Abstract
The main reason for mortality after lung transplantation is the bronchiolitis obliterans syndrome (BOS), which represents chronic rejection. As soluble CD30, which is produced mainly by activated T helper 2 (Th2) cells, was shown to be related to development of BOS, we aimed to investigate the relation between development of BOS and Th2 chemoattractant thymus and activation regulated chemokine (TARC/CCL17). In 54 patients we measured serum TARC levels prior to transplantation by enzyme-linked immunosorbent assay, and in 44 of these patients sera were analysed at months 1, 2 and 3 after lung transplantation. In addition, longitudinal measurements were performed in sera from eight healthy controls and 14 patients, the latter taken over a period of 2 years post-transplantation from seven patients developing BOS plus seven clinically matched BOS-free patients. Median serum TARC levels post-transplantation of patients who developed BOS were significantly lower than those of the matched BOS-free patients (P = 0·05). A receiver operating characteristics analysis (area under the curve 0·77), together with a Kaplan–Meyer analysis, showed that serum TARC levels below 325 pg/ml in the first month post-transplantation can predict development of BOS post-transplantation (P = 0·001). In contrast, pretransplant serum TARC levels were not significantly different between patients developing BOS, BOS-free patients or healthy controls. In conclusion, pretransplantation serum TARC levels do not predict the development of BOS post-transplantation, but measurement of the serum TARC levels in the first month directly after transplantation can provide us with a tool to identify the group at risk of developing BOS.
Keywords: bronchiolitis obliterans syndrome, lung transplantation, TARC/CCL17
Introduction
Lung transplantation (LTx) is the final treatment option in end-stage lung disease. The proportion of patients living 5 years after LTx is limited to approximately 50%; the main cause of long-term morbidity and mortality is the bronchiolitis obliterans syndrome (BOS), which represents chronic lung allograft rejection [1–3]. Data have shown that 58% of the recipients are diagnosed with BOS within 5 years post-LTx with a median of diagnosis between 16 and 20 months, and it is generally considered that most recipients who survive operative and infectious complications will ultimately develop BOS [4,5].
Due to airflow obstruction and decline of graft function, BOS manifests as the development in a progressive deterioration in forced expiratory volume in 1 second (FEV1), and can be diagnosed by the definitive decline of 20% in FEV1 of the baseline value with no indication for other complications, including infections, acute rejection and suture problems, among others [6–8]. Although the pathogenesis of BOS is unclear, the disease has a patchy character of fibroproliferation and obliteration of the small airways [9]. Several risk factors have been identified, including acute rejection, primary graft dysfunction and ischaemic time of the graft during transplantation, and viral infections such as cytomegalovirus (CMV), gastro-oesophageal reflux disease and human leucocyte antigen mismatches [3,10–13]. None of these factors, however, can be used as clinical markers for the early onset of the disease.
High sCD30 levels prior to LTx were also identified as a risk factor for the development of BOS [14–16]. CD30 is expressed on the surface of T helper 2 (Th2) cells and secreted in the bloodstream as a soluble form (sCD30) upon activation [17]. The relation between sCD30 and BOS led us to speculate that chemokines involved in the recruitment of Th2 cells might also be associated with the development of BOS. The thymus and activation regulated chemokine (TARC/CCL17) can act as a chemoattractant for Th2 cells by binding to the chemokine receptor CCR4 on the surface of these cells [18]. TARC induces recruitment and migration of Th2 cells [18,19]. The chemokine is expressed by various cells, including endothelial cells, dendritic cells, epidermal keratinocytes, fibroblasts, platelets and activated bronchial epithelial cells, and can be up-regulated by proinflammatory cytokines such as tumour necrosis factor (TNF)-α, interleukin (IL)-1 and interferon (IFN)-γ[20–23].
The objective of this study was to investigate whether TARC levels prior to and post-LTx can predict the onset and development of BOS.
Material and methods
Patients
A total of 57 patients (M/F 28/29, average age 46 years, range 18–61) who underwent LTx at the Heart Lung Center in Utrecht, the Netherlands, between October 2001 and July 2007 and who survived more than 3 months were included in this study.
The BOS was defined as a decline of the FEV1 from the post-operative baseline at two distinct time-points of more than 20% in the absence of infection or other aetiology [7].
Standard immunosuppressive therapy consisted of basiliximab, tacrolimus, mycophenolate mofetil and prednisone for all patients. No surveillance bronchoscopies were performed. In patients who had a decline in lung function, infections were diagnosed by cultures of bronchoalveolar lavage fluid and polymerase chain reaction for CMV and Epstein–Barr virus (EBV). When infections were excluded as the cause of FEV1 decline, the patients were treated with corticosteroids and azithromycine. When no increase in lung function was observed, the diagnosis of BOS was made.
Patient follow-up began in September 2004, after approval by the medical–ethical committee and informed consent was obtained from each patient. Forty-four patients donated blood every month in the first year post-transplantation and once every 3 months in the following years. Sera stored for diagnostic purposes from 13 other patients were also included in this study, although they were either transplanted before this date or the serum sampling was not performed systematically, as described above. Pretransplant serum was present from 54 of 57 patients, and sera were available taken monthly from 44 of 57 patients after transplantation up to month 3. TARC levels were determined in these sera and also in sera collected longitudinally up to 25 months post-transplantation in a group of 14 patients, consisting of seven patients who developed BOS who could be matched closely for gender, age, primary disease and follow-up to seven patients who did not develop BOS. Three patients who developed BOS were not included in this longitudinal analysis, due to lack of follow-up time, lack of available serum samples or no clinical match to a non-BOS patient. Characteristics are given in Table 1.
Table 1.
Characteristics of study group
| Matched patient group | Other LTx patients | |||
|---|---|---|---|---|
| BOS | Non-BOS | BOS | Non-BOS | |
| Total number | 7 | 7 | 3 | 40 |
| BOS grade | ||||
| I | 0 | n.a. | 0 | n.a. |
| II | 3 | n.a. | 0 | n.a. |
| III | 4 | n.a. | 3 | n.a. |
| Mean follow-up | 38(69–9) | 39(65–32) | 21(17–33) | 17(76–6) |
| Mean age | 51(24–61) | 50(22–61) | 39(23–58) | 41(17–64) |
| Primary disease | ||||
| CF | 1(14%) | 1(14%) | 2(67%) | 16(40%) |
| Emphysema | 4(57%) | 4(57%) | 0(0%) | 14(35%) |
| Fibrotic disease | 2(29%) | 2(29%) | 1(33%) | 10(25%) |
| Infections | ||||
| CMV | 0(0%) | 1(14%) | 0(0%) | 2(5%) |
| EBV | 0(0%) | 0(0%) | 0(0%) | 1(2·5%) |
| Pseudomonas | 3(42%) | 1(14%) | 2(67%) | 18(45%) |
Characteristics of 14 matched patients for longitudinal study, and the 43 other lung transplant (LTx) patients included in this study. A division is made between the patients who developed bronchiolitis obliterans syndrome (BOS) and the patients who have not developed BOS. Three patients had a cytomegalovirus (CMV) infection; two patients were CMV-negative and received lungs from a CMV-positive donor, one CMV-positive patient received lungs from a CMV-negative donor. One patient had an Epstein–Barr virus (EBV) reactivation. CF, cystic fibrosis; n.a., not available.
Eight healthy [M/F = 5/3, mean age 35 years (range 26–46)] non-allergic and non-smoking controls donated blood every 2 weeks for 6 months and once 5 years later. In total, 442 samples were measured for serum TARC levels.
Enzyme-linked immunosorbent assay
Serum TARC levels were measured in duplicate as described previously [24]. Ninety-six-well enzyme-linked immunosorbent assay plates (Becton Dickinson, Franklin Lakes, NJ, USA) were coated with a murine monoclonal capturing antibody directed against anti-human TARC (MAB364; R&D Systems, Abingdon, UK). Human serum diluted (1/2) was added and standard concentrations (range: 4000 pg/ml–16 pg/ml) were prepared with recombinant human TARC (364-DN; R&D Systems) in phosphate-buffered saline containing 1% bovine serum albumin. Goat polyclonal biotinylated anti-human TARC antibody (BAF364; R&D Systems) was used as the detecting antibody. Horseradish peroxidase–streptavidin conjugate (Zymed, San Francisco, CA, USA) and substrate [3.3′, 5.5′-tetramethylbenzidine substrate; Pierce, Rockford, IL, USA] were used according to the manufacturer's manual. Optical densities were measured at 450 nm with a Thermo Labsystems Multiskan RC plate reader (Waltham, MA, USA). The minimal concentration of TARC that could be detected was 16 pg/ml.
Statistical analysis
To compare the healthy controls with the group of patients for the data prior to transplantation the Mann–Whitney rank-sum test was used. In order to evaluate the median of the non-BOS versus BOS group post-transplantation, or between the patients before and after transplantation, the Wilcoxon signed-rank test was performed. To assess whether serum TARC levels post-transplantation can serve as a BOS predicting factor, a receiver operating curve (ROC) and Kaplan–Meyer curve with a log-rank test were used.
Results
In order to study the relation between serum TARC levels in LTx patients and the development of BOS, 57 patientsand eight healthy controls were included in this study. The median follow-up time of patients after transplantation was 11 months, with a range from 4 to 75 months. Ten patients (19%) developed BOS and five patients died during the course of the study, three of whom were associated with BOS. The median age of the patient population was 50 years (range 17–64 years); their gender was divided equally (M/F = 27/27) and 35%, 36% and 29% suffered from cystic fibrosis (CF), emphysema or fibrotic diseases (fibrosis, sarcoidosis and connective tissue diseases) respectively.
Pretransplant serum TARC and BOS
Analysis of TARC concentrations showed no difference between amounts of TARC present in serum taken prior to transplantation (605 pg/ml ± 380) compared with those in healthy controls (685 pg/ml ± 430). No associations were found between serum TARC concentrations, pre- or post-transplantation, and age, gender or primary disease. Furthermore, the 10 patients eventually developing BOS had the same amounts of pretransplant serum TARC levels as those not developing BOS. These data indicate that pretransplant serum TARC levels were not associated with any of the clinical parameters investigated.
Effect of transplantation and immune suppression on serum TARC levels
To determine whether the transplantation procedure in combination with immunosuppressive therapy had an effect on the serum TARC levels of LTx patients, 44 patients were selected in whom serum TARC was measured pre- and 1 month post-transplantation. As shown in Fig. 1, serum TARC levels decreased in 15 patients; in 14 patients it remained constant, whereas in 15 patients an increasewas found in serum TARC levels. Overall, no significant difference was found between pre- and 1 month post-transplantation TARC levels. The patients who developed BOS are marked by closed symbols. Also for this group, no differences were found pre- and 1 month post-transplantation, as five patients had a decrease, two patients remained the same and three patients had an increase in serum TARC levels. Apparently, the transplantation procedure did not have an effect on serum TARC levels.
Fig. 1.
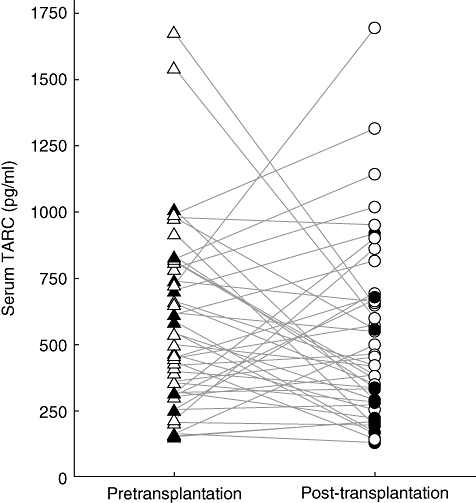
The transplantation procedure does not influence serum thymus and activation regulated chemokine (TARC) levels. Serum TARC levels were measured through enzyme-linked immunosorbent assay for 44 lung transplant patients pretransplantation (triangles) and 1 month post-transplantation (circles). Ten patients who eventually developed BOS are indicated by the filled triangles and circles.
Fourteen patients were followed-up over time and donated blood once every month during the first year and once every 3 months during the years following post-transplantation. The median follow-up time was 40·5 months (range 9–74 months). Seven patients who developed BOS and could be followed longitudinally were matched closely for underlying disease (CF 14%, fibrotic disease 29% and emphysema 57%), age (median age 49 years, range 22–61), gender (male 29%) and follow-up time to seven BOS-free patients.
Serum TARC levels were determined up to 25 months post-transplantation, and for comparison levels were also measured in eight non-allergic non-smoking healthy controls every 2 weeks for 6 months and once 5 years later. This resulted in an average of 13 (9–17) measurements per individual, providing a bandwidth of serum TARC concentration from 22 people, as shown in Fig. 2. The values of serum TARC levels post-transplantation in patients (P1–P14) are in the same range of the healthy controls (N1–N8), indicating that the transplantation plus immune suppression used did not cause the patient's serum TARC levels to differ from that in healthy individuals.
Fig. 2.
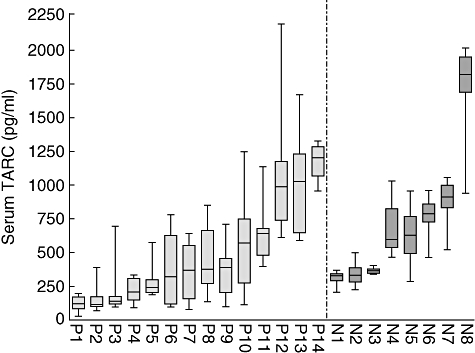
The serum thymus and activation regulated chemokine (TARC) levels of lung transplant patients do not differ from those of healthy controls over time. Longitudinally, an average of 13 (nine to 17) measurements, enzyme-linked immunosorbent assay for serum TARC determined the bandwidth for 22 people. Patients (P1–P14) and healthy controls (N1–N8; non-smoking non-allergic) were ordered by increasing median of serum TARC.
Post-transplant serum TARC and BOS
We next examined whether the course of serum TARC levels is associated with development of BOS, CMV or EBV reactivation or colonization with Pseudomonas. No change was found between the serum TARC levels post-transplantation prior to, during or after onset of BOS or CMV reactivation or Pseudomonas colonization in the group of 14 patients followed longitudinally. A relation with EBV appearance could not be investigated, as none of the 14 patients experienced a primary EBV infection or reactivation (data not shown). Patients experiencing a decline in FEV1, who were treated with corticosteroids and azithromycine, showed no alteration in serum TARC levels; also, no difference in TARC levels could be found between the three patients who developed BOS grade II versus the four patients who developed BOS grade III.
To study whether there was a difference in serum TARC levels post-transplantation between the group of seven patients who developed BOS versus those seven who did not develop BOS, the median of all measurements per person post-transplantation was calculated and the result of the matched patient pairs is displayed in Fig. 3. As shown, for six of seven fully matched patient pairs, the median serum TARC levels of the patients who developed BOS were lower compared with the serum TARC levels of the patients who did not develop BOS. Statistical analysis indicated that median levels of TARC were significantly lower in the patients eventually developing BOS compared with the BOS-free patients (P = 0·05, Wilcoxon's rank sum test), indicating that low levels of TARC post-transplant are a risk factor for BOS.
Fig. 3.
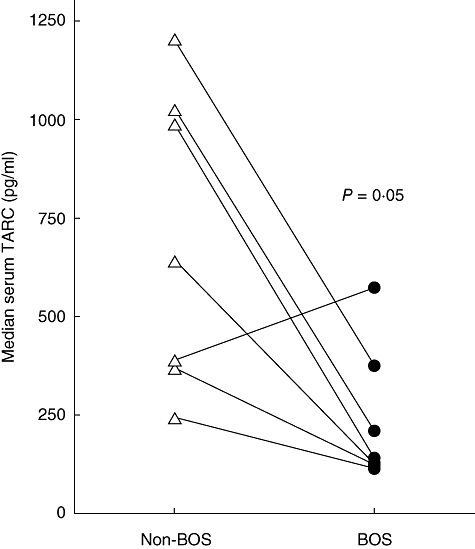
Median serum thymus and activation regulated chemokine (TARC) level is higher post-transplantation in patients without bronchiolitis obliterans syndrome (BOS) (open triangles) compared with patients who developed BOS (closed circles). Patient pairs are connected. Fourteen patients, who were matched closely, were followed longitudinally post-transplantation. An average of 13 samples per patient was used to calculate the median serum TARC levels. Six of seven stable lung transplant patients have a higher median TARC post-transplantation than their matched patient with BOS.
Serum TARC levels as a BOS predicting factor
A ROC curve was used to assess the possibility of predicting BOS by serum TARC levels prior to or during the first 3 months post-transplantation. Measurements of serum TARC were included at the fixed time-points of months 0, 1, 2 or 3 of 44 patients who survived at least 6 months post-transplantation.
Prior to transplantation the ROC curve showed an AUC of 0·53, with a cut-off value of 590 pg/ml. This, however, did not result in a significant difference between the high or low serum TARC group prior to transplantation, nor did any other cut-off value (Fig. 4a).
Fig. 4.
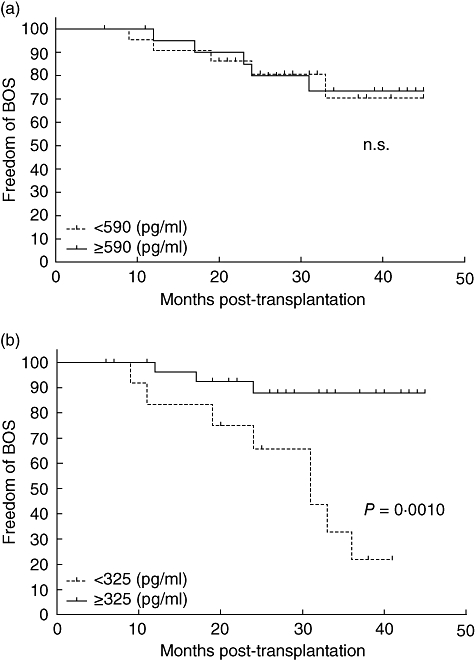
Thymus and activation regulated chemokine (TARC) is a predicting factor for development of bronchiolitis obliterans syndrome (BOS). (a) Prior to transplantation, no significant differences can be seen with regard to freedom from BOS between, respectively, the 21 versus 23 patients with serum TARC levels above versus below 590 pg/ml. (b) The 30 patients with serum TARC levels above 325 pg/ml in the first month post-transplantation show a significantly higher freedom of BOS (P = 0·001) compared with the 14 patients with serum TARC levels below this concentration.
For the 1-month post-transplantation time-point the curve resulted in an AUC of 0·77 (0·59–0·94), which indicates a good predictive factor for the development of BOS within 5 years post-transplantation. The cut-off value defining whether a patient is at risk of developing BOS in the first years post-transplantation, with highest specificity as well as sensitivity, was found at approximately 325 pg/ml serum (range 290–326 pg/ml) TARC, showing a specificity of 71% and a sensitivity of 80%.
This value was used for a Kaplan–Meyer analysis, whichis shown in Fig. 4b. The difference between the group with high serum TARC levels in the first month post-transplantation from those with low serum TARC levels at this time-point was significant (P = 0·001, log-rank test). The group with low serum TARC levels in the first month post-transplantation showed a higher incidence of developing BOS within the first 5 years post-transplantation. However, analysis at the time-points of 2 or 3 months post-transplantation did not reveal significant differences between the high and low serum TARC groups. Therefore, a low serum TARC level, < 325 pg/ml, in the first month post-transplantation is a risk factor for developing BOS.
Discussion
As patients with BOS generally respond poorly to augmented immunosuppressive therapy there is clearly a need for markers that predict the decline in graft performance, allowing the development of a strategy for treatment of patients at risk before onset of BOS. The object of this study was to investigate whether serum TARC levels are associated with the onset and development of BOS. To our knowledge, this is the first study showing that measurement of serum TARC levels after LTx has a predictive value for the development of BOS.
Although the immunosuppressive regimen consisting of tacrolimus and mycophenolate mofetil used in this study is known to suppress cellular (allo)immune responses efficiently, their influence on TARC production is not well known. In studies with atopic dermatitis and allergic asthma patients it was shown that TARC protein and mRNA levels decreased upon treatment with either cyclosporin A, tacrolimus and dexamethasone, or in combination [24–27]. It is unknown, however, whether this decrease in TARC levels was due to a direct effect on TARC production or an indirect effect caused by diminishing of disease activity. Furthermore, TARC can be produced by endothelial cells, dendritic cells, fibroblasts, epidermal keratinocytes and activated bronchial epithelial cells, all which can be affected differentially by immunosuppressives. The main source of TARC in atopic dermatitis seems to be keratinocytes in skin lesions, whereas in allergic asthma it appears to be produced mainly by lung macrophages, indicating that the source of circulating TARC could be actually dependent upon clinical conditions.
In our study we did not see a difference in serum TARC levels measured in a period without or with immune suppression that is previous versus 1-month post-transplantation. Moreover, the levels of serum TARC of LTx patients measured longitudinally after transplantation were comparable to those found in healthy controls. This is an unexpected finding, as up-regulation shortly after organ transplantation has been shown for many other cyto- and chemokines, including IP-10, monocyte chemoattractant protein-1, IL-1β, IL-2, IL-12p40, IL-15, IL-2R, IL-6, IL-8 and IL-1Rα, although IL-10 was found to be decreased [28–31]. We assume that TARC production after transplantation is up-regulated by vigorous allogeneic responses leading to production of known TARC-stimulatory cytokines such as IL-1, IFN-γ and TNF-α[20–23], but inhibited by the immune suppression employed, resulting in serum levels similar to those found in healthy controls. The actual reason for the low serum TARC levels directly after transplantation in patients who will eventually develop BOS remains unknown. It has been suggested that pre-existing subclinical inflammation – with its associated chemokine production – present in the donor lungs prior to transplantation is associated with graft dysfunction and poorer prognosis after transplantation [31]. Alternatively, lowered serum TARC levels also could be due to functional polymorphisms in the promoter region, such as found previously in Japanese individuals [32].
The relation found between low levels of circulating TARC and the development of BOS may be explained by its role as chemoattractant. Recently, it was shown that a subpopulation of Th2 cells expressing CCR4, the receptor for TARC, is characterized by CD4+CD25+ T regulatory (Treg) cells, and it was postulated that antigen-presenting cells in the lungs and activated bronchial epithelium cells can recruit Treg cells towards a site of inflammation through the secretion of TARC [33]. Recruitment of Tregs down-regulates inflammatory responses and limits tissue damage or autoimmunity. A lowered local production of TARC in the lung after transplantation might lead, according to the model described above, to an insufficient recruitment of Treg cells to the sites of ongoing inflammation, which would result in deficient clearing of the chronic inflammatory responses in BOS. The role of Tregs in allograft rejection was also supported in a mouse model using cardiac allografts. In this model, up-regulation of forkhead box P3 expression was shown in the allografts displaying donor-specific tolerance combined with recruitment of Tregs to the allografts through the action of CCR4 and its ligands. [34]. Interestingly, both the Th2 cytokine IL-10, known to suppress inflammatory responses, and IL-12 were also found to be decreased in the BAL of patients with BOS [30,35]. This Treg hypothesis may not seem to fit with published data showing up-regulation of sCD30 prior to BOS [14]. However, in our patient cohort we were not able to reproduce this finding and found instead unaltered sCD30 levels prior to BOS under the current immune suppressive regimen [36]. Moreover, shedding of CD30 from Tregs resulting in increased serum sCD30 levels has not yet been reported.
As TARC is a small molecule of 10·5 kD and leaks to circulation without restriction, it can be expected that serum levels measured after LTx reflect quantities produced locally in the lung, e.g. by mature dendritic cells, monocytes and activated macrophages. This notion is supported by a recent study, showing that TARC levels in serum correlate well with those in BAL in acute eosinophilic pneumonia [37]. A small-scale study showing up-regulation of CCL 19, CCL20 and CCL22 in patients developing BOS did not show an indication of TARC levels in BAL predictive for BOS at months 3 and 6 after transplantation [38]. These data are in line with our results showing no predictive value for serum TARC levels 3 months after transplantation. We conclude that median serum TARC levels post-transplantation in LTx patients without BOS is significantly higher than in those who developed BOS within 5 years after transplantation, and that low serum TARC levels during the first month after LTx is a predictive factor for the development of BOS. These data need to be confirmed in a larger cohort of patients, and the cut-off value of 325 pg/ml with a range of 290–326 pg/ml should be set more precisely in such a study.
Measurement of serum TARC levels in combination with other known risk factors may allow the identification of LTx patients at risk for development of BOS.
References
- 1.Boehler A, Estenne M. Post-transplant bronchiolitis obliterans. Eur Respir J. 2003;22:1007–18. doi: 10.1183/09031936.03.00039103. [DOI] [PubMed] [Google Scholar]
- 2.Hertz MI, Mohacsi PJ, Boucek MM, et al. The registry of the international society for heart and lung transplantation: past, present and future. J Heart Lung Transplant. 2002;21:945–9. doi: 10.1016/s1053-2498(02)00499-0. [DOI] [PubMed] [Google Scholar]
- 3.Trulock EP, Edwards LB, Taylor DO, Boucek MM, Keck BM, Hertz MI. Registry of the international society for heart and lung transplantation: twenty-second official adult lung and heart-lung transplant report–2005. J Heart Lung Transplant. 2005;24:956–67. doi: 10.1016/j.healun.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 4.Boehler A, Kesten S, Weder W, Speich R. Bronchiolitis obliterans after lung transplantation: a review. Chest. 1998;114:1411–26. doi: 10.1378/chest.114.5.1411. [DOI] [PubMed] [Google Scholar]
- 5.Trulock EP. Lung transplantation. Am J Respir Crit Care Med. 1997;155:789–818. doi: 10.1164/ajrccm.155.3.9117010. [DOI] [PubMed] [Google Scholar]
- 6.Burke CM, Theodore J, Dawkins KD, et al. Posttransplant obliterative bronchiolitis and other late lung sequelae in human heart–lung transplantation. Chest. 1984;86:824–9. doi: 10.1378/chest.86.6.824. [DOI] [PubMed] [Google Scholar]
- 7.Estenne M, Hertz MI. Bronchiolitis obliterans after human lung transplantation. Am J Respir Crit Care Med. 2002;166:440–4. doi: 10.1164/rccm.200201-003pp. [DOI] [PubMed] [Google Scholar]
- 8.Estenne M, Maurer JR, Boehler A, et al. Bronchiolitis obliterans syndrome 2001: an update of the diagnostic criteria. J Heart Lung Transplant. 2002;21:297–310. doi: 10.1016/s1053-2498(02)00398-4. [DOI] [PubMed] [Google Scholar]
- 9.Al-Githmi I, Batawil N, Shigemura N, et al. Bronchiolitis obliterans following lung transplantation. Eur J Cardiothorac Surg. 2006;30:846–51. doi: 10.1016/j.ejcts.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 10.Daud SA, Yusen RD, Meyers BF, et al. Impact of immediate primary lung allograft dysfunction on bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 2007;175:507–13. doi: 10.1164/rccm.200608-1079OC. [DOI] [PubMed] [Google Scholar]
- 11.Jaramillo A, Smith MA, Phelan D, et al. Adetected anti-HLA antibodies precedes the development of bronchiolitis obliterans syndrome and correlates with progressive decline in pulmonary function after lung transplantation. Transplantation. 1999;67:1155–61. doi: 10.1097/00007890-199904270-00012. [DOI] [PubMed] [Google Scholar]
- 12.Reinsmoen NL, Nelson K, Zeevi A. Anti-HLA antibody analysis and crossmatching in heart and lung transplantation. Transpl Immunol. 2004;13:63–71. doi: 10.1016/j.trim.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Sharples LD, McNeil K, Stewart S, Wallwork J. Risk factors for bronchiolitis obliterans: a systematic review of recent publications. J Heart Lung Transplant. 2002;21:271–81. doi: 10.1016/s1053-2498(01)00360-6. [DOI] [PubMed] [Google Scholar]
- 14.Bauwens AM, van de Graaf EA, van Ginkel WG, van Kessel DA, Otten HG. Pre-transplant soluble CD30 is associated with bronchiolitis obliterans syndrome after lung transplantation. J Heart Lung Transplant. 2006;25:416–9. doi: 10.1016/j.healun.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 15.Fields RC, Bharat A, Steward N, et al. Elevated soluble CD30 correlates with development of bronchiolitis obliterans syndrome following lung transplantation. Transplantation. 2006;82:1596–601. doi: 10.1097/01.tp.0000241076.46033.4c. [DOI] [PubMed] [Google Scholar]
- 16.Golocheikine AS, Saini D, Ramachandran S, Trulock EP, Patterson A, Mohanakumar T. Soluble CD30 levels as a diagnostic marker for bronchiolitis obliterans syndrome following human lung transplantation. Transpl Immunol. 2008;18:260–3. doi: 10.1016/j.trim.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Del Prete G, De Carli M, D’Elios MM, et al. CD30-mediated signaling promotes the development of human T helper type 2-like T cells. J Exp Med. 1995;182:1655–61. doi: 10.1084/jem.182.6.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imai T, Baba M, Nishimura M, Kakizaki M, Takagi S, Yoshie O. The T cell-directed CC chemokine TARC is a highly specific biological ligand for CC chemokine receptor 4. J Biol Chem. 1997;272:15036–42. doi: 10.1074/jbc.272.23.15036. [DOI] [PubMed] [Google Scholar]
- 19.Bonecchi R, Bianchi G, Bordignon PP, et al. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med. 1998;187:129–34. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berin MC, Eckmann L, Broide DH, Kagnoff MF. Regulated production of the T helper 2-type T-cell chemoattractant TARC by human bronchial epithelial cells in vitro and in human lung xenografts. Am J Respir Cell Mol Biol. 2001;24:382–9. doi: 10.1165/ajrcmb.24.4.4360. [DOI] [PubMed] [Google Scholar]
- 21.Fujisawa T, Fujisawa R, Kato Y, et al. Presence of high contents of thymus and activation-regulated chemokine in platelets and elevated plasma levels of thymus and activation-regulated chemokine and macrophage-derived chemokine in patients with atopic dermatitis. J Allergy Clin Immunol. 2002;110:139–46. doi: 10.1067/mai.2002.126079. [DOI] [PubMed] [Google Scholar]
- 22.Panina-Bordignon P, Papi A, Mariani M, et al. The C-C chemokine receptors CCR4 and CCR8 identify airway T cells of allergen-challenged atopic asthmatics. J Clin Invest. 2001;107:1357–64. doi: 10.1172/JCI12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sekiya T, Miyamasu M, Imanishi M, et al. Inducible expression of a Th2-type CC chemokine thymus- and activation-regulated chemokine by human bronchial epithelial cells. J Immunol. 2000;165:2205–13. doi: 10.4049/jimmunol.165.4.2205. [DOI] [PubMed] [Google Scholar]
- 24.Hijnen D, De Bruin-Weller M, Oosting B, et al. Serum thymus and activation-regulated chemokine (TARC) and cutaneous T cell- attracting chemokine (CTACK) levels in allergic diseases: TARC and CTACK are disease-specific markers for atopic dermatitis. J Allergy Clin Immunol. 2004;113:334–40. doi: 10.1016/j.jaci.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 25.Furukawa H, Nakamura K, Zheng X, et al. Enhanced TARC production by dust-mite allergens and its modulation by immunosuppressive drugs in PBMCs from patients with atopic dermatitis. J Dermatol Sci. 2004;35:35–42. doi: 10.1016/j.jdermsci.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 26.Kakinuma T, Nakamura K, Wakugawa M, et al. Thymus and ctivationregulated chemokine in atopic dermatitis: Serum thymus and activation-regulated chemokine level is closely related with disease activity. J Allergy Clin Immunol. 2001;107:535–41. doi: 10.1067/mai.2001.113237. [DOI] [PubMed] [Google Scholar]
- 27.Kurokawa M, Kokubu F, Matsukura S, et al. Effects of corticosteroid on the expression of thymus and ctivationregulated chemokine in a murine model of allergic asthma. Int Arch Allergy Immunol. 2005;137:60–8. doi: 10.1159/000085434. [DOI] [PubMed] [Google Scholar]
- 28.Belperio JA, Keane MP, Burdick MD, et al. Critical role for the chemokine MCP-1/CCR2 in the pathogenesis of bronchiolitis obliterans syndrome. J Clin Invest. 2001;108:547–56. doi: 10.1172/JCI12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bharat A, Narayanan K, Street T, et al. Early posttransplant inflammation promotes the development of alloimmunity and chronic human lung allograft rejection. Transplantation. 2007;83:150–8. doi: 10.1097/01.tp.0000250579.08042.b6. [DOI] [PubMed] [Google Scholar]
- 30.Reynaud-Gaubert M, Marin V, Thirion X, et al. Upregulation of chemokines in bronchoalveolar lavage fluid as a predictive marker of post-transplant airway obliteration. J Heart Lung Transplant. 2002;21:721–30. doi: 10.1016/s1053-2498(02)00392-3. [DOI] [PubMed] [Google Scholar]
- 31.Scholma J, Slebos DJ, Boezen HM, et al. Eosinophilic granulocytes and interleukin-6 level in bronchoalveolar lavage fluid are associated with the development of obliterative bronchiolitis after lung transplantation. Am J Respir Crit Care Med. 2000;162:2221–5. doi: 10.1164/ajrccm.162.6.9911104. [DOI] [PubMed] [Google Scholar]
- 32.Sekiya T, Tsunemi Y, Miyamasu M, et al. Variations in the human Th2-specific chemokine TARC gene. Immunogenetics. 2003;54:742–5. doi: 10.1007/s00251-002-0520-2. [DOI] [PubMed] [Google Scholar]
- 33.Iellem A, Mariani M, Lang R, et al. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4(+)CD25(+) regulatory T cells. J Exp Med. 2001;194:847–53. doi: 10.1084/jem.194.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee I, Wang L, Wells AD, et al. T regulatory cells mediating allograft tolerance depends on the CCR4 chemokine receptor. J Exp Med. 2005;201:1037–44. doi: 10.1084/jem.20041709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meloni F, Vitulo P, Cascina A, et al. Bronchoalveolar lavage cytokine profile in a cohort of lung transplant recipients: a predictive role of interleukin-12 with respect to onset of bronchiolitis obliterans syndrome. J Heart Lung Transplant. 2004;23:1053–60. doi: 10.1016/j.healun.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 36.Kwakkel- van Erp JM, Otten HG, Paantjens AWM, et al. Soluble CD30 measured after lung transplantation does not predict the bronchiolitis obliterans syndrome in a tacrolimus/mycophenolate mofetil based immunosuppressive regimen. J Heart Lung Transplant. doi: 10.1016/j.healun.2008.06.007. in press. [DOI] [PubMed] [Google Scholar]
- 37.Miyazaki E, Nureki S, Ono E, et al. Circulating thymus- and activation-regulated chemokine/CCL17 is a useful biomarker for discriminating acute eosinophilic pneumonia from other causes of acute lung injury. Chest. 2007;131:1726–34. doi: 10.1378/chest.06-2596. [DOI] [PubMed] [Google Scholar]
- 38.Meloni F, Solari N, Miserere S, et al. Chemokine redundancy in BOS pathogenesis. A possible role also for the CC chemokines: MIP3-beta, MIP3-alpha, MDC and their specific receptors. Transpl Immunol. 2008;18:275–80. doi: 10.1016/j.trim.2007.08.004. [DOI] [PubMed] [Google Scholar]


