SUMMARY
Deciding in which direction to move is a ubiquitous feature of animal behavior, but the neural substrates of locomotor choices are not well understood. The superior colliculus (SC) is a midbrain structure known to be important for controlling the direction of gaze, particularly when guided by visual or auditory cues, but which may play a more general role in behavior involving spatial orienting. To test this idea, we recorded and manipulated activity in the SC of freely-moving rats performing an odor-guided spatial choice task. In this context, not only did a substantial majority of SC neurons encode choice direction during goal-directed locomotion, but many also predicted the upcoming choice and maintained selectivity for it after movement completion. Unilateral inactivation of SC activity profoundly altered spatial choices. These results indicate that the SC processes information necessary for spatial locomotion, suggesting a broad role for this structure in sensory-guided orienting and navigation.
INTRODUCTION
Animals use stimulus cues to guide spatial choices required for seeking out desired resources and avoiding potential hazards in their environment. Despite the importance of sensory-guided locomotion, little is known about its neural bases, in part due to the relative difficulty of performing recordings in freely-moving animals. Although multiple interconnected cortical and subcortical regions are likely to be involved in the selection, execution, and evaluation of spatial choices, a variety of data suggest that the superior colliculus (SC), a midbrain structure with sensory inputs and motor outputs, may play a central role in spatial decision-making critical to directed locomotion.
Across several species, the SC (or optic tectum, in non-mammalian vertebrates) has been intensively studied as an essential component of the neural circuitry controlling orienting (Sparks, 1986; Sparks, 1999). In fish and amphibians, the optic tectum is the principle structure responsible for spatial orienting (Angeles Luque et al., 2005; Ingle and Crews, 1985), while in mammals, the intermediate and deep layers of the SC constitute a final common pathway for coordinated orienting movements of the eyes and the head via descending projections to several motor nuclei (Freedman and Sparks, 1997; May, 2005; Sparks, 1999; Sparks and Hartwich-Young, 1989). The activity of intermediate- and deep-layer SC neurons is correlated with the initiation of contralateral eye and head movements (Cooper et al., 1998; Freedman and Sparks, 1997; Harris, 1980; Horwitz and Newsome, 2001; Mohler and Wurtz, 1976; Schiller and Koerner, 1971; Wurtz and Goldberg, 1971; Wurtz and Goldberg, 1972), and lesions disrupt saccades and induce neglect of contralateral stimuli(Hikosaka and Wurtz, 1985; Ingle, 1973; Schiller et al., 1980; Sinnamon and Garcia, 1988). SC microstimulation in head-fixed animals triggers eye movements (McHaffie and Stein, 1982; Robinson, 1972) and activates neck muscles (Corneil et al., 2002), consistent with observations in unrestrained animals that microstimulation produces movements of the head and body (Dean et al., 1988; Freedman et al., 1996; Harris, 1980; Roucoux et al., 1980; Sahibzada et al., 1986; Salas et al., 1997).
The SC (or optic tectum) is important for more than the control of motor output. In non-mammalian vertebrates, the optic tectum is the principle site of sensory-motor integration (King, 2004). In primates, several pieces of data suggest that the SC is important not only for executing movements but for planning them as well (Carello and Krauzlis, 2004; Glimcher and Sparks, 1992; Horwitz et al., 2004; Horwitz and Newsome, 1999; Horwitz and Newsome, 2001; McPeek and Keller, 2004), and even for covertly orienting attention to a particular region of space (Kustov and Robinson, 1996; Muller et al., 2005). Although most studies have focused on orienting responses to visual cues, the SC also mediates movements triggered by auditory and somatosensory stimuli (Groh and Sparks, 1996; Jay and Sparks, 1987). Thus, the SC may be considered a critical part of the circuitry for sensory-guided orienting decisions (Hikosaka et al., 2006; Krauzlis et al., 2004; Lo and Wang, 2006).
In this study, we sought to examine the role of the SC in spatial choices made by freely-moving animals. Despite the extensive literature discussed above, very few studies have recorded from the SC of unrestrained animals (Pond et al., 1977; Weldon and Best, 1992; Weldon et al., 2007; Weldon et al., 2008), and fewer still have focused on locomotor behavior (Cooper et al., 1998). We hypothesized that, because spatial orientation and directed locomotion are tightly coupled, the SC would be critical to spatial locomotor choices. To study this, we used tetrodes to record simultaneously from several single neurons in the SC of rats performing a sensory-guided spatial choice task (Uchida and Mainen, 2003). We focused on the intermediate and deep layers of the SC because these layers are thought to mediate motor output (Freedman and Sparks, 1997; May, 2005; Sparks, 1999; Sparks and Hartwich-Young, 1989). In this task, an arbitrary odor cue presented at a central port determines whether water will be delivered upon entry into the left or right reward port. After sampling the odor, a well-trained rat will, in one fluid movement, withdraw from the odor port, orient left or right, and enter the selected reward port. This task thus requires that a freely moving animal make a spatial choice but also affords highly reliable timing of task events and a large number of trials. To test our hypothesis, we first analyzed locomotor-related activity before, during, and after the spatial choice, and found that the activity of overlapping populations of cells encoded the spatial choice during all of these periods. We then unilaterally inhibited the SC with muscimol (Martin and Ghez, 1999), and found that spatial choices were affected in a manner predictable from the neural data. Our results suggest that the rat SC is critical for executing goal-directed locomotor choices cued by sensory stimuli, and may play a role in planning such choices and associating them with their outcomes.
RESULTS
We recorded from 258 well-isolated neurons in the intermediate and deep layers of the left SC of four rats performing the odor-guided spatial choice task (Figure 1 and Figure 2; Experimental Procedures). Briefly, the task requires the rat to first sample an odor stimulus presented at a central port, and then to move to either the left or right reward port to receive water (Figures 1A and 1B). In versions of the task in which the stimulus ensemble is limited to well-learned pure odors, rats achieve nearly perfect performance (Uchida and Mainen, 2003). Since a lack of error trials introduces ambiguity in our analyses (i.e., odor identity can not be dissociated from the spatial choice), for each rat we increased the difficulty of the task in one of two ways: either by requiring the rat to learn a novel odor discrimination in each session (Quinlan et al., 2004) (Figure 1C), or by using binary odor mixtures (Uchida and Mainen, 2003) (Figure 1D; Experimental Procedures). Because we focus here on the neural representations of spatial choices and their significance for behavior, and we observed no difference in the data collected during the two paradigms, data were combined across paradigms in all subsequent analyses. For all rats, odor sampling duration (the time from odor valve opening until the rat withdraws its snout from the odor port; Figure 1E) and movement time (odor port withdrawal until reward port entry; Figure 1F) were consistent with previous studies (Feierstein et al., 2006; Uchida and Mainen, 2003). In the following sections, we describe our analyses of the neural activity recorded during, preceding, and following locomotion to the reward port.
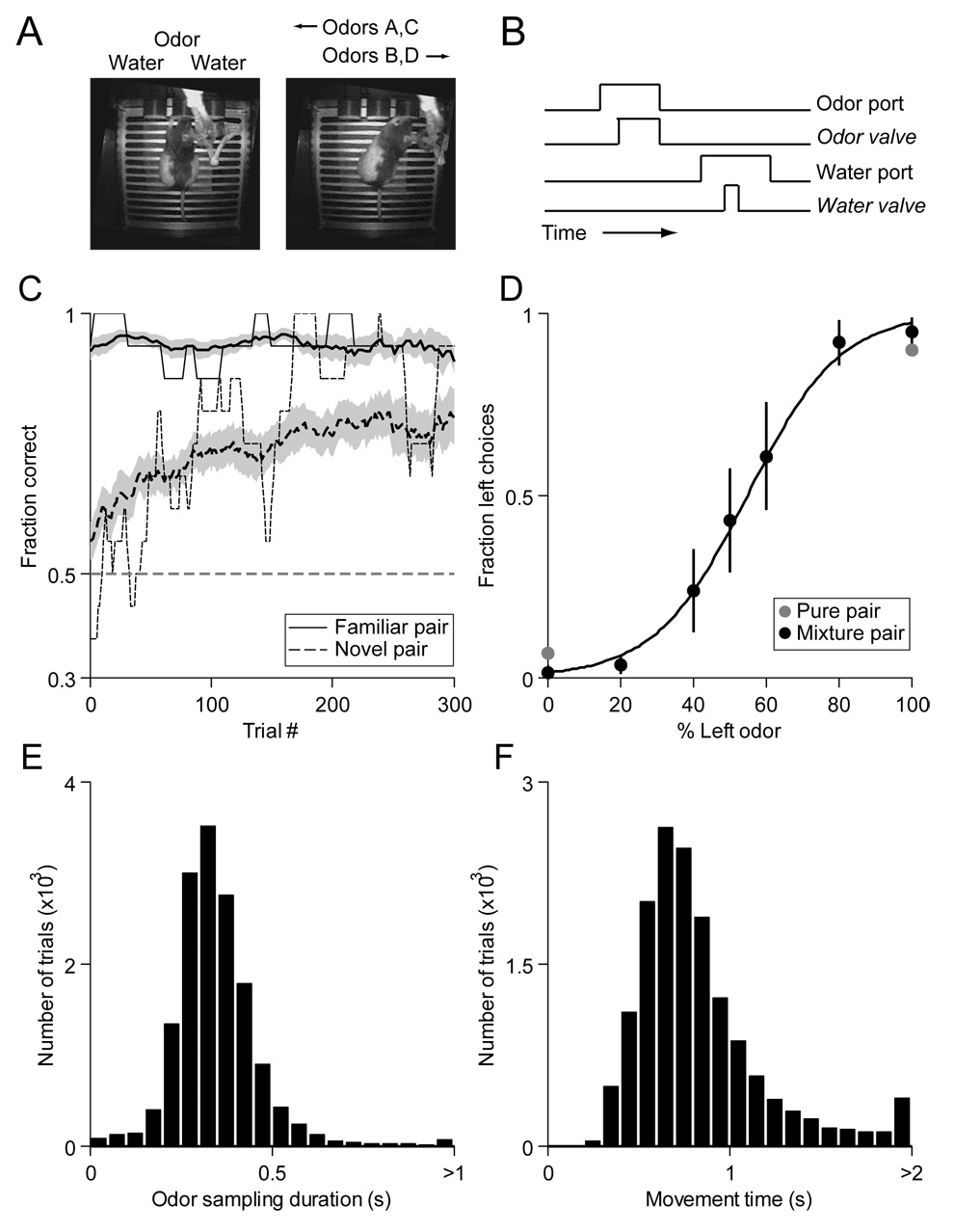
Figure 1. Odor-guided spatial choice task and behavioral performance.
(A) The task environment, showing the implanted rat in the odor port (left image) and the right reward port (right image). In each session, two odors instructed the rat to enter the left reward port, and two instructed the rat to enter the right reward port (Experimental Procedures).
(B) Timing of task events.
(C) Performance in the novel odors paradigm (2 rats). Running average (over 16 trials) of fraction correct as a function of trial number for familiar odors (the pair presented in each session [caproic acid vs. hexanol for one rat; S(+)-2-octanol vs. R(−)-2-octanol for one rat]) and novel odors (new pair each session [Table S1]). Thick line and shading, mean ± SEM. Thin line, example session.
(D) Performance in the mixtures paradigm (2 rats). Line shows best-fit logistic function. Error bars, ± SEM across sessions.
(E) Odor sampling duration (time between opening of odor valve and odor port exit) across all trials, sessions, and rats. Note that this duration does not account for the delay between the odor valve opening and the odor reaching the rat, since the delay is not relevant to the analyses performed here.
(F) Movement time (time between odor port exit and reward port entry) across all trials, sessions, and rats. Long movement times indicate trials in which the rat may not have moved directly from the odor port to the ultimately selected reward port; trials with movement times > 1 s were thus excluded from all subsequent analyses.
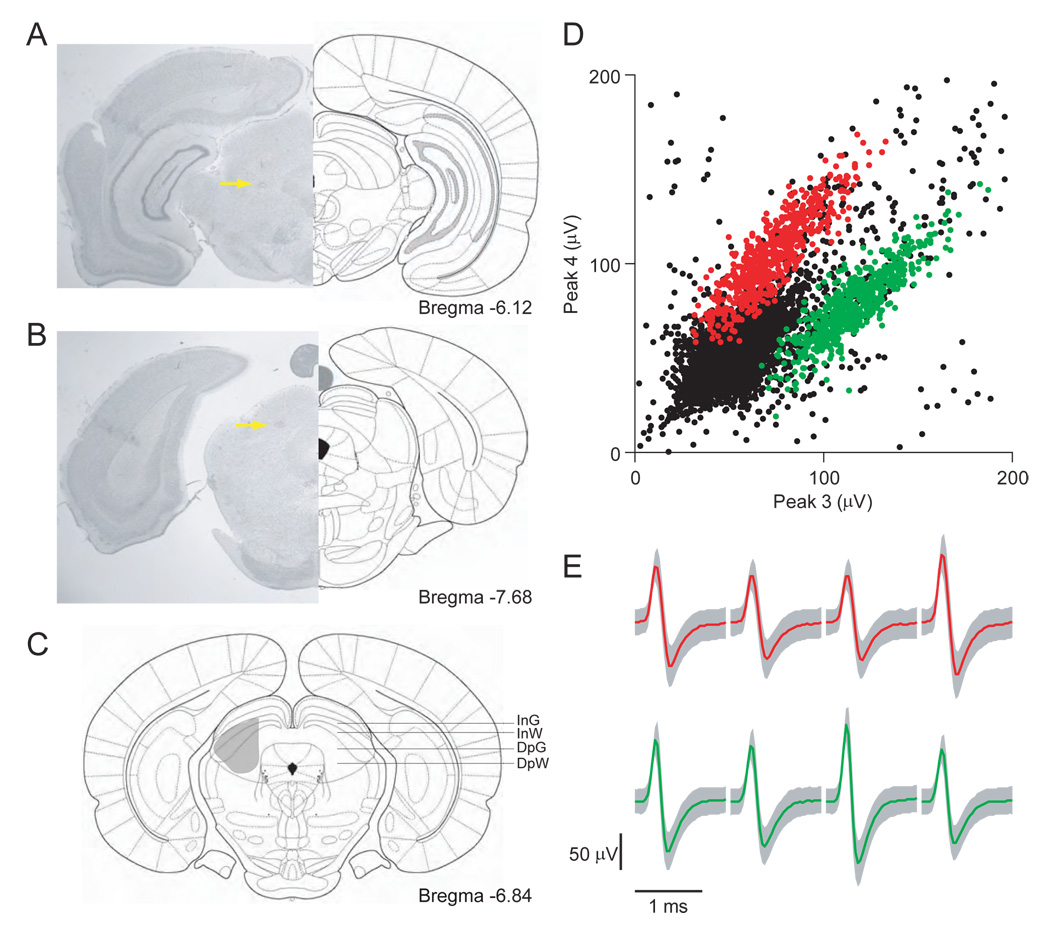
Figure 2. Localization of recording sites and spike clustering.
(A) Rostral-most confirmed recording site. Arrow shows representative electrolytic lesion made after final recording session. Note tetrode track visible above lesion.
(B) Caudal-most confirmed recording site.
(C) Shaded area shows estimated mediolateral and dorsoventral extent of recordings, reconstructed from lesions and visible tetrode tracks. InG, intermediate gray layer; DpG, deep gray layer; InW, intermediate white layer; DpW, deep white layer.
(D) Peaks of waveforms from lead 4 plotted against peaks of waveforms from lead 3 of one tetrode for a representative recording session. Red and green points show waveform peaks recorded from distinct cells. 10,000 points are shown.
(E) Mean ± SD waveforms recorded on all 4 leads, corresponding to red and green points in (D).
Direction Selectivity During Locomotion
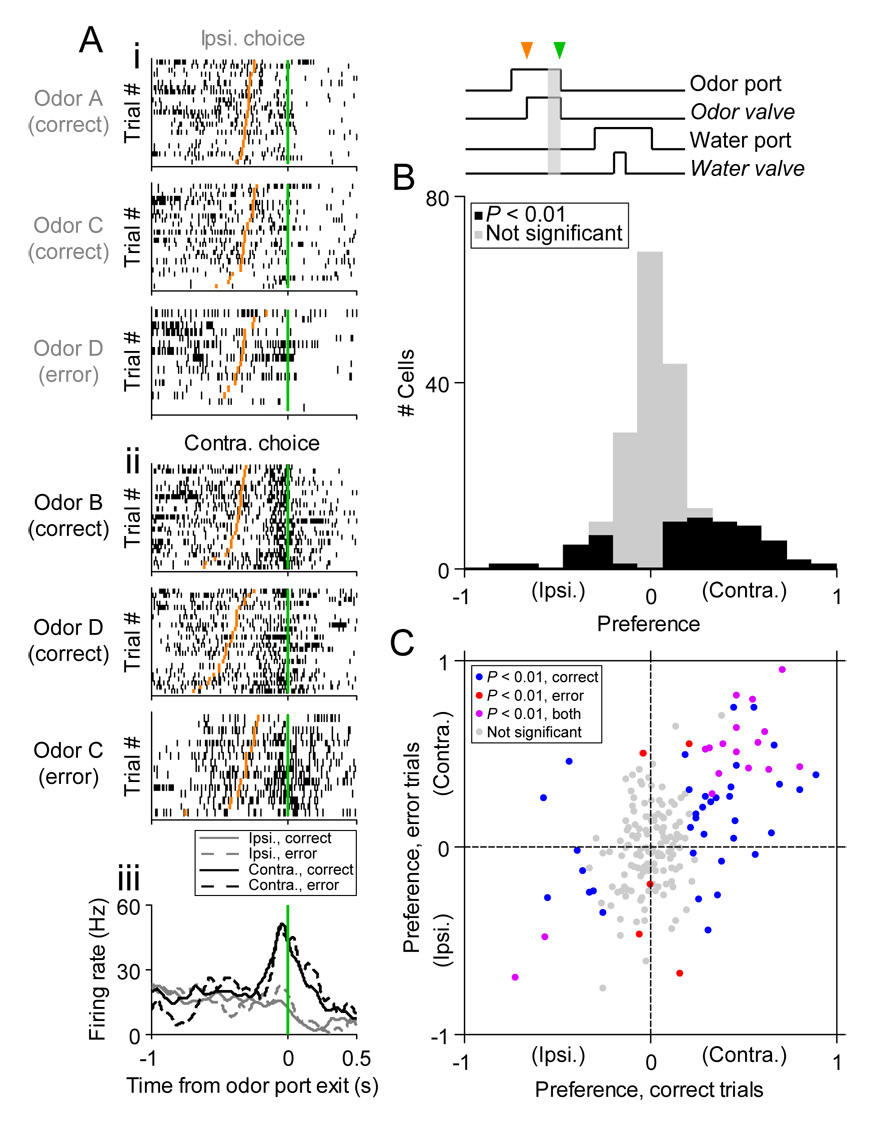
We first focused on neural activity as the rat moved from the odor port to the reward port. For most of the cells recorded, we found that firing rate depended on whether the movement was toward the reward port ipsilateral or contralateral to the recording site (always the left SC; Figures 3A and 3B). In order to quantify the dependence of firing rate on movement direction, we used an ROC analysis to calculate a “preference” index for each cell (Feierstein et al., 2006; Green and Swets, 1966) (Experimental Procedures). The ROC metric reflects how often an ideal observer can correctly discriminate whether a given firing rate was recorded during leftward or rightward locomotion. Preference ranges from −1 to 1, where negative values reflect a higher firing rate during leftward movement (“prefers ipsilateral”), positive values reflect a higher firing rate during rightward movement (“prefers contralateral”), and a larger magnitude corresponds to more accurate discrimination by the ideal observer. We determined the significance of the preference using a permutation test (Experimental Procedures). Across the population, we found that many cells significantly preferred locomotion in one direction (P < 0.01, permutation test), and the proportion of significant preference for ipsilateral and contralateral choices was not significantly different (P = 0.34, χ2 test; Figure 3C).
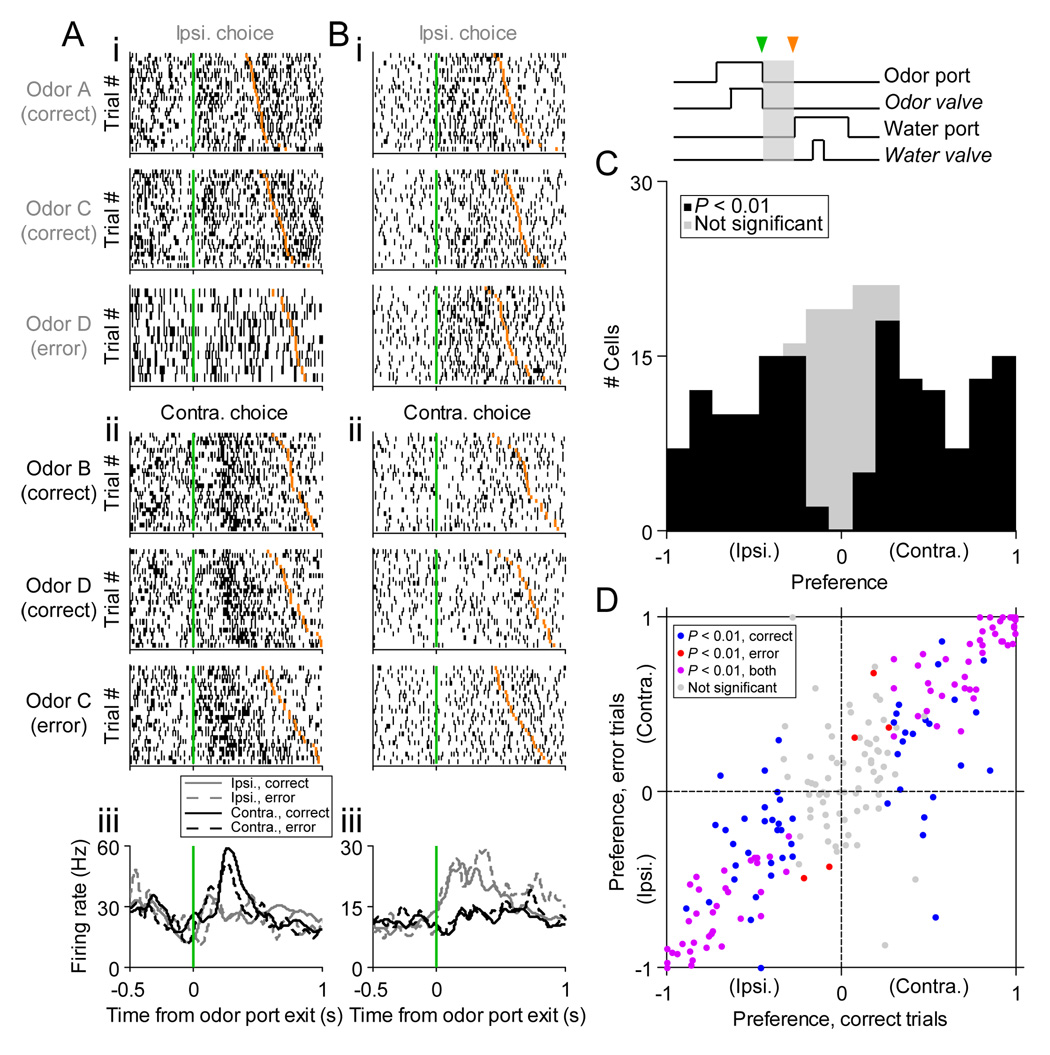
Figure 3. Direction preference during locomotion to reward port.
(A) Rasters and perievent histograms for an example cell recorded during the novel odors paradigm that prefers contralateral movement. i: Trials in which the reward port ipsilateral to the recording site (left SC) was selected. Each row shows spikes (black ticks) in 1 trial, aligned to time of odor port exit (green line). Orange ticks, times of reward port entry. Trials are grouped by odor and within each group are sorted by movement time (Figure 1F). For these and subsequent rasters, 25 pseudorandomly selected trials are shown per category (unless fewer than 25 trials in that category were performed). Note that no Odor B trials are shown because the rat did not choose the ipsilateral (left) reward port during Odor B trials in this session. ii: Trials in which the contralateral (right) reward port was selected, organized as above. iii: Perievent histograms showing average activity across trials. Histograms are averaged across odors, and smoothed with a Gaussian filter (σ2 = 23 ms).
(B) Same as (A), for a second cell preferring the ipsilateral choice during locomotion.
(C) Histogram of choice preferences across population (210 cells that met criteria for trials and firing rate [Experimental Procedures]). Gray box in task events diagram shows epoch in which preference was calculated; green arrowhead, odor port exit; orange arrowhead, reward port entry).
(D) Preference calculated during correct trials plotted against preference calculated during error trials, within each odor pair. Similar values for correct and error trials indicate that firing rate is modulated by movement direction, and not by odor identity.
Since most trials were performed correctly (Figures 1C and 1D), odor identity and choice direction were correlated across trials. It is therefore possible that the preference that we have attributed to direction could more accurately reflect a preference for the recently sampled odor. However, since multiple odors were associated with each reward port, and since a sufficient number of errors were made, we can dissociate preference for odor and direction. As shown in the example (Figure 3A), the activity of the cell during ipsilateral movement (its preferred direction) did not depend on which of three odors (A, C, or D) was presented. To address this issue across the population, we calculated the direction preference separately for correct trials and for error trials, within each odor pair. For a cell that prefers a particular direction, the preference calculated during correct and error trials would be approximately equal (within the limits imposed by firing rate variability across trials), falling along the line x = y. Cells that prefer a particular odor should show preference values of the opposite sign for correct and error trials, falling along the line x = −y. Clearly, preference is correlated for correct and error trials (Figure 3D), demonstrating that SC activity during locomotion reflects the current direction of movement, and not the identity of the recently sampled odor.
The examples shown in Figure 3 indicate that, during the movement, the time course of direction preference varies across neurons. The cell in Figure 3A shows a sharp peak in firing rate during rightward locomotion approximately 400 ms after movement initiation, while the cell in Figure 3B shows increased activity immediately after the start of an ipsilateral movement that seems to last until the rat enters the reward port. To quantify the dynamics of direction preference, for each cell we calculated a “preference curve” by computing the preference and its significance in short windows during and preceding the movement (in overlapping 200 ms windows, starting every 20 ms). These curves reveal how the direction preference of each cell evolves as the movement is planned and executed (Figure 4B). We then calculated three measures from each preference curve:
The time at which a significant preference for direction was first evident (P < 0.01, permutation test; Figure 4C).
The time corresponding to the center of mass of the significant points (P < 0.01, permutation test) of the preference curve (Figure 4D). Calculating the center of mass based on all points (regardless of their significance) in the preference curve yields similar results.
The duration over which direction preference was significant (P < 0.01, permutation test; Figure 4E).
Although the behavior of the population was heterogeneous, many cells were selective for direction very early during, or even before the initiation of, the movement, and remained selective for a large fraction of the movement, often until its completion. These results were independent of the size of the window in which preference was calculated (Figure S1). In the next sections, we focus on neural activity preceding and following the movement.
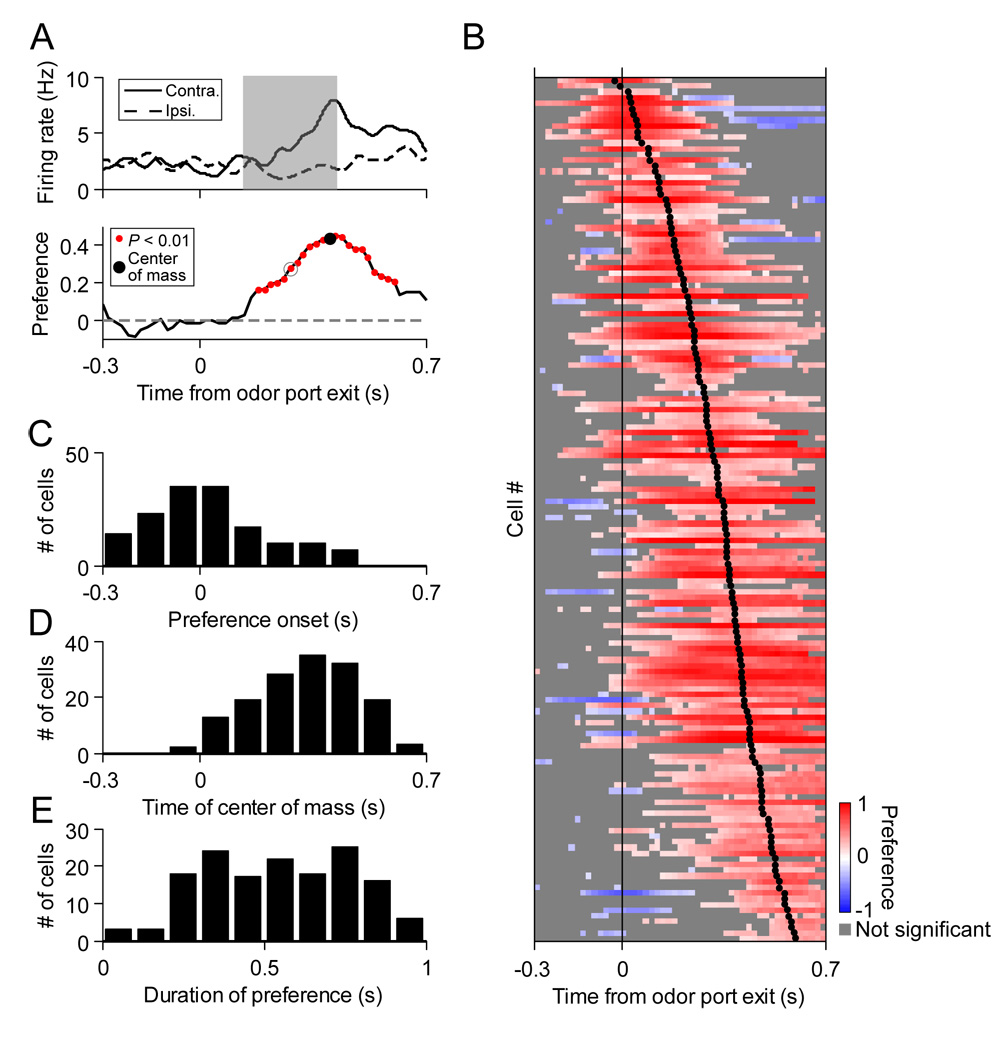
Figure 4. Dynamics of direction preference preceding and during locomotion.
(A) Upper, perievent histograms during locomotion for one cell. Lower, corresponding “preference curve.” Each point (e.g., in gray circle) corresponds to the direction preference (Experimental Procedures) calculated in the surrounding 200 ms window (e.g., gray box in perievent histograms).
(B) Preference curves for all significantly direction-selective cells (P < 0.01, permutation test; 152 cells), sorted by time of center of mass of preference curve. Each row corresponds to one cell. Preference curves were calculated by sliding the 200 ms window by 20 ms increments. Trials are aligned to odor port exit. Color scale shows significant preferences (P < 0.01, permutation test; positive values correspond to the preferred direction calculated during the entire movement time [as in Figure 3C]). Gray boxes indicate bins with nonsignificant preferences (P > 0.01, permutation test) or with fewer than 15 ipsilateral or contralateral trials. Black dots, centers of mass of preference curves. Note that for some cells, the preferred direction changes during locomotion (corresponding to the blue bins).
(C) Time of first significant preference bin, relative to odor port exit, for each cell.
(D) Time of center of mass of preference curve, relative to odor port exit, for each cell.
(E) Duration of significant positive preference for each cell.
Direction Selectivity Preceding Locomotion
Many cells appear to be prospectively direction-selective for the movement that is about to be initiated (Figure 4B and Figure 5A). We quantified this by calculating the preference for the direction of the upcoming choice based on the firing rate during the 100 ms preceding movement initiation. Across the population, in contrast to the distribution of preferences during the movement itself (Figure 3C), more SC neurons significantly preferred (P < 0.01, permutation test) upcoming contralateral movements to ipsilateral movements (P < 10−5, χ2 test; Figure 5B; the results were similar when we considered the entire odor sampling duration [Figure S2A]). Since in this analysis direction preference was calculated while the odor was presented, we again asked whether this metric could reflect the identity of the odor, and not the direction of movement. The example (Figure 5A) suggests that this is not the case: Firing rate is higher preceding contralateral movements than ipsilateral movements, independent of the odor presented. Across the population, we performed the same error analysis as described above (Figure 3D), which again demonstrated a preference for the direction of locomotion, and not odor identity (Figure 5C). Thus, the increase in activity of a subpopulation of rat SC neurons signals the early phase of execution, and perhaps the initiation, of contralateral locomotor choices.
Figure 5. Direction preference preceding locomotion to reward port.
(A) Rasters and perievent histograms for an example cell that prefers upcoming contralateral choice. i: Trials in which the ipsilateral reward port was selected, aligned to time of odor port exit (green line). Orange ticks, times at which odor valve opened. Trials are grouped by odor and within each group are sorted by odor sampling duration (Figure 1E). ii: Trials in which the contralateral reward port was selected, organized as above. iii: Perievent histograms showing average activity across trials.
(B) Histogram of direction preferences across population (199 cells that met criteria for trials and firing rate [Experimental Procedures]). Gray box in task events diagram shows epoch in which preference was calculated (100 ms preceding odor port exit); orange arrowhead, odor valve open; green arrowhead, odor port exit.
(C) Preference calculated during correct trials plotted against preference calculated during error trials, within each odor pair.
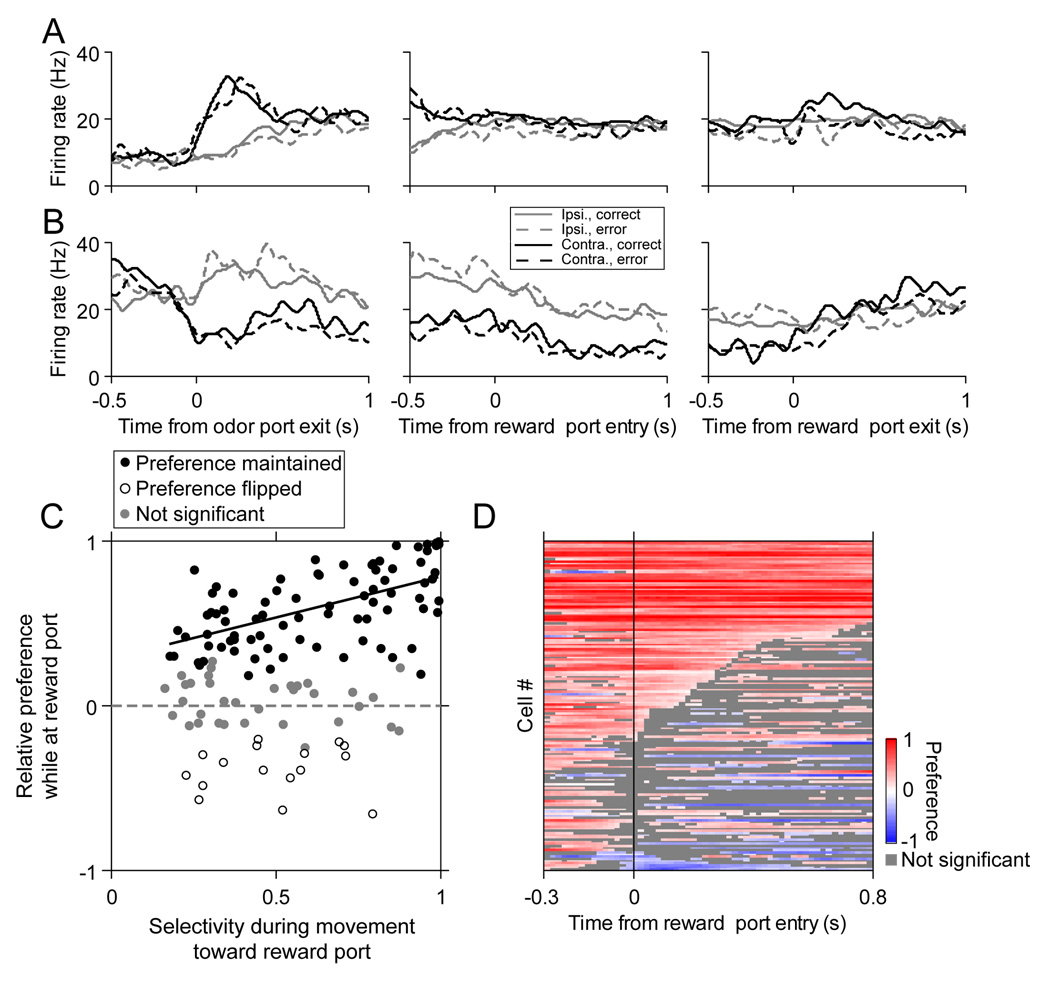
Direction Selectivity Following Locomotion
Our analyses of the dynamics of direction selectivity during locomotion suggest that many cells remained direction-selective at least until the end of the movement (Figure 4B). Note that such a cell may not appear to exhibit significant direction preference at the maximum time bin shown in Figure 4B (500 to 700 ms following reward port entry) because the movement is often completed well before that time (Figure 1F). Does the selectivity disappear once the movement is completed, or does it persist? By aligning neural activity to the time of reward port entry and exit, we can see that some cells were direction-selective only during locomotion (Figure 6A), but other cells remained selective after movement completion, until the rat exited the reward port to return to the odor port for the next trial (Figure 6B). We quantified this persistence by calculating ipsilateral vs. contralateral preference, as above, during the 500 ms following entry into the reward port, and comparing this value to the direction preference calculated during locomotion. We found that, for a significant fraction of the direction-selective cells, the preferred reward port (ipsilateral or contralateral) corresponded to the preferred direction during locomotion (Figure 6C; P < 10−5, χ2 test; only correct trials were included in this analysis because the rat often exited the reward port quickly if it was not rewarded). Within this population of cells, the magnitude of the preference calculated while the rat was at the reward port depended on the magnitude of direction selectivity during movement (Figure 6C, solid black line; positive slope of best-fit line: P < 0.01, bootstrap resampling; similar results were obtained when we calculated preference during the 1000 ms following reward port entry [Figure S2B]). For how long is preference maintained after reward port entry? To address this, we calculated the preference for the ipsilateral or contralateral reward port in overlapping 200 ms windows (as described above for Figure 4B), aligned to reward port entry. We found that across the population there was a wide range of times during which selectivity persisted (Figure 6D; note that activity following exit from the reward port in each trial is excluded). Although our focus is on locomotion toward the reward, we also analyzed left/right preference during the return of the rat to the odor port in order to initiate the next trial. We found that some cells maintained their directional preference during locomotion back to the odor port (e.g., they prefer moving to the right reward port and from the left reward port), but more cells actually maintained their spatial preference (e.g., they prefer moving to and from the right reward port, independent of the direction of motion) (P < 0.05, χ2 test; Figure S3). Thus, preference for a particular spatial choice during locomotion often persisted long after the movement itself was completed.
Figure 6. Persistence of direction selectivity after locomotion.
(A) Perievent histograms, aligned to different task events (odor port exit, reward port entry, and reward port exit), for an example cell that prefers contralateral choice only during locomotion to the reward port.
(B) As in (A), for a cell in which ipsilateral preference is maintained while the rat is at the reward port.
(C) Abscissa shows selectivity (i.e., magnitude of preference) during locomotion to the reward port. Ordinate shows relative preference while at the reward port (starting at reward port entry and lasting 500 ms). Positive values indicate preference for the same side while at the reward port as during locomotion, negative values indicate preference for the opposite side. Only cells that significantly prefer a direction during movement (P < 0.01, permutation test) and that met criteria for trials and firing rate (Experimental Procedures) are shown (132 cells). Black circles, cells with significant preference at the reward port (P < 0.01, permutation test) for the same (filled) or the opposite (open) side as during the movement; gray filled circles, no significant preference at the reward port. Black line, best fit line to solid black points. Gray dashed line, y = 0.
(D) Preference curves (calculated as described in Figure 4) for all significantly direction-selective cells (P < 0.01, permutation test; 152 cells), aligned to reward port entry and sorted by length of uninterrupted time following reward port entry during which direction selectivity persisted. Color scale as in Figure 4B.
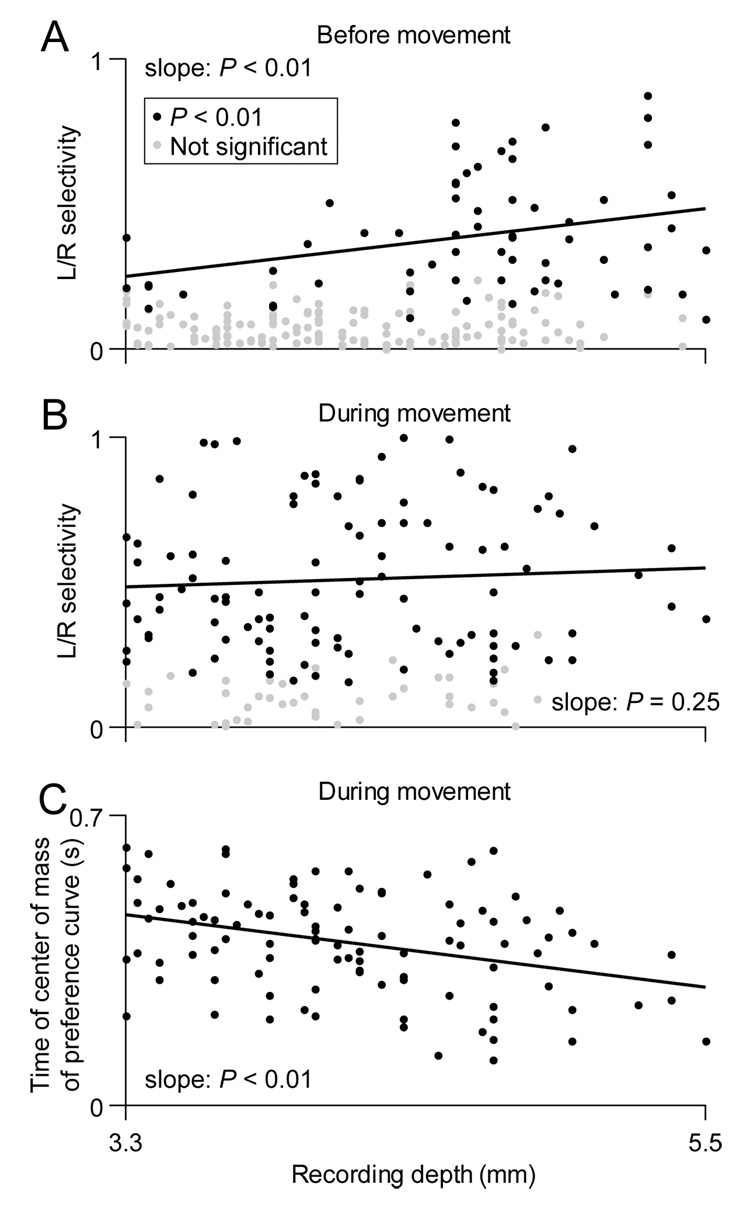
Depth-Dependence of Selectivity
The SC can be subdivided into several anatomical layers that have been shown to mediate specific functions, such as the processing of visual input superficially and motor output in deep layers (Huerta and Harting, 1984). We therefore analyzed whether the direction and outcome selectivity that we have described were dependent on the depth of the recording site. We found that deeper cells tended to exhibit stronger direction selectivity during the early phase of movement execution (Figure 7A; positive slope of best-fit line: P < 0.01, bootstrap resampling). During locomotion, however, the direction selectivity of cells that were not already direction-selective before movement was independent of depth (Figure 7B; non-zero slope of best-fit line: P =0.25, bootstrap resampling). We next looked at how the dynamics of direction selectivity during locomotion (Figure 4) depended on depth for those cells that were not selective preceding locomotion. We found that deeper cells tended to reach their peak selectivity (measured as in Figure 4D) earlier during the movement period (Figure 7C; negative slope of best-fit line: P < 0.01, bootstrap resampling). Thus, the timing, but not the strength, of selectivity during locomotion depended on depth. Together, these results suggest a dorsoventral organization of spatial computations within the rat SC.
Figure 7. Depth-dependence of direction and outcome selectivity.
(A) Direction selectivity preceding locomotion (Figure 5) as a function of recording depth, which ranged from the dorsal-most aspect of the intermediate layers to the ventral- most aspect of the deep layers (Figure 2). Data shown are from same cells as in Figure 5B. Slope of regression was significantly positive (P < 0.01, bootstrap resampling).
(B) Direction selectivity during locomotion (Figure 3) as a function of recording depth. Data shown are from same cells as in Figure 3C. Slope of regression (black line) was not significantly different from zero (P = 0.25, bootstrap resampling).
(C) Time of center of mass of preference curve during locomotion (Figure 4) as a function of recording depth. Only cells that were direction-selective during, but not preceding, locomotion are included (103 cells that met criteria for trials and firing rate). Slope of regression was significantly negative (P < 0.01, bootstrap resampling).
Unilateral Inactivation
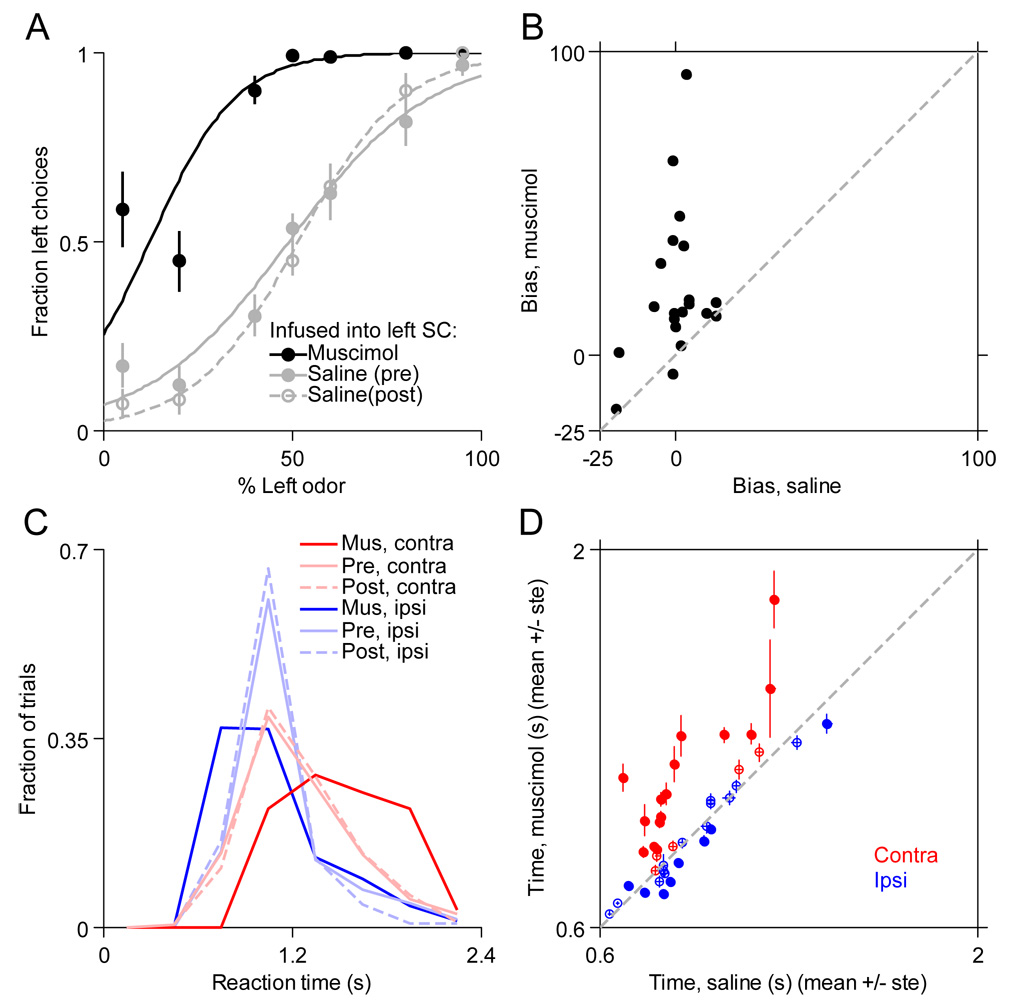
In order to determine whether the locomotor choice-related selectivity we have observed is necessary for, or simply correlated with, the execution of goal-directed locomotion, we unilaterally inactivated the SC in four rats performing the spatial choice task with odor mixtures (Experimental Procedures). This allowed us to vary the strength of the sensory cue from trial-to-trial and thereby obtain a psychometric choice function under control and unilateral inactivation conditions. On alternate days, prior to the behavioral session, we infused 0.5 µl of either saline or the reversible GABAA agonist muscimol (0.04 mg/ml) via a chronically implanted cannula into the intermediate and deep layers of either the left or right SC (Experimental Procedures; Figure S4C). We first asked whether muscimol affected the probability of choosing the left or right reward port, by comparing the psychometric functions for the muscimol session and its preceding and following saline sessions. Since many more SC cells preferred contralateral rather than ipsilateral choices during the initial phase of locomotion (Figure 5C), we expected SC inactivation to bias the rat toward the reward port ipsilateral to the inactivated side. This is clearly the case for the example sessions shown, in which the left SC was inactivated (Figure 8A). We quantified the magnitude and direction of this choice bias from each psychometric function, where positive values reflect ipsilateral bias (Experimental Procedures). We found that the bias was larger (i.e., more ipsilateral) during muscimol sessions than the corresponding saline sessions (Figure 8B; P < 0.001, t-test; 20 muscimol sessions). Note that the bias during saline sessions was occasionally nonzero because animals sometimes developed an idiosyncratic preference for one side, but even in these cases the rats were still biased more ipsilaterally during muscimol than saline sessions. Thus, inactivation of a given SC biased the rats toward the inactivated side, as predicted from our analyses of the neural activity.
Figure 8. Unilateral reversible SC inactivation.
(A) Psychometric curves during example sessions in which 0.5 μl of either 0.04 mg/ml muscimol (black circles) or 0.9% saline (gray circles) was infused into the left SC. Saline was infused during the session before (filled circles) and after (open circles) the muscimol session. 1 session was performed per day. Lines show best-fit logistic functions. Error bars, ± SEM.
(B) Biases of psychometric functions for saline sessions (mean of pre and post) and corresponding muscimol sessions, calculated from the best-fit logistic function (Experimental Procedures). Positive values reflect ipsilateral bias (i.e., a preponderance of choices ispilateral to the side of infusion). Bias was more ipsilateral during muscimol than saline sessions in nearly all cases.
(C) Distribution of reaction times (odor sampling duration + movement time) for ipsilateral (blue) and contralateral (red) trials during the same sessions shown in (A) (see legend).
(D) Mean reaction times during muscimol sessions plotted against mean reaction times during corresponding saline sessions for contralateral (red) and ipsilateral (blue) choices. Filled circles show individual sessions in which muscimol reaction times were different from saline reaction times (P < 0.01, t-test). Error bars, ± SEM.
Although unilateral muscimol infusion biased the rats ipsilaterally, they still made some contralateral choices (e.g., Figure 8A). When such choices were made, were they identical during muscimol and saline sessions? For each trial, we calculated the reaction time as the time from the opening of the odor valve to entry into the reward port (i.e., the combined odor sampling duration [Figure 1E] and movement time [Figure 1F]). In Figure 8C, the probability density functions of reaction times are shown separately for contralateral and ipsilateral choices during the same example sessions shown in Figure 8A. Reaction times for contralateral choices tend to be longer during muscimol than saline sessions, while reaction times for ipsilateral choices tend to be shorter during muscimol than saline sessions. This was the case for contralateral choices in 15/20 individual sessions (P < 0.01, t-test) and for ipsilateral choices in 7/21 individual sessions (P < 0.01, t-test), and when the means of the distributions were considered as a group (Figure 8D; P < 0.02 for means of ipsilateral and contralateral distributions, t-tests; note that in 1 session, no contralateral choices were made). These results suggest that SC activity is necessary for normal spatial locomotor choices.
DISCUSSION
In this study, we examined representations in the SC that underlie the goal-directed locomotion demanded by an odor-cued spatial choice task. We found that the neural activity of overlapping populations of neurons was dependent on the spatial choice (left vs. right) made before, during, and after movement execution, and that unilateral inactivation of the SC biases spatial choices ipsilateral to the inactivated side. These results suggest that the SC is involved in spatial choices during goal-directed locomotion.
Previous research in primates has shown that the SC is a critical component of the circuitry responsible for orienting gaze and attention toward salient stimuli through eye and head movements (Freedman and Sparks, 1997; Horwitz and Newsome, 2001; Sparks, 1999; Wurtz and Goldberg, 1972). Our data (Figure 5) are consistent with the idea that the SC is involved in similar processes in rats (Dean et al., 1989; McHaffie and Stein, 1982; Redgrave and Gurney, 2006; Sahibzada et al., 1986), and extend previous findings by demonstrating that in freely-moving animals, the SC is also important for the execution of spatially-specific locomotor responses. Moreover, while the SC is known to be important for orienting to auditory and somatosensory in addition to visual stimuli (Groh and Sparks, 1996; Jay and Sparks, 1987), here we show that SC is also critical for orienting triggered by olfactory stimuli. Together, our findings suggest an even broader role for the SC in the orientation of attention and the execution of orientation-dependent actions than had previously been appreciated.
Saccade-related SC neurons in primates tend to show a build-up or burst of activity prior to an eye movement with a rapid reduction after its initiation, and in most cells there is little activity after movement completion (Munoz and Wurtz, 1995; Wurtz and Goldberg, 1972). In contrast, we typically observed direction-selectivity during movement (Figure 3 and Figure 4), which often endured long after the movement was completed (Figure 6 and Figure S3). What is the function of this persistent direction selectivity? One possibility is that the activity signals the discrepancy between the preferred movement amplitude for the neuron under study and the actual amplitude of the executed movement (Waitzman et al., 1988). Another possibility is that it serves to integrate representations of spatial choice (Figure 3) with task outcome (i.e., whether reward was received), which could be important for learning the relationship between actions and their consequences and therefore the value of performing a particular action in a given context (Sutton and Barto, 1998). Indeed, recent studies have shown that SC activity in rats is modulated by the presence or magnitude of reward (Weldon et al., 2007; Weldon et al., 2008), and it has been suggested that the SC is responsible for assigning value to stimuli and actions via its projection to the substantia nigra (Redgrave and Gurney, 2006; Redgrave et al., 2007).
Is the neural activity described here actually necessary for, or simply correlated with, locomotor choices? To address this, we studied how movements were affected by inactivating the activity in one SC with muscimol (Figure 8). Consistent with previous observations (Sinnamon and Garcia, 1988; Wang and Redgrave, 1997), we found that choices were biased ipsilateral to the inhibited SC (Figure 7Aand Figure 7B) and that contralateral reaction times were increased (Figure 7C and Figure 7D). These data suggest an essential role for the SC in producing contralateral locomotor responses (Figure 5B). Interestingly, we also found that reaction times for locomotion ipsilateral to the inactivated SC were decreased (Figure 7C and Figure 7D). This observation supports a model in which locomotion direction is determined by the “winner” of a competition between the left and right SC (Lo and Wang, 2006; McPeek and Keller, 2004). The idea is that inactivating the, e.g., left SC increases the probability that the right SC will dominate the competition, resulting in a leftward choice. The competition may be sharpened by inhibition between the left and right SC (Edwards, 1977), such that decreased activity in one SC directly leads to increased activity in the other. Thus, our reaction time analysis suggests that competitive interactions in the rat SC may be involved in selecting upcoming choice direction (McPeek and Keller, 2004), a process that the SC has been proposed to mediate in primates (Carello and Krauzlis, 2004; Glimcher and Sparks, 1992; Horwitz et al., 2004; McPeek and Keller, 2004).
SC activity early in movement execution (Figure 5) may reflect the implementation of a selection process, or a command to initiate movement, that occurred in an efferent brain region, such as the motor cortex or basal ganglia (Hikosaka et al., 2006; Lo and Wang, 2006). It is also possible that the process of movement selection is distributed among several regions along the sensorimotor pathway, from areas that process sensory input to those required for motor output (Koulakov et al., 2005; Shadlen and Newsome, 2001). It is difficult to dissociate neural activity underlying movement selection from that underlying movement execution in the context of the task described here, since the rat is free to execute its movement as soon as it selects a direction. Future electrophysiology studies could address the role of the SC in movement selection more directly; for example, by recording neural activity during a delayed-response version of the spatial choice task, in which the movement time and the presumed decision time are temporally dissociated (Shadlen and Newsome, 2001).
Although we found some dependence of neural response properties on the depth of the recording site (Figure 7), it is perhaps surprising that, given the differences in connectivity and morphology between the intermediate and deep layers, we did not observe more striking differences across layers (indeed, this is why they were combined in most of our analyses). This may be due to a high degree of within-layer variability resulting from the fact that there are several distinct cell classes within each layer of the rat SC (Saito and Isa, 1999), each of which may exhibit a different pattern of activity. As molecular tools are developed that allow for recordings targeted to specific cell-types (Aravanis et al., 2007), we may be able to identify how each of these classes contributes to overall SC function.
Since we did not record muscle activity or attempt to analyze detailed eye and head movements, we do not know the precise relationship between the SC activity described here and the many individual motor components underlying spatially-directed locomotor actions. For instance, although we know that neural activity was recorded during, for example, locomotion to the reward port (Figure 3 and Figure 4), we do not know whether this activity is most directly coupled to the movement of the body in space, the head relative to the body, or even to the spatial orientation of the head or body (Muller et al., 1996). It is likely that the locomotor actions required by this task are accompanied by characteristic orientation of the head, neck, and eyes, any of which could result in a systematic neural correlate of the spatial choice. Furthermore, SC activity might also reflect motor commands sent in the absence of overt movements (Corneil et al., 2002; Hadjidimitrakis et al., 2007). Although this is a limitation of our results, it is worth noting that, for similar reasons, attributing SC activity to the appropriate component of a gaze shift (i.e., a commanded eye or head movement) is considered problematic in head-fixed primate studies as well (Sparks, 1999).
Several other brain regions in rodents are known to represent spatial and directional variables, such as the hippocampus (O'Keefe and Dostrovsky, 1971), entorhinal cortex (Fyhn et al., 2004), subiculum (Taube et al., 1990), orbitofrontal cortex (Feierstein et al., 2006), and posterior cortex (Chen et al., 1994). It has been suggested that the SC provides spatial input to some of these areas (Cooper et al., 1998). The fact that so many different areas represent spatial information may reflect the importance that rats place on using spatial cues for wayfinding (Moser et al., 2008). Future studies can build on the paradigm and findings described here to address how the SC interacts with these other areas to mediate the processes necessary for goal-directed spatial locomotion.
EXPERIMENTAL PROCEDURES
For more detailed explanations of procedures, see Supplemental Data.
Animal Subjects
Animal use procedures were approved by the Cold Spring Harbor Laboratory Institutional Animal Care and Use Committee and carried out in accordance with National Institutes of Health standards. Eight male Long-Evans hooded rats were used in these experiments. Rats had free access to food but water was restricted to the behavioral session and approximately 1 additional hour per day.
Odor-Guided Spatial Choice Task
Rats were trained and tested on a two-alternative odor-guided spatial choice task in which the identity of an odor was associated with the location of a water reward (Uchida and Mainen, 2003). In each trial of the task, the rat first entered the odor port, triggering the delivery of an odor, and then moved to one of the reward ports to harvest the reward, if any (Figure 1B). Odors were mixed with a pure air carrier and delivered at a flow rate of 1 l/min using a custom-built olfactometer (Island Motion, Tappan, NY). In order to decorrelate the timing of port entry and the delivery of odor (or water), opening of the odor (or water) valve was delayed following entry into the odor (or reward) port by 200–500 ms (uniformly distributed). For pure odor discrimination trials, the rat was rewarded at the left reward port following presentation of one stimulus (e.g., S(+)-2- octanol), and at the right following presentation of the other stimulus (e.g., R(−)-2-octanol). An incorrect port entry, or an absence of a port entry, resulted in no reward. In mixture discrimination trials, the odor stimulus consisted of some fraction of each of the two odors, achieved by setting differential rates of air flow through the two odor sources. Using the odors in the example above, the rat was rewarded at the left if the dominant component in the mixture was S(+)-2-octanol, and at the right if the dominant component was R(−)-2-octanol. For mixtures of equal concentrations, left and right choices were rewarded with a probability of 0.5.
Two paradigms were used to determine the odors delivered in each session: a “mixtures” paradigm, and a “novel odors” paradigm. For the neuronal recordings, each paradigm was used for two rats. In each trial of the mixtures paradigm, the rat received either a pure odor (S(+)-carvone or R(−)-carvone) or a mixture of two odors (S(+)-2- octanol and R(−)-2-octanol). In each trial of the novel odors paradigm, the rat received either one of two familiar pure odors (for one rat, caproic acid or hexanol; for the second rat, S(+)-2-octanol or R(−)-2-octanol), which were used during every session, or one of two novel pure odors, which had not been used prior to that session (Table S1). The odor presented in each trial was determined pseudorandomly. Data collected during the two paradigms were combined for all analyses of neuronal activity. For the inactivation experiments, the mixtures paradigm was used for all four rats.
Surgery
For the recording experiments, each rat was surgically implanted with a custom-made drive (Feierstein et al., 2006) containing 6−12 independently adjustable tetrodes targeted to the left SC (6.8 mm posterior to bregma and 1.7 mm lateral to the midline (Paxinos and Watson, 1998)). For the inactivation experiments, each rat was implanted with a steel cannula assembly (guide and dummy cannulae, Plastics One, Roanoke, VA) targeted to the SC (4 mm from the brain surface). Rats were allowed to recover for 5 days before water restriction resumed and the recording or inactivation sessions began.
Neural Recording
Individual tetrodes consisted of four twisted polyimide-coated nichrome wires (H.P. Reid, Inc., Palm Coast, FL; single-wire diameter 12.5 μm) gold-plated to 0.2–0.4 MΩ impedance. Electrical signals were amplified and recorded using the NSpike multichannel acquisition system (L. Frank, J. MacArthur). Multiple single units were isolated offline by a combination of an automated expectation maximization algorithm (Klustakwik, K. D. Harris) and by manually clustering spike features derived from the sampled waveforms using MCLUST software (A. D. Redish; Figures 2D and 2E). Tetrode depths were adjusted prior to each recording session in order to sample an independent population of cells across sessions, and their locations during each recording session were estimated based on their depth and later confirmed histologically based on electrolytic lesions and on the visible tetrode tracks (Figures 2A, 2B, and 2C). Cells were not selected based on any criteria prior to beginning a recording session. Rats performed between 180 and 500 trials per session (mean ± SD, 316 ± 69), one session was performed per day, and a total of 44 recording sessions were obtained from all four rats.
Neural Data Analysis
All data analysis was performed using MATLAB (Mathworks, Natick, MA). To quantify the dependence of firing rate on task variables (e.g., direction of locomotion), we used an algorithm based on ROC analysis that calculates the ability of an ideal observer to classify whether a given spike rate was recorded in one of two conditions (e.g., during leftward or rightward movement) (Feierstein et al., 2006; Green and Swets, 1966). We defined “preference” as 2(ROCarea − 0.5), a measure ranging from −1 to 1, where −1 signifies the strongest possible preference for one alternative and 1 signifies the strongest possible preference for the other alternative. “Selectivity” was defined as 2(|ROCarea − 0.5|), ranging from 0 to 1, where 0 signifies not selective, and 1 signifies maximal selectivity. Note that selectivity is equivalent to the absolute value of the preference. Statistical significance was determined with a permutation test: We recalculated the preference after randomly reassigning all firing rates to either of the two groups arbitrarily, repeated this procedure a large number of times (500 repeats for analyses of dynamics [Figure 4 and Figure 6D], 1000 repeats for all other analyses) to obtain a distribution of values, and calculated the fraction of random values exceeding the actual value. For all analyses, we tested for significance at α = 0.01. This analysis is sensitive to both absolute and relative differences in firing rates, and yielded very similar results to another common metric of selectivity, (Figure S5). Only cells with a minimum number of four trials for each analyzed condition, and with a firing rate above two spikes/s for either of the analyzed conditions, were included in that analysis. For analyses based on movement from the odor port to the reward port, trials in which the movement time was >1 s were excluded. Our results were independent of the specific values selected for these criteria.
Inactivation experiments
To determine the appropriate dose of muscimol (Sigma-Aldrich, St. Louis, MO), for one rat, we tested how the magnitude of the bias depended on the amount of muscimol infused. As expected, larger doses of muscimol tended to produce larger ipsilateral biases (Figures S4A and S4B). Since we observed an effect on choice behavior, but no gross behavioral deficits, with 0.175 nmol of muscimol, we selected this dosage for our main experiments. Prior to each session, the rat was anesthetized with 2% isoflurane (Vetland, Louisville, KY) and an infusion pump (Harvard Apparatus, Holliston, MA) was used to administer 0.5 μl of either muscimol (test sessions) or saline (control sessions) at a rate of 0.25 μl/min (Narayanan et al., 2006). Animals recovered for at least 20 minutes before beginning the behavioral session.
Psychometric functions were fitted to where x is the proportion of the left odor in the mixture ratio, p is the fraction of left choices, and a and b are the best- fit free parameters. The bias of the curve was calculated as . Depending on whether the left or right SC was inactivated, the sign of the bias was flipped such that positive values reflect ipsilateral bias. For our analyses of reaction times (Figure 8C and Figure 8D), we chose to combine odor sampling duration and movement time because of the limits imposed by our method of measuring the time of odor port exit on the accuracy of estimating these epochs separately.
Histology
In order to verify the ultimate location of the tetrodes, electrolytic lesions were produced after the final recording session (Figures 2A and 2B). To verify the location of the cannula in the inactivation experiments, DiI (Molecular Probes, Eugene, OR) diluted in 0.9% sterile saline was infused into the SC after the final session (Figure S4C). Rats were then deeply anesthetized with a cocktail of ketamine (Fort Dodge, Overland Park, KS) and medetomidine (Pfizer, New York, NY) and perfused transcardially with 4% paraformaldehyde. The brain was removed and stored in 4% paraformaldehyde, and was then sectioned at 50 µm and Nissl-stained.
Supplementary Material
Detailed experimental procedures.
Table S1. Odor pairs used in the novel odors paradigm.
Figure S1. Dependence of dynamics of direction preference on window size.
Figure S2. Dependence of preference on bin size.
Figure S3. Neural activity during movement from reward port to odor port.
Figure S4. Unilateral SC inactivation.
Figure S5. Dependence of preference on firing rate.
ACKNOWLEDGMENTS
We thank Joshua Sanders for assistance with recording experiments, Robert Klein for assistance with muscimol inactivation experiments, Barry Burbach for technical assistance, and Matthew Smear and Takashi Sato for helpful comments and discussions. This work was supported by the Swartz Center for Computational Neuroscience (G.F.), the National Institutes of Health (NIDCD) (Z.F.M.), and the Center for the Neural Mechanisms of Cognition at Cold Spring Harbor Laboratory (Z.F.M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors maybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Angeles Luque M, Pilar Perez-Perez M, Herrero L, Torres B. Involvement of the optic tectum and mesencephalic reticular formation in the generation of saccadic eye movements in goldfish. Brain Res. Brain Res. Rev. 2005;49:388–397. doi: 10.1016/j.brainresrev.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Aravanis AM, Wang LP, Zhang F, Meltzer LA, Mogri MZ, Schneider MB, Deisseroth K. An optical neural interface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. J. Neural Eng. 2007;4:S143–S156. doi: 10.1088/1741-2560/4/3/S02. [DOI] [PubMed] [Google Scholar]
- Carello CD, Krauzlis RJ. Manipulating intent: evidence for a causal role of the superior colliculus in target selection. Neuron. 2004;43:575–583. doi: 10.1016/j.neuron.2004.07.026. [DOI] [PubMed] [Google Scholar]
- Chen LL, Lin LH, Green EJ, Barnes CA, McNaughton BL. Head-direction cells in the rat posterior cortex. I. Anatomical distribution and behavioral modulation. Exp. Brain Res. 1994;101:8–23. doi: 10.1007/BF00243212. [DOI] [PubMed] [Google Scholar]
- Cooper BG, Miya DY, Mizumori SJ. Superior colliculus and active navigation: role of visual and non-visual cues in controlling cellular representations of space. Hippocampus. 1998;8:340–372. doi: 10.1002/(SICI)1098-1063(1998)8:4<340::AID-HIPO4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Corneil BD, Olivier E, Munoz DP. Neck muscle responses to stimulation of monkey superior colliculus. I. Topography and manipulation of stimulation parameters. J. Neurophysiol. 2002;88:1980–1999. doi: 10.1152/jn.2002.88.4.1980. [DOI] [PubMed] [Google Scholar]
- Dean P, Mitchell IJ, Redgrave P. Contralateral head movements produced by microinjection of glutamate into superior colliculus of rats: evidence for mediation by multiple output pathways. Neuroscience. 1988;24:491–500. doi: 10.1016/0306-4522(88)90344-2. [DOI] [PubMed] [Google Scholar]
- Dean P, Redgrave P, Westby GW. Event or emergency? Two response systems in the mammalian superior colliculus. Trends Neurosci. 1989;12:137–147. doi: 10.1016/0166-2236(89)90052-0. [DOI] [PubMed] [Google Scholar]
- Edwards SB. The commissural projection of the superior colliculus in the cat. J. Comp. Neurol. 1977;173:23–40. doi: 10.1002/cne.901730103. [DOI] [PubMed] [Google Scholar]
- Feierstein CE, Quirk MC, Uchida N, Sosulski DL, Mainen ZF. Representation of spatial goals in rat orbitofrontal cortex. Neuron. 2006;51:495–507. doi: 10.1016/j.neuron.2006.06.032. [DOI] [PubMed] [Google Scholar]
- Freedman EG, Sparks DL. Activity of cells in the deeper layers of the superior colliculus of the rhesus monkey: evidence for a gaze displacement command. J. Neurophysiol. 1997;78:1669–1690. doi: 10.1152/jn.1997.78.3.1669. [DOI] [PubMed] [Google Scholar]
- Freedman EG, Stanford TR, Sparks DL. Combined eye-head gaze shifts produced by electrical stimulation of the superior colliculus in rhesus monkeys. J. Neurophysiol. 1996;76:927–952. doi: 10.1152/jn.1996.76.2.927. [DOI] [PubMed] [Google Scholar]
- Fyhn M, Molden S, Witter MP, Moser EI, Moser MB. Spatial representation in the entorhinal cortex. Science. 2004;305:1258–1264. doi: 10.1126/science.1099901. [DOI] [PubMed] [Google Scholar]
- Glimcher PW, Sparks DL. Movement selection in advance of action in the superior colliculus. Nature. 1992;355:542–545. doi: 10.1038/355542a0. [DOI] [PubMed] [Google Scholar]
- Green DM, Swets JA. Signal Detection Theory and Psychophysics. New York: Wiley; 1966. [Google Scholar]
- Groh JM, Sparks DL. Saccades to somatosensory targets. II. motor convergence in primate superior colliculus. J. Neurophysiol. 1996;75:428–438. doi: 10.1152/jn.1996.75.1.428. [DOI] [PubMed] [Google Scholar]
- Hadjidimitrakis K, Moschovakis AK, Dalezios Y, Grantyn A. Eye position modulates the electromyographic responses of neck muscles to electrical stimulation of the superior colliculus in the alert cat. Exp. Brain Res. 2007;179:1–16. doi: 10.1007/s00221-006-0765-3. [DOI] [PubMed] [Google Scholar]
- Harris LR. The superior colliculus and movements of the head and eyes in cats. J. Physiol. 1980;300:367–391. doi: 10.1113/jphysiol.1980.sp013167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O, Nakamura K, Nakahara H. Basal ganglia orient eyes to reward. J. Neurophysiol. 2006;95:567–584. doi: 10.1152/jn.00458.2005. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Modification of saccadic eye movements by GABA-related substances. I. Effect of muscimol and bicuculline in monkey superior colliculus. J. Neurophysiol. 1985;53:266–291. doi: 10.1152/jn.1985.53.1.266. [DOI] [PubMed] [Google Scholar]
- Horwitz GD, Batista AP, Newsome WT. Representation of an abstract perceptual decision in macaque superior colliculus. J. Neurophysiol. 2004;91:2281–2296. doi: 10.1152/jn.00872.2003. [DOI] [PubMed] [Google Scholar]
- Horwitz GD, Newsome WT. Separate signals for target selection and movement specification in the superior colliculus. Science. 1999;284:1158–1161. doi: 10.1126/science.284.5417.1158. [DOI] [PubMed] [Google Scholar]
- Horwitz GD, Newsome WT. Target selection for saccadic eye movements: prelude activity in the superior colliculus during a direction-discrimination task. J. Neurophysiol. 2001;86:2543–2558. doi: 10.1152/jn.2001.86.5.2543. [DOI] [PubMed] [Google Scholar]
- Huerta MF, Harting JK. Connectional organization of the superior colliculus. Trends Neurosci. 1984;7:286–289. [Google Scholar]
- Ingle D. Two visual systems in the frog. Science. 1973;181:1053–1055. doi: 10.1126/science.181.4104.1053. [DOI] [PubMed] [Google Scholar]
- Ingle D, Crews D. Vertebrate neuroethology: definitions and paradigms. Annu Rev. Neurosci. 1985;8:457–494. doi: 10.1146/annurev.ne.08.030185.002325. [DOI] [PubMed] [Google Scholar]
- Jay MF, Sparks DL. Sensorimotor integration in the primate superior colliculus. I. Motor convergence. J. Neurophysiol. 1987;57:22–34. doi: 10.1152/jn.1987.57.1.22. [DOI] [PubMed] [Google Scholar]
- King AJ. The superior colliculus. Curr. Biol. 2004;14:R335–R338. doi: 10.1016/j.cub.2004.04.018. [DOI] [PubMed] [Google Scholar]
- Koulakov AA, Rinberg DA, Tsigankov DN. How to find decision makers in neural networks. Biol. Cybern. 2005;93:447–462. doi: 10.1007/s00422-005-0022-z. [DOI] [PubMed] [Google Scholar]
- Krauzlis RJ, Liston D, Carello CD. Target selection and the superior colliculus: goals, choices and hypotheses. Vision Res. 2004;44:1445–1451. doi: 10.1016/j.visres.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Kustov AA, Robinson DL. Shared neural control of attentional shifts and eye movements. Nature. 1996;384:74–77. doi: 10.1038/384074a0. [DOI] [PubMed] [Google Scholar]
- Lo CC, Wang XJ. Cortico-basal ganglia circuit mechanism for a decision threshold in reaction time tasks. Nat. Neurosci. 2006;9:956–963. doi: 10.1038/nn1722. [DOI] [PubMed] [Google Scholar]
- Martin JH, Ghez C. Pharmacological inactivation in the analysis of the central control of movement. J. Neurosci. Methods. 1999;86:145–159. doi: 10.1016/s0165-0270(98)00163-0. [DOI] [PubMed] [Google Scholar]
- May PJ. The mammalian superior colliculus: laminar structure and connections. Prog. Brain. Res. 2005;151:321–378. doi: 10.1016/S0079-6123(05)51011-2. [DOI] [PubMed] [Google Scholar]
- McHaffie JG, Stein BE. Eye movements evoked by electrical stimulation in the superior colliculus of rats and hamsters. Brain Res. 1982;247:243–253. doi: 10.1016/0006-8993(82)91249-5. [DOI] [PubMed] [Google Scholar]
- McPeek RM, Keller EL. Deficits in saccade target selection after inactivation of superior colliculus. Nat. Neurosci. 2004;7:757–763. doi: 10.1038/nn1269. [DOI] [PubMed] [Google Scholar]
- Mohler CW, Wurtz RH. Organization of monkey superior colliculus: intermediate layer cells discharging before eye movements. J. Neurophysiol. 1976;39:722–744. doi: 10.1152/jn.1976.39.4.722. [DOI] [PubMed] [Google Scholar]
- Moser EI, Kropff E, Moser MB. Place Cells, Grid Cells, and the Brain's Spatial Representation System. Annu. Rev. Neurosci. 2008 doi: 10.1146/annurev.neuro.31.061307.090723. [DOI] [PubMed] [Google Scholar]
- Muller JR, Philiastides MG, Newsome WT. Microstimulation of the superior colliculus focuses attention without moving the eyes. Proc. Natl. Acad. Sci. U. S. A. 2005;102:524–529. doi: 10.1073/pnas.0408311101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller RU, Ranck JB, Jr, Taube JS. Head direction cells: properties and functional significance. Curr. Opin. Neurobiol. 1996;6:196–206. doi: 10.1016/s0959-4388(96)80073-0. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Wurtz RH. Saccade-related activity in monkey superior colliculus. I. Characteristics of burst and buildup cells. J. Neurophysiol. 1995;73:2313–2333. doi: 10.1152/jn.1995.73.6.2313. [DOI] [PubMed] [Google Scholar]
- Narayanan NS, Horst NK, Laubach M. Reversible inactivations of rat medial prefrontal cortex impair the ability to wait for a stimulus. Neuroscience. 2006;139:865–876. doi: 10.1016/j.neuroscience.2005.11.072. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1998. [Google Scholar]
- Pond FJ, Sinnamon HM, Adams DB. Single unit recording in the midbrain of rats during shock-elicited fighting behavior. Brain. Res. 1977;120:469–484. doi: 10.1016/0006-8993(77)90400-0. [DOI] [PubMed] [Google Scholar]
- Quinlan EM, Lebel D, Brosh I, Barkai E. A molecular mechanism for stabilization of learning-induced synaptic modifications. Neuron. 2004;41:185–192. doi: 10.1016/s0896-6273(03)00874-2. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Gurney K. The short-latency dopamine signal: a role in discovering novel actions? Nat. Rev. Neurosci. 2006;7:967–975. doi: 10.1038/nrn2022. [DOI] [PubMed] [Google Scholar]
- Redgrave P, Gurney K, Reynolds J. What is reinforced by phasic dopamine signals? Brain Res. Rev. 2007 doi: 10.1016/j.brainresrev.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Robinson DA. Eye movements evoked by collicular stimulation in the alert monkey. Vision Res. 1972;12:1795–1808. doi: 10.1016/0042-6989(72)90070-3. [DOI] [PubMed] [Google Scholar]
- Roucoux A, Guitton D, Crommelinck M. Stimulation of the superior colliculus in the alert cat. II. Eye and head movements evoked when the head is unrestrained. Exp. Brain Res. 1980;39:75–85. doi: 10.1007/BF00237071. [DOI] [PubMed] [Google Scholar]
- Sahibzada N, Dean P, Redgrave P. Movements resembling orientation or avoidance elicited by electrical stimulation of the superior colliculus in rats. J. Neurosci. 1986;6:723–733. doi: 10.1523/JNEUROSCI.06-03-00723.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y, Isa T. Electrophysiological and morphological properties of neurons in the rat superior colliculus. I. Neurons in the intermediate layer. J. Neurophysiol. 1999;82:754–767. doi: 10.1152/jn.1999.82.2.754. [DOI] [PubMed] [Google Scholar]
- Salas C, Herrero L, Rodriguez F, Torres B. Tectal codification of eye movements in goldfish studied by electrical microstimulation. Neuroscience. 1997;78:271–288. doi: 10.1016/s0306-4522(97)83048-5. [DOI] [PubMed] [Google Scholar]
- Schiller PH, Koerner F. Discharge characteristics of single units in superior colliculus of the alert rhesus monkey. J. Neurophysiol. 1971;34:920–936. doi: 10.1152/jn.1971.34.5.920. [DOI] [PubMed] [Google Scholar]
- Schiller PH, True SD, Conway JL. Deficits in eye movements following frontal eye-field and superior colliculus ablations. J. Neurophysiol. 1980;44:1175–1189. doi: 10.1152/jn.1980.44.6.1175. [DOI] [PubMed] [Google Scholar]
- Shadlen MN, Newsome WT. Neural basis of a perceptual decision in the parietal cortex (area LIP) of the rhesus monkey. J. Neurophysiol. 2001;86:1916–1936. doi: 10.1152/jn.2001.86.4.1916. [DOI] [PubMed] [Google Scholar]
- Sinnamon HM, Garcia EJ. Lateral neglect in a head movement task: more impairment with unilateral than bilateral lesions of the superior colliculus in the rat. Behav. Brain Res. 1988;27:131–143. doi: 10.1016/0166-4328(88)90039-3. [DOI] [PubMed] [Google Scholar]
- Sparks DL. Translation of sensory signals into commands for control of saccadic eye movements: role of primate superior colliculus. Physiol. Rev. 1986;66:118–171. doi: 10.1152/physrev.1986.66.1.118. [DOI] [PubMed] [Google Scholar]
- Sparks DL. Conceptual issues related to the role of the superior colliculus in the control of gaze. Curr. Opin. Neurobiol. 1999;9:698–707. doi: 10.1016/s0959-4388(99)00039-2. [DOI] [PubMed] [Google Scholar]
- Sparks DL, Hartwich-Young R. The deep layers of the superior colliculus. Rev. Oculomot. Res. 1989;3:213–255. [PubMed] [Google Scholar]
- Sutton RS, Barto AG. Reinforcement Learning: An Introduction. Cambridge, MA: MIT Press; 1998. [Google Scholar]
- Taube JS, Muller RU, Ranck JB., Jr Head-direction cells recorded from the postsubiculum in freely moving rats. I. Description and quantitative analysis. J. Neurosci. 1990;10:420–435. doi: 10.1523/JNEUROSCI.10-02-00420.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida N, Mainen ZF. Speed and accuracy of olfactory discrimination in the rat. Nat. Neurosci. 2003;6:1224–1229. doi: 10.1038/nn1142. [DOI] [PubMed] [Google Scholar]
- Waitzman DM, Ma TP, Optican LM, Wurtz RH. Superior colliculus neurons provide the saccadic motor error signal. Exp. Brain Res. 1988;72:649–652. doi: 10.1007/BF00250610. [DOI] [PubMed] [Google Scholar]
- Wang S, Redgrave P. Microinjections of muscimol into lateral superior colliculus disrupt orienting and oral movements in the formalin model of pain. Neuroscience. 1997;81:967–988. doi: 10.1016/s0306-4522(97)00191-7. [DOI] [PubMed] [Google Scholar]
- Weldon DA, Best PJ. Changes in sensory responsivity in deep layer neurons of the superior colliculus of behaving rats. Behav. Brain Res. 1992;47:97–101. doi: 10.1016/s0166-4328(05)80257-8. [DOI] [PubMed] [Google Scholar]
- Weldon DA, DiNieri JA, Silver MR, Thomas AA, Wright RE. Reward-related neuronal activity in the rat superior colliculus. Behav. Brain Res. 2007;177:160–164. doi: 10.1016/j.bbr.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Weldon DA, Patterson CA, Colligan EA, Nemeth CL, Rizio AA. Single unit activity in the rat superior colliculus during reward magnitude task performance. Behav. Neurosci. 2008;122:183–190. doi: 10.1037/0735-7044.122.1.183. [DOI] [PubMed] [Google Scholar]
- Wurtz RH, Goldberg ME. Superior colliculus cell responses related to eye movements in awake monkeys. Science. 1971;171:82–84. doi: 10.1126/science.171.3966.82. [DOI] [PubMed] [Google Scholar]
- Wurtz RH, Goldberg ME. Activity of superior colliculus in behaving monkey. 3. Cells discharging before eye movements. J. Neurophysiol. 1972;35:575–586. doi: 10.1152/jn.1972.35.4.575. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Detailed experimental procedures.
Table S1. Odor pairs used in the novel odors paradigm.
Figure S1. Dependence of dynamics of direction preference on window size.
Figure S2. Dependence of preference on bin size.
Figure S3. Neural activity during movement from reward port to odor port.
Figure S4. Unilateral SC inactivation.
Figure S5. Dependence of preference on firing rate.