed least EPO activity among the different antigens tested. The higher number of eosinophils and EPO activity in the EAP group mice can be attributed to increased IL-5, which is a key mediator of eosinophil activation. These observations indicate severe airway inflammation similar to asthma in mice administered with EAP or EE. Changes in airway tissues such as hyperplasia of epithelial cells, goblet cell metaplasia, thickening of basement membrane, subepithelial fibrosis and increased smooth muscle mass are associated with asthma [9,33].
The levels of endogenous proteases in body are regulated tightly. Dysregulation in levels of endogenous proteases, e.g. matrix metalloproteases (MMP-9) and neutrophilic elastase, show a correlation with the occurrence of asthma [34]. Enzyme allergens (exogenous proteases) possibly induce an inflammatory response via a mechanism similar to that triggered by endogenous proteases [34]. A recent study showed that basophils are activated directly by protease allergens and produce Th2 cytokines, including IL-4 [35]. Further, it was demonstrated that the allergic response specific to corresponding proteases, e.g. cysteine protease, serine protease, aspartic protease and metalloprotease, was reduced by protease inhibitors [36]. In the present study, a low concentration of PMSF (1 mM) used during extraction is not able to block the protease activity of EE, whereas 50 µg PMSF/µg of purified Epi p 1 could inhibit >90% of proteolytic activity of Epi p 1. Because of this, inactivated protease showed a low Th2 response than active Epi p 1 in mice. However, T cell proliferation in the EIAP group mice was significantly higher than saline control.
In conclusion, the active protease Epi p 1of E. purpurascens plays an important role in driving the allergic response in mice airways. The inactivated protease (Epi p1) with reduced allergenicity has the potential for immunotherapy of allergic disorders.
Acknowledgments
One of the authors (N. K.) received financial assistance from Council of Scientific and Industrial Research, Delhi, India. The authors thank the CSIR Task force for financial assistance.
References
- 1.Jeong SK, Kim HJ, Youm JK, et al. Mite and cockroach allergens activate protease activated receptor-2 and delay epidermal permeability barrier recovery. J Invest Dermatol. 2008;128:1930–9. doi: 10.1038/jid.2008.13. [DOI] [PubMed] [Google Scholar]
- 2.Runswick S, Mitchell T, Davies P, Robinson C, Garrod DR. Pollen proteolytic enzymes degrade tight junctions. Respirology. 2007;12:834–42. doi: 10.1111/j.1440-1843.2007.01175.x. [DOI] [PubMed] [Google Scholar]
- 3.Shen HD, Tam MF, Tang RB, Chou H. Aspergillus and Penicillium allergens: focus on proteases. Curr Allergy Asthma Rep. 2007;7:351–6. doi: 10.1007/s11882-007-0053-8. [DOI] [PubMed] [Google Scholar]
- 4.Ghaemmaghami AM, Gough L, Sewell HF, Shakib F. The proteolytic activity of the major dust mite allergen Der p 1 conditions dendritic cells to produce less interleukin-12: allergen-induced Th2 bias determined at the dendritic cell level. Clin Exp Allergy. 2002;32:1468–75. doi: 10.1046/j.1365-2745.2002.01504.x. [DOI] [PubMed] [Google Scholar]
- 5.Gough L, Schulz O, Sewell HF, Shakib F. The cysteine protease activity of the major dust mite allergen Der p 1 selectively enhances the immunoglobulin E antibody response. J Exp Med. 1999;190:1897–902. doi: 10.1084/jem.190.12.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gough L, Campbell E, Bayley D, Van Heeke G, Shakib F. Proteolytic activity of the house dust mite allergen Der p 1 enhances allergenicity in a mouse inhalation model. Clin Exp Allergy. 2003;33:1159–63. doi: 10.1046/j.1365-2222.2003.01716.x. [DOI] [PubMed] [Google Scholar]
- 7.Kikuchi Y, Takai T, Kuhara T, et al. Crucial commitment of proteolytic activity of a purified recombinant major house dust mite allergen Der p1 to sensitization toward IgE and IgG responses. J Immunol. 2006;177:1609–17. doi: 10.4049/jimmunol.177.3.1609. [DOI] [PubMed] [Google Scholar]
- 8.Schwab CJ, Cooley JD, Jumper CJ, Graham SC, Straus DC. Allergic inflammation induced by a Penicillium chrysogenum conidia-associated allergen extract in a murine model. Allergy. 2004;59:758–65. doi: 10.1111/j.1398-9995.2004.00481.x. [DOI] [PubMed] [Google Scholar]
- 9.Shin SH, Lee YH, Jeon CH. Protease-dependent activation of nasal polyp epithelial cells by airborne fungi leads to migration of eosinophils and neutrophils. Acta Otolaryngol. 2006;126:1286–94. doi: 10.1080/00016480500395179. [DOI] [PubMed] [Google Scholar]
- 10.Tai HY, Tam MF, Chou H, et al. Pen ch 13 allergen induces secretion of mediators and degradation of occludin protein of human lung epithelial cells. Allergy. 2006;61:382–8. doi: 10.1111/j.1398-9995.2005.00958.x. [DOI] [PubMed] [Google Scholar]
- 11.Kurup VP, Xia JQ, Shen HD, et al. Alkaline serine proteinase from Aspergillus fumigatus has synergistic effects on Asp-f-2-induced immune response in mice. Int Arch Allergy Immunol. 2002;129:129–37. doi: 10.1159/000065882. [DOI] [PubMed] [Google Scholar]
- 12.Kheradmand F, Kiss A, Xu J, Lee SH, Kolattukudy PE, Corry DB. A protease-activated pathway underlying Th cell type 2 activation and allergic lung disease. J Immunol. 2002;169:5904–11. doi: 10.4049/jimmunol.169.10.5904. [DOI] [PubMed] [Google Scholar]
- 13.Kauffman HF, Tomee JF, van de Riet MA, Timmerman AJ, Borger P. Protease-dependent activation of epithelial cells by fungal allergens leads to morphologic changes and cytokine production. J Allergy Clin Immunol. 2000;105:1185–93. doi: 10.1067/mai.2000.106210. [DOI] [PubMed] [Google Scholar]
- 14.Bisht V, Singh BP, Arora N, Sridhara S, Gaur SN. Allergens of Epicoccum nigrum grown in different media for quality source material. Allergy. 2000;55:274–80. doi: 10.1034/j.1398-9995.2000.00371.x. [DOI] [PubMed] [Google Scholar]
- 15.Dixit AB, Lewis WH, Wedner HJ. The allergens of Epicoccum nigrum link. I. Identification of the allergens by immunoblotting. J Allergy Clin Immunol. 1992;90:11–20. doi: 10.1016/s0091-6749(06)80006-0. [DOI] [PubMed] [Google Scholar]
- 16.Bisht V, Arora N, Singh BP, Pasha S, Gaur SN, Sridhara S. Epi p 1, an allergenic glycoprotein of Epicoccum purpurascens is a serine protease. FEMS Immunol Med Microbiol. 2004;42:205–11. doi: 10.1016/j.femsim.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Kukreja N, Singh BP, Arora N, Gaur SN, Sridhara S. Identification of Epicoccum purpurascens allergens by two-dimensional immunoblotting and mass spectrometry. Immunobiology. 2008;213:65–73. doi: 10.1016/j.imbio.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Bisht V, Arora N, Singh BP, Gaur SN, Sridhara S. Purification and characterization of a major cross-reactive allergen from Epicoccum purpurascens. Int Arch Allergy Immunol. 2004;133:217–24. doi: 10.1159/000076827. [DOI] [PubMed] [Google Scholar]
- 19.Kukreja N, Arora N, Singh BP, Das HR, Sridhara S. Role of glycoproteins isolated from Epicoccum purpurascens in host-pathogen interaction. Pathobiology. 2007;74:186–92. doi: 10.1159/000103378. [DOI] [PubMed] [Google Scholar]
- 20.Schneider T, van Velzen D, Moqbel R, Issekutz AC. Kinetics and quantitation of eosinophil and neutrophil recruitment to allergic lung inflammation in a brown Norway rat model. Am J Respir Cell Mol Biol. 1997;17:702–12. doi: 10.1165/ajrcmb.17.6.2849. [DOI] [PubMed] [Google Scholar]
- 21.Inoue K, Takano H, Hiyoshi K, et al. Naphthoquinone enhances antigen related airway inflammation in mice. Eur Respir J. 2007;29:259–67. doi: 10.1183/09031936.00033106. [DOI] [PubMed] [Google Scholar]
- 22.Cui T, Kusunose M, Hamada A, et al. Relationship between the eosinophilia of bronchoalveolar lavage fluid (BALF) and the severity of pulmonary fibrosis induced by bleomycin in rats. Biol Pharm Bull. 2003;26:959–63. doi: 10.1248/bpb.26.959. [DOI] [PubMed] [Google Scholar]
- 23.Shankar J, Singh BP, Gaur SN, Arora N. Recombinant glutathione-S-transferase a major allergen from Alternaria alternata for clinical use in allergy patients. Mol Immunol. 2006;43:1927–32. doi: 10.1016/j.molimm.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 24.Thompson PJ. Unique role of allergens and the epithelium in asthma. Clin Exp Allergy. 1998;28:110–16. doi: 10.1046/j.1365-2222.1998.028s5110.x. [DOI] [PubMed] [Google Scholar]
- 25.Sun G, Stacey MA, Schmidt M, Mori L, Mattoli S. Interaction of mite allergens Der p 3 and Der p 9 with protease-activated receptor-2 expressed by lung epithelial cells. J Immunol. 2001;167:1014–21. doi: 10.4049/jimmunol.167.2.1014. [DOI] [PubMed] [Google Scholar]
- 26.Su NY, Yu CJ, Shen HD, Pan FM, Chow LP. Pen c 1, a novel enzymic allergen protein from Penicillium citrinum. Purification, characterization, cloning and expression. Eur J Biochem. 1999;261:115–23. doi: 10.1046/j.1432-1327.1999.00242.x. erratum in: Eur J Biochem 1999; 261:821. [DOI] [PubMed] [Google Scholar]
- 27.Dudler T, Machado DC, Kolbe L, et al. A link between catalytic activity, IgE-independent mast cell activation, and allergenicity of bee venom phospholipase A2. J Immunol. 1995;155:2605–13. [PubMed] [Google Scholar]
- 28.Chapman MD, Wunschmann S, Pomes A. Proteases as Th2 adjuvants. Curr Allergy Asthma Rep. 2007;7:363–7. doi: 10.1007/s11882-007-0055-6. [DOI] [PubMed] [Google Scholar]
- 29.Hewitt CR, Brown AP, Hart BJ, Pritchard DI. A major house dust mite allergen disrupts the immunoglobulin E network by selectively cleaving CD23: innate protection by antiproteases. J Exp Med. 1995;182:1537–44. doi: 10.1084/jem.182.5.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shakib F, Schulz O, Sewell H. A mite subversive: cleavage of CD23 and CD25 by Der p 1 enhances allergenicity. Immunol Today. 1998;19:313–16. doi: 10.1016/s0167-5699(98)01284-5. [DOI] [PubMed] [Google Scholar]
- 31.Wan H, Winton HL, Soeller C, et al. Quantitative structural and biochemical analyses of tight junction dynamics following exposure of epithelial cells to house dust mite allergen Der p 1. Clin Exp Allergy. 2000;30:685–98. doi: 10.1046/j.1365-2222.2000.00820.x. [DOI] [PubMed] [Google Scholar]
- 32.Wan H, Winton HL, Soeller C, et al. The transmembrane protein occludin of epithelial tight junctions is a functional target for serine peptidases from faecal pellets of Dermatophagoides pteronyssinus. Clin Exp Allergy. 2001;31:279–94. doi: 10.1046/j.1365-2222.2001.00970.x. [DOI] [PubMed] [Google Scholar]
- 33.Havaux X, Zeine A, Dits A, Denis O. A new mouse model of lung allergy induced by the spores of Alternaria alternata and Cladosporium herbarum moulds. Clin Exp Immunol. 2005;139:179–88. doi: 10.1111/j.1365-2249.2004.02679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simpson JL, Scott RJ, Boyle MJ, Gibson PG. Differential proteolytic enzyme activity in eosinophilic and neutrophilic asthma. Am J Respir Crit Care Med. 2005;172:559–65. doi: 10.1164/rccm.200503-369OC. [DOI] [PubMed] [Google Scholar]
- 35.Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2007;9:310–18. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suzuki M, Itoh M, Ohta N, et al. Blocking of protease allergens with inhibitors reduces allergic responses in allergic rhinitis and other allergic diseases. Acta Otolaryngol. 2006;126:746–51. doi: 10.1080/00016480500475625. [DOI] [PubMed] [Google Scholar]


