Abstract
Oestrogen, acting through its cognate receptor oestrogen receptor-α (ERα), is a critical regulator of uterine endometrial epithelial proliferation. Although the dynamic communication between endometrial stromal and epithelial cells is considered to be an important component in this process, key molecular players in particular compartments remain poorly defined. Here, we used mice null for Krüppel-like factor 9 (KLF9) to evaluate the contribution of this nuclear protein in stromal-epithelial interactions underlying proliferative effects of oestrogen. We find that in ovariectomized mice administered estradiol-17β (E2) for 24 h, Klf9 null mutation resulted in lack of E2-induced proliferative response in all endometrial compartments. We demonstrate a negative association between Klf9 expression and nuclear levels of ERα transcriptional corepressor prohibitin (PHB) 2 in uterine stromal and epithelial cells of E2-treated wildtype (WT) and Klf9 null mice. In early pregnancy uteri of WT mice, the temporal pattern of Klf9 transcript levels was inversely associated with that of Phb2. Deletion of Klf9 up-regulated uterine Phb2 expression and increased PHB2 nuclear localization in endometrial stromal and epithelial cells, with no effects on the expression of the related Phb1. In the human endometrial stromal cell line HESC treated with E2 for 24 h, KLF9 siRNA targeting augmented PHB2 transcript and increased nuclear PHB2 protein levels, albeit this effect was not to the extent seen in vivo with Klf9 null mutants. Our findings suggest a novel mechanism for control of oestrogen-induced luminal epithelial proliferation involving stromal KLF9 regulation of paracrine factor(s) to repress epithelial expression of corepressor PHB2.
Keywords: oestrogen, oestrogen receptor, Krüppel-like factor 9, prohibitin, endometrial proliferation
Introduction
Oestrogen (E) control of cell proliferation is a complex process that is subject to regulation at many levels. The nuclear receptor/transcription factor estrogen receptor-α (ERα) is the key regulatory participant, transducing E action by binding the ligand to form a complex that upon homodimerization, interacts with various co-regulators to target E-responsive gene promoters, leading to transcriptional activation, and the synthesis of gene products that modify cellular phenotype and behavior (Tsai and O’Malley, 1994; Hall et al. 2001). Nuclear receptor coregulator proteins, acting as coactivators or corepressors contribute to ERα transactivity and exert their effects by direct or indirect interactions with ERα (McKenna and O’Malley, 2002; Lonard and O’Malley, 2006; Green and Carroll, 2007). Examples of co-factors that directly interact with ligand-bound ERα at its activation function domains include the forkhead protein FOXA1 and the p160 protein family members SRC1/ERAP140/ERA160/NcoA1, SRC2/GRIP1/TIF2/NcoA2, and SRC3/AIB1/Rac3/NcoA3 (Yahata et al. 2001; Hu et al. 2002; Duterte and Smith, 2003; Wang et al. 2007). Coregulators which influence ERα transactivation of target genes indirectly alter chromatin structure by facilitating histone acetylation/deacetylation and methylation/demethylation, and by post-translational modifications of other coregulators and transcription factors within the transcriptional complex (McKenna and O’Malley, 2002; Stenoien et al. 2001; Green and Carroll, 2007). The reports on the formation of an extranuclear complex between ligand-bound ERα and SRC-3/AIB1/NcoA3 (Zheng et al. 2005) and the increased transcription of ERα target genes upon MAPK phosphorylation of SRC-3 (Font de Mora and Brown, 2000) provide evidence for the increasingly complex and less predictable mechanisms underlying coregulator participation in ERα signaling.
We previously reported that the nuclear protein Krüppel-like factor 9 (KLF9), previously designated Basic Transcription Element Binding Protein-1 (BTEB1) (Imataka et al. 1992) and a member of the Sp-family of transcription factors (Suske et al. 2005) may function as a coregulator of steroid hormone receptor signaling in the uterine endometrium (Zhang et al. 2002; Zhang et al. 2003). We found that KLF9 exerts its effects on progesterone receptor (PGR) and ERα actions by distinct mechanisms. KLF9 promotes PGR transactivation through functional and physical interactions with PGR-B and to a lesser extent, PGR-A at progesterone (P)-responsive gene promoters (Zhang et al. 2003; Velarde et al. 2006). By contrast, KLF9 can inhibit ERα transactivity in vitro in a high estrogen environment by promoting estradiol-dependent down-regulation of ERα expression through enhancement of the association of ERα to GC-rich motifs within its promoter (Velarde et al. 2007). Based on these studies, we concluded that KLF9 may influence both PGR and ERα genomic pathways to favor P-induced cell differentiation (Velarde et al. 2007). Nonetheless, while the latter presented an attractive model integrating KLF9 in the opposing actions of E and P in the uterus, it did not account for our earlier findings that in the endogenous E-dominated environment of early pregnancy, uterine endometrial cells of Klf9 null mice exhibited delayed (by 24 h) and attenuated proliferation relative to WT counterparts (Velarde et al. 2005). The decreased numbers of implanting embryos in Klf9 null mutants suggested that the altered pattern of proliferation of endometrial cells with Klf9 ablation resulted in developmental asynchrony between the uterine luminal epithelium and the implantation-ready embryo, leading to subfertility (Simmen et al. 2004; Velarde et al. 2005). Thus, KLF9 may function as a positive regulator of ERα signaling to influence cell proliferation, with important consequences on pregnancy outcome.
Prohibitin (PHB) 2, also designated as Repressor of Estrogen Receptor Activity (REA) is a 37 kDa protein exhibiting high homology to the putative tumor suppressor protein prohibitin (Phb1) (Montano et al. 1999). PHB1 and PHB2 are highly conserved proteins in eukaryotic cells, with similar functions as inhibitors of cellular proliferation (Mishra et al. 2006). Unlike PHB1, however, which represses signaling of numerous steroid hormone receptors (Wang et al. 2004; Mussi et al. 2006; Gamble et al. 2007; He et al. 2007), PHB2 demonstrates selective repression of ER activity (Montano et al. 1999), possibly in a cell-specific context (Wang et al. 2004), and cooperates with the Chicken Ovalbumin Upstream Promoter binding transcription factors I and II to decrease transcription (Kurtev et al. 2004). PHB2 inhibits ERα transcriptional activity by competing with p160 coregulators such as SRC-1/NcoA1 and SRC-3/NcoA3 for binding to ERα in the presence of E (Montano et al. 1999; Delage-Morroux et al. 2000; Wang et al. 2004), and by recruiting class I and class II histone deacetylases (Kurtev et al. 2004). Whereas genetic deletion of both Phb2 alleles resulted in embryonic lethality, heterozygous mice displayed phenotypes that are characteristic of overactivated ERα signaling including uterine epithelial hyperplasia coincident with higher expression levels of E-responsive genes, enhanced mammary gland morphogenesis, and delayed mammary gland involution (Park et al. 2005; Mussi et al. 2006). These collective results indicate that PHB2 functions as an important mediator of E action in vivo.
In the present study, we show that endometrial epithelial cells of ovariectomized (Ovx) mice with Klf9 null mutation were unresponsive to E2-induced proliferation. We hypothesized that stromal KLF9 may mediate E2 effects on uterine epithelial cell proliferation by influencing the expression of specific ERα coregulators. We demonstrate that the KLF9-mediated increase in proliferation with E2 was negatively associated with uterine Phb2 but not Phb1, expression and with nuclear localization of PHB2 in all endometrial compartments. We further show that this negative linkage between KLF9 and nuclear PHB2 also occurs during the physiological condition of early pregnancy and in an E2-treated human endometrial stromal cell line HESC. Our results suggest that the repression of epithelial PHB2 expression involving stromal KLF9 signaling may be a necessary component in the paracrine regulation of uterine epithelial proliferation by oestrogen.
Materials and Methods
Animals and treatments
Experiments were carried out in accordance with protocols approved by the Institutional Animal Care and Use Committee of the University of Arkansas for Medical Sciences. WT and Klf9 mutant (KO) mice lines were propagated and genotyped as previously described (Morita et al. 2003; Simmen et al. 2004). For steroid hormone treatment studies, approximately 8-wk-old WT and KO female littermates were subjected to bilateral ovariectomy. Two weeks later, mice were treated with vehicle [sesame oil; 0.25 ml] or 17β-estradiol (E2, 125 ng in 0.25 ml sesame oil), and uteri were collected after 24 h. Uterine tissues from WT and KO mice at day post-coitum (dpc) 2.5, 3.5, and 4.5 were isolated as previously described (Velarde et al. 2005). The presence of vaginal plug was considered 0.5dpc.
Immunohistochemistry
Paraffin-embedded uteri from ovariectomized (Ovx) WT and KO females (n=3-5 mice per genotype per treatment) were serially sectioned, dewaxed in xylene and rehydrated through a graded alcohol series. Tissue sections were treated with 3% H2O2 to quench endogenous peroxidase activity. Antigen unmasking was performed by boiling the sections in Citra Plus (Biogenex, San Ramon, CA) in a microwave oven for 105 sec at power 10 and then for 10 min at power 1. After blocking with horse IgG (Vectastain Elite ABC kit, Vector Laboratories, Inc., Burlingame, CA) for 30 min, sections were incubated with the following antibodies to detect expression of proteins: (a) mouse monoclonal antibody to PCNA (PC-10, Dako) at 1:500 dilution; (b) rabbit polyclonal antibody to ERα (MC-20, Santa Cruz Biotechnology, Inc) at 1:500 dilution; and c) rabbit polyclonal antibody to Phb2 (Bethyl Laboratories Inc.) at 1:250 dilution. Incubations with anti-PCNA and ERα were carried out for 1 h at room temperature, whereas incubation with anti-PHB2 antibody was performed overnight at room temperature. Following incubation with secondary antibody (horse anti-mouse IgG at 1:1600 dilution or goat anti-rabbit IgG at 1:2000 dilution) for 30 min, sections were stained with 3,3’-diaminobenzidine tetra-hydrochloride (Dako) and counter-stained with hematoxylin. Control sections were processed similarly with the omission of the primary antibody. A total of 1000 stromal, 300 luminal epithelial, and 200 glandular epithelial cells were counted on average from at least three randomly selected fields (200X magnification) per slide; one to two slides/mouse with n=3-5 mice per treatment group per genotype, were evaluated. Results are expressed as % nuclear-immunopositive cells ([number of nuclear positively-staining cells/number of total cells counted] x 100).
RNA isolation and quantitative RT-PCR
Total cellular RNA was prepared from whole uteri or cells by TriZol reagent (Invitrogen). Integrity of isolated RNAs was confirmed using the RNA 6000 NanoLabChip kit (Agilent Biotechnologies, Palo Alto, CA). RNA samples were reverse-transcribed using random primers and a cDNA synthesis kit (Applied Biosystems, Foster City, CA). SYBR Green quantitative RT-PCR was performed as previously described (Velarde et al. 2006). Primer sets were designed to flank an intron to prevent amplification of genomic DNA, using PrimerExpress (Applied Biosystems). Synthetic oligonucleotides were obtained from Integrated DNA Technologies, Inc. (Coralville, IA). The sense and antisense primers for the mouse genes, and the resultant PCR product sizes (in parentheses) were: Klf9, 5’-CGT TGC CCA CTG TGT GAG AA-3’ and 5’-TTG ATC ATG CTG GGA TGG AA-3’ (92 bp); Phb2/Rea, 5’-AGC AGG AAC AGC AC AGA AGA-3’ and 5’-CGG AGC TTG ATA TAG CCA GGA T-3’ (103 bp); and Phb1, 5’-TCC CTT GGG TAC AGA AAC CAA TTA-3’ and 5’-TGTGAT ATT GAC GTT CTG CAA GTC T-3 (101 bp). For each sample, gene expression was normalized to that of cyclophilin A (Ppia) (sense: 5’-AGA TGC CAG GAC CTG TAT GCT T-3’; antisense: 5’-TGT GCC AGG GTG GTG ACT TTA-3’) as internal reference. Human primers (sense and antisense, respectively) for KLF9, PHB2/REA, and the internal reference gene RPL7 were as follows: KLF9, 5’-TGG CTG TGG GAA AGT CTA TGG-3’ and 5’-CTC GTC TGA GCG GGA GAAA CT-3’ (124 bp); PHB2/REA, 5’-GAA CAG CGG CAG AAA ATT GTG-3’ and 5’-CGA ATC TTG CGA AGT TTG ATG T-3’ (105 bp), and RPL7, 5’-TGCTGT GCC AGA AAC CCT TAA-3’ and 5’-GCT TCC TCC TTG CCT TTC G-3’ (110 bp).
Cell culture, transfection with siRNAs, immunofluorescence, and Western blots
The human endometrial stromal cell line HESC was a generous gift of Dr. Graziella Krikun (Yale University, New Haven, CT). The cell line was maintained in phenol-red free DMEM and Ham’s F12 (1:1 vol/vol) medium supplemented with 10% charcoal-stripped calf serum and 1% antibiotic/antimycotic solution in an atmosphere of 5% CO2, as previously described (Krikun et al. 2004). Transfection with Klf9 or scrambled (non-specific) siRNAs (Dharmacon) at a final concentration of 50 nM was performed using Lipofectamine 2000 according to the manufacturer’s protocol when cells were ~60% confluent. After 6 h, the transfection mix was replaced with medium containing 2% charcoal-stripped calf serum. Cells were incubated for an additional 24 h in 2% charcoal-stripped calf serum with added 17β-estradiol (E2, 10 nM) and collected for RNA analyses. Immunofluorescence was performed essentially as previously described (Velarde et al. 2007). In brief, cells were seeded on sterile 22-mm glass cover slides at a density of 1.8 x105 cells per well and grown overnight. After transfection with siRNAs, cells were incubated in medium containing E2 (10 nM) in DMSO for 24 h. Cells were fixed in 4% paraformaldehyde for 10 min and permeabilized in 0.1% Triton-PBS for 45 min, with 1 X PBS washes after each step. Cells were sequentially incubated in goat serum blocking solution (Vectastain Elite ABC kit) for 30 min and with anti-PHB2 polyclonal antibody (1:250 dilution) overnight. After incubation with biotinylated secondary antibody (Vectastain Elite ABC kit), fluorescence signals were developed, visualized, and quantified for percentage of nuclear-staining cells (relative to total number of cells; ~250 per treatment group) (Velarde et al. 2007). Western immunoblots were performed (Velarde et al. 2007) using anti-rat KLF9 antibody generated in-house (Zhang D et al. 2002) and anti-α-actinin antibody (Santa Cruz Biotechnology) for loading control.
Data analysis
Values are presented as mean ± Standard Error of the Mean (SEM). Data were analyzed using SigmaStat (SPSS Science, Chicago, IL) and evaluated for differences between groups by Student’s t test or two-way ANOVA, followed by Tukey’s test. P values ≤ 0.05 were considered statistically significant.
Results
Uterine endometrial cells are unresponsive to E2-induced proliferation with Klf9 null mutation
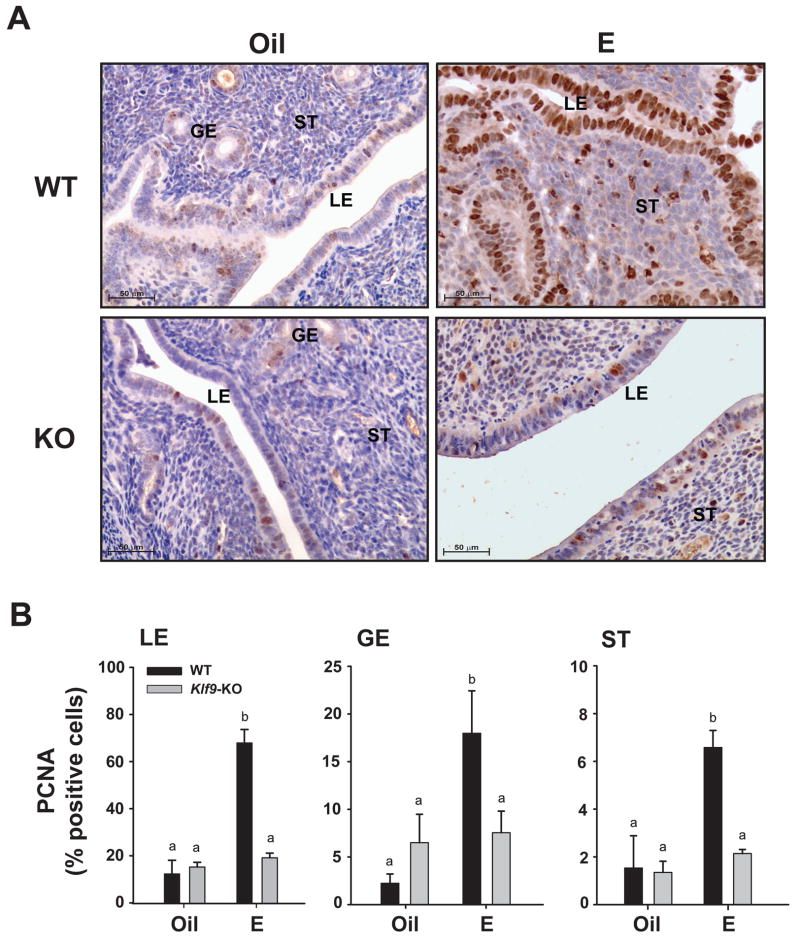
To evaluate the proliferative response of uterine endometrial cells to E as a function of KLF9 expression status, age-matched Ovx WT and Klf9 KO littermates were subcutaneously administered E2 in sesame oil or sesame oil alone (control), and corresponding uteri were isolated after 24 h. Tissue sections from WT and KO mice were immunostained for the proliferation marker PCNA (Fig. 1A), and the numbers of immunopositive cells in each endometrial compartment were counted. In WT mice, E2 administration increased PCNA expression in LE, GE, and ST cells by at least four-fold over those of corresponding control cells (Fig. 1B). Basal and E2-induced PCNA expression was significantly greater for LE than for GE and ST. By contrast, PCNA immunoreactivity in all endometrial compartments of E2-treated Klf9 null mice did not rise above those of corresponding control cells.
Figure 1.
Nuclear PCNA levels in uterine endometrial cells of Oil (vehicle) and E2-treated ovariectomized WT and Klf9 null mice. (A) Representative PCNA immunostaining of glandular epithelium (GE), luminal epithelium (LE), and stromal (ST) compartments are shown at 200X magnification. (B) The percentages of nuclear-staining cells are presented as mean ± SEM (n=3–5 mice per treatment per genotype). Significant differences were identified by two-way ANOVA, followed by Tukey’s test. Means with different superscripts differed at P<0.05.
Since E2 predominantly signals through ERα to exert its proliferative effects in the uterus (Dupont et al. 2000), the nuclear levels of this protein in endometrial compartments were evaluated by immunohistochemistry as a function of KLF9 status. Ovx oil-treated WT mice showed robust ERα expression in the uterine endometrium. E2 treatment had no effect on nuclear levels of this protein for all cell types, in the presence or absence of KLF9. The percentages of cells positive for nuclear ERα in LE from control (15.10±7.43%) and E2-treated (22.91±5.91%) WT mice did not differ from those of LE in corresponding control (10.67±2.25%) and E2-treated Klf9 null mice (11.86±2.34%). Similarly, ST cells from WT (control: 45.02±10.38%; E2-treated: 38.51±6.19%) and Klf9 null (control: 35.86±11.59%; E2-treated: 37.78±6.27%) mice and GE cells from WT (control: 29.21±11.42%; E2-treated: 33.04±3.23%) and Klf9 null (control: 21.44±6.65%; E2-treated: 36.23±13.56%) mice showed no differences in percentages of cells positive for nuclear ERα.
KLF9 is negatively associated with uterine PHB2 expression and nuclear localization
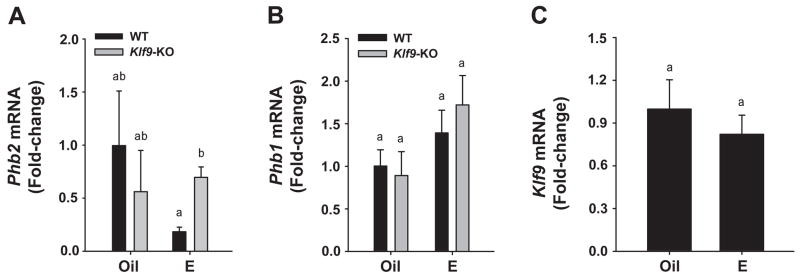
To understand the mechanism by which ablation of Klf9 expression (predominantly in the stroma; Simmen et al. 2004) resulted in the lack of E2-mediated proliferative response in all endometrial compartments, we examined the possibility that KLF9 negatively regulates the expression of the selective ERα transcriptional corepressor PHB2 (Montano et al. 1999). Uterine tissues from control (oil) and E2-treated Ovx WT and KO mice were quantified for Phb2 mRNA abundance by QPCR. Control (oil-treated) WT and KO mice showed comparable levels of Phb2 transcripts (Fig. 2A). E2 administration appeared to decrease (P=0.10) Phb2 expression in WT mice, relative to corresponding control, and this trend was reversed with loss of Klf9 (Fig. 2A). The expression of the related gene Phb1 was not affected by E2 or by KLF9 expression status (Fig. 2B). Similarly, E2 had no effect on Klf9 transcript levels (Fig. 2C).
Figure 2.
Transcript levels of (A) Phb2, (B) Phb1, and (C) Klf9 in uteri of Oil (vehicle) and E2-treated ovariectomized WT and/or Klf9 null mice. mRNA expression was quantified by QPCR and normalized to that of the control gene Ppia. Transcript levels (mean ± SEM) are expressed as fold-change relative to WT oil treatment group (n=3–5 mice per treatment per genotype). Means with different superscripts differed at P<0.05.
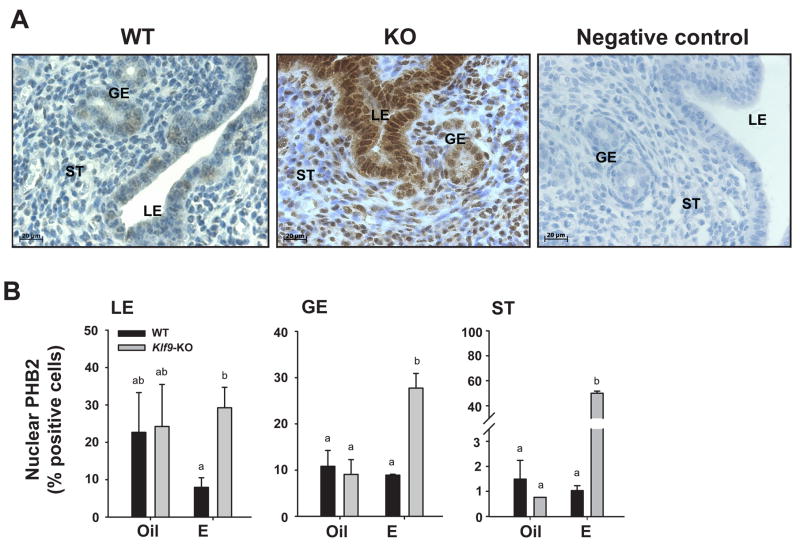
We evaluated the presence of PHB2 protein in uterine tissue sections by immunohistochemistry to determine its spatial distribution pattern, as a function of E2 treatment and KLF9 expression. Using a previously characterized anti-PHB2 antibody (Montano et al. 1999), we found immunoreactive PHB2 in both cytoplasmic and nuclear compartments of GE, LE, and ST cells (Fig. 3A), consistent with the reported localization of this protein in the mitochondria and nucleus (Fusaro et al. 2003; Mishra et al. 2006). Since E-dependent interactions between PHB2 and ERα occur predominantly in the nucleus to inhibit ERα transactivity, nuclear PHB2 expression in endometrial GE, LE, and ST cells was determined in control and E2-treated mice. In oil-treated Ovx WT and KO mouse uteri, the amounts of nuclear PHB2 (measured as %Phb2-immunopositive cells) were significantly greater in LE (~20%) than in GE (10%) and ST (1%) cells (Fig. 3B). With E2-treatment, the levels of nuclear PHB2 in GE and ST cells of WT uteri did not differ from those of oil-treated controls whereas in LE cells, nuclear PHB2 levels showed a trend to decrease (Fig. 3B), following that seen for corresponding mRNA in whole uteri (Fig. 2A). Loss of Klf9 reversed the possible inhibitory effect of E2 on nuclear PHB2 expression in LE cells to the level of control (non-E2 treated) cells, and increased the percent of nuclear PHB2-immunopositive GE and ST cells, with the latter demonstrating amost dramatic (~40-fold) enhancement (Fig. 3B).
Figure 3.
PHB2 levels in uterine endometrial cells of Oil (vehicle) and E2-treated ovariectomized WT and Klf9 null mice. (A) Representative PHB2 immunostaining of GE, LE, and ST cells of E2-treated WT and Klf9 null (KO) mice are shown at 400X magnification to demonstrate nuclear localization of immunoreactive PHB2. Negative control was tissue from E2-treated WT mice processed similarly except for omission of primary antibody. (B) The percentages of PHB2 nuclear-staining cells are presented as mean ± SEM (n=3–5 mice per treatment per genotype). Significant differences were identified by two-way ANOVA, followed by Tukey’s test. Means with different superscripts differed at P<0.05.
Uterine Phb2 and Klf9 expression are inversely associated in early pregnancy
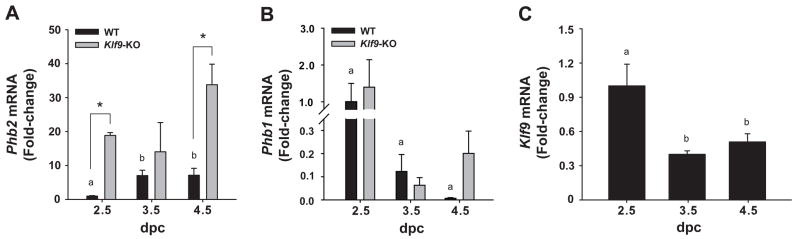
We have previously shown that during the E-dominated condition of early pregnancy at dpc2.5, uterine endometrial cells of Klf9 null mice had negligible BrdU labeling relative to those of WT mice; however, KO cells displayed peak BrdU labeling one-day later at dpc3.5, similar to the level of WT cells at dpc2.5 (Velarde et al. 2005). To determine whether the delayed proliferative response to E2 in Klf9 null uteri was associated with a transient increase in Phb2 expression, the same WT and KO uterine tissues previously analyzed for proliferation status (Velarde et al. 2005) were evaluated for Phb2 mRNA abundance. In WT mice, Phb2 expression was low at dpc2.5, significantly increased at dpc 3.5, and remained at that level at dpc 4.5 (Fig. 4A). By contrast, Phb1 mRNA abundance in WT mice (relative to WT dpc 2.5) progressively decreased at early pregnancy (Fig. 4B). The large variations in Phb1 abundance reflect its very low levels of expression, relative to Phb2. The pattern of Phb2 mRNA expression was inversely associated with that of Klf9 (Fig. 4C), the latter concordant with the peak of proliferation of luminal epithelial cells at dpc 2.5 and subsequently decreasing at dpc 3.5 and dpc 4.5 (Velarde et al. 2005). In KO mice, Phb2 gene expression was significantly higher than for WT mice at dpc 2.5 and dpc 4.5, while at dpc 3.5, no difference in Phb2 expression levels was noted between genotypes (Fig. 4A). Phb1 expression was not affected by loss of Klf9 expression (Fig. 4B).
Figure 4.
Uterine transcript levels for Phb2 (A) and Phb1 (B) in WT and Klf9 null mice and for Klf9 in WT mice (C) during early pregnancy. mRNA expression was quantified by QPCR and normalized to that of the control gene Ppia. Transcript levels (mean ± SEM) were determined at dpc 2.5, 3.5 and 4.5 in WT (Phb2; Phb1; Klf9) and Klf9 KO (Phb2; Phb1) mice and expressed as fold-change relative to WT (dpc 2.5) group. Significant difference (*) due to genotype at P<0.05 was identified by Student’s t-test for dpc 2.5 and 4.5. One-way ANOVA was used to compare Phb2 and Phb1 mRNA levels of WT among pregnancy days. Means with different superscripts differed at P<0.05.
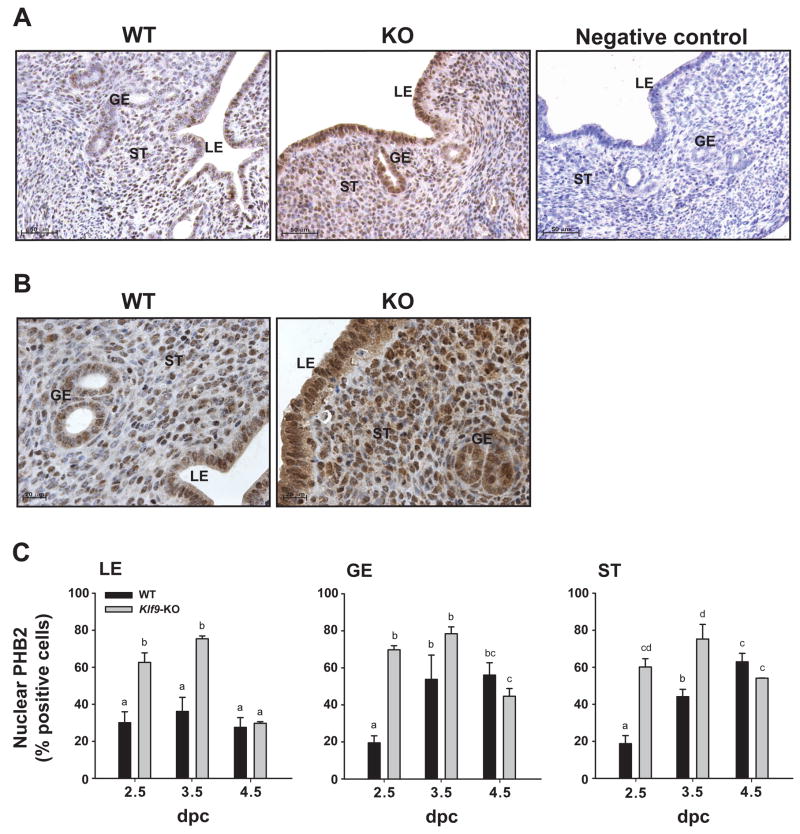
Next, immunoreactive PHB2 was localized in uteri of WT and KO mice at dpc2.5, 3.5, and 4.5. Representative immunostained uterine sections from WT and KO mice at dpc 2.5 are shown (Fig. 5A). In WT mice, immunoreactive PHB2 was present in all endometrial cell types and in the myometrium (not shown), and was localized to both cytoplasmic and nuclear compartments (Fig. 5B). The percentage of nuclear PHB2-positive cells in WT mice was higher in GE and ST cells at dpc 3.5 and dpc 4.5 than at dpc 2.5 (Fig. 5C), following the temporal pattern observed for corresponding mRNA in whole uteri; by contrast, the percentage of nuclear PHB2-positive LE cells did not change across these pregnancy days (Fig. 4A). At dpc 2.5, there was a greater level (by 2-3-fold) in the percentage of nuclear PHB2-immunostaining cells for all endometrial cell types of Klf9 null mice, relative to WT counterparts (Fig. 5C). At dpc 3.5, LE and ST, but not GE cells showed similar significant increases in nuclear PHB2 levels with Klf9 ablation, whereas at dpc 4.5, nuclear PHB2 expression for all cell types was independent of Klf9 (Fig. 5C).
Figure 5.
Nuclear PHB2 levels in uterine endometrial cells of WT and Klf9 null mice during early pregnancy. (A) Representative immunostaining for uterine PHB2 in pregnant WT and Klf9 null mice at dpc 2.5. GE, glandular epithelium; LE, luminal epithelium; ST, stroma. Panels are shown at 200X magnification. Negative control was tissue from Klf9 KO mice processed similarly except for omission of primary antibody. (B) Representative PHB2 immunostaining of GE, LE, and ST cells of pregnant WT and Klf9 null (KO) mice at dpc 2.5 are shown at 400X magnification to demonstrate nuclear localization of immunoreactive PHB2. (C) The percentages of nuclear-immunostained cells are presented as mean ± SEM (n=3-5 mice per treatment per genotype). Significant differences were identified by two-way ANOVA, followed by Tukey’s test. Means with different superscripts differed at P<0.05.
Phb2 expression in human endometrial stromal cells with Klf9 knock-down
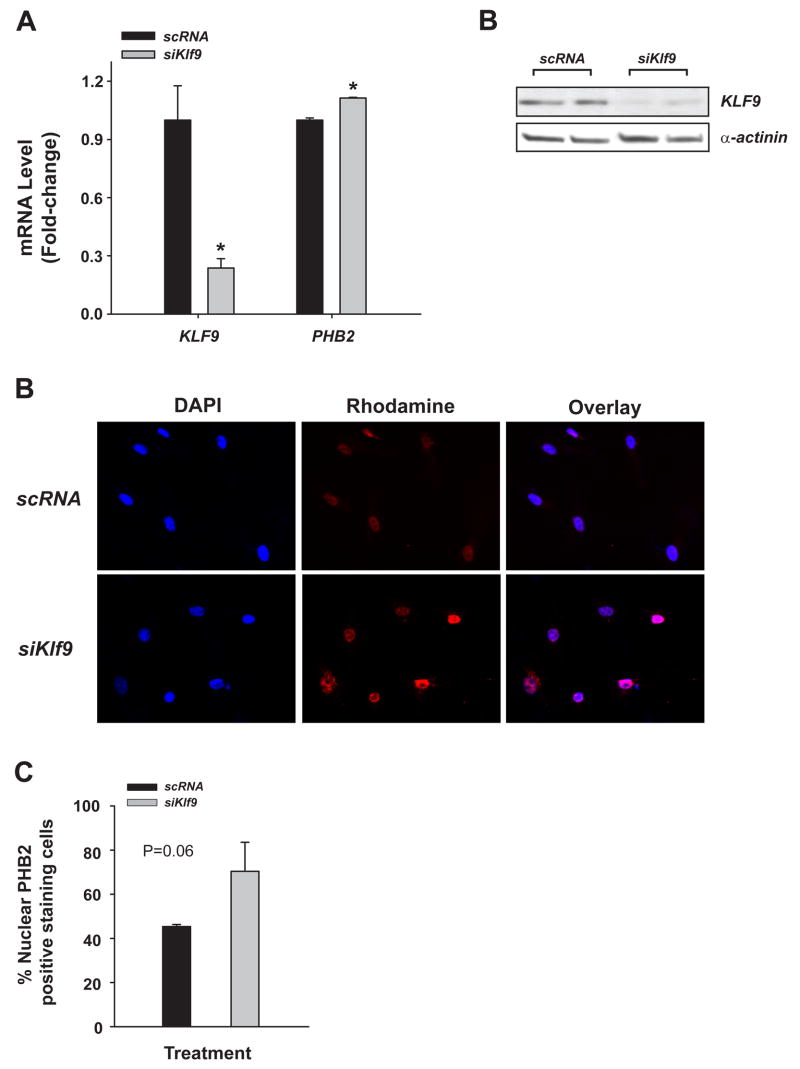
To evaluate if KLF9 directly inhibits PHB2 expression in an E2-dominated environment as suggested in vivo (above), a human endometrial stromal cell line HESC was used in siRNA targeting of human KLF9 mRNA. PHB2 expression in siKLF9 mRNA-transfected cells, relative to those transfected with non-specific (scrambled) siRNAs, was subsequently determined at the transcript and nuclear protein levels. Transfection of HESC with a pool of KLF9 siRNAs (Dharmacon) followed by E2-treatment diminished KLF9 gene expression by at least 70%, relative to similarly treated cells transfected with scrambled siRNAs (Fig. 6A); this level of knock-down at the transcript level resulted in a comparable decrease in KLF9 protein levels, as determined by Western blots (Fig. 6B). The decrease in KLF9 expression significantly (P=0.009), albeit modestly increased (by 20%) PHB2 transcript levels (Fig. 6A). Immunofluorescence studies with anti-PHB2 antibody confirmed the up-regulated expression of PHB2 at the level of the protein (Fig. 6C). Relative to scrambled siRNA, KLF9 siRNA increased the percentage of nuclear-localized PHB2 in E2-treated HESC (Fig. 6D). Parallel studies using anti-ERα antibody showed no effect of KLF9 status on the nuclear levels of this protein (data not shown), consistent with the findings for uterine ST cells of Ovx, E2-treated Klf9 null mice (described in text, above).
Figure 6.
PHB2 levels in human endometrial stromal cells as a function of KLF9 expression status. HESC were incubated in phenol-red free DMEM/Ham’s F12 medium containing 10% charcoal-stripped serum and E2 (10 nM) in the presence of siRNA to scrambled (negative control) mRNA or siRNA to KLF9 (50 nM). (A) Harvested cells were analyzed for KLF9 and PHB2 transcripts by QPCR and normalized to control gene RPL7. Results (means ± SEM; relative to cells treated with scrambled siRNA) shown are representative of 2–3 independent experiments, with each experiment conducted in triplicates. (B) Western blots of whole cell lysates prepared from HESC cells as a function of Klf9 knockdown. Cells transfected with siRNA to scrambled sequence (negative control) or to Klf9 were subjected to Western blots using anti-KLF9 or anti-α-actinin antibodies. Each lane represents an independent experiment. (C) HESC treated with E2 for 24 h were immunostained for PHB2/REA and counterstained for DAPI. Immunopositive cells were visualized using fluorescent antibodies. Overlay of anti-PHB2 (red) and DAPI (blue)-staining cells showed nuclear localization of PHB2. (D) The percentages of nuclear PHB2-staining cells were expressed relative to the total number of cells counted. Results (mean ± SEM) were normalized to those of scrambled siRNA-treated cells and were from two independent experiments, with each experiment performed in triplicates. Differences between groups were determined by t-test (P=0.06)
Discussion
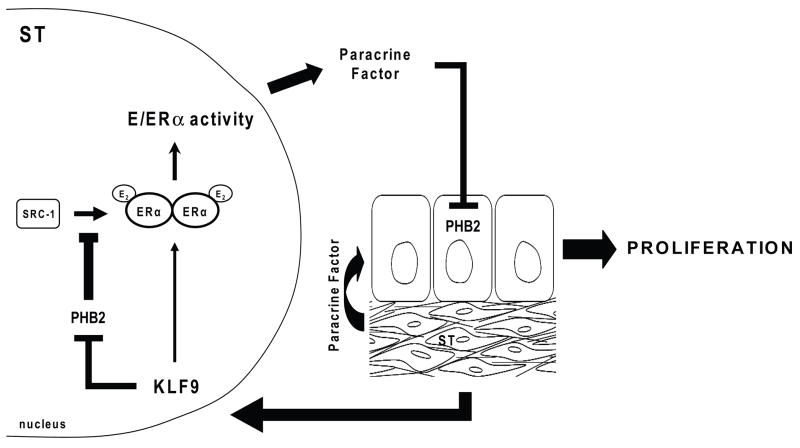
Results from the present study suggest the participation of the transcription factor KLF9 in paracrine signaling to regulate E2-induced proliferation of uterine endometrial epithelial cells. Using Ovx, E2-treated Klf9 null mutant mice and corresponding WT littermates as controls, we showed that the stromal KLF9 regulation of the proliferative response of endometrial LE cells toE2 is negatively associated with uterine Phb2 expression and nuclear localization of PHB2 in endometrial epithelial cells. We confirmed the physiological relevance of the negative linkage between KLF9 and PHB2 by demonstrating that the transiently lower proliferative response of uterine endometrial epithelial cells of Klf9 null mutants relative to WT counterparts at dpc 2.5 (Velarde et al. 2005) corresponded with increased expression of nuclear PHB2 expression in these cells. Finally, we demonstrated that in the E2-treated human endometrial stromal cell line HESC, a reduction in KLF9 expression resulted in increased transcript and nuclear protein levels for PHB2. Taken together, our results suggest a working model whereby E2 control of epithelial cell proliferation can be dynamically influenced by stromal KLF9 (Fig. 7). By attenuating expression of stromal ERα corepressor PHB2 which can interact with ERα coactivators such as SRC-1 (Montano et al. 1999) and histone deacetylases (Kurtev et al. 2004), KLF9 may promote ERα-mediated transactivation of yet unknown paracrine factor(s) to repress epithelial expression of PHB2, leading to increased proliferative responsiveness to E2.
Figure 7.
Postulated model for E2 control of uterine epithelial cell proliferation involving stromal KLF9. By attenuating expression of stromal corepressor PHB2 which can inhibit the interaction of ERα coactivators such as SRC-1 with ERα, KLF9 may promote ligand-dependent ERα-mediated transactivation of paracrine factor(s) yet unknown to repress epithelial expression of PHB2, leading to cell proliferation.
The current study provides the first direct evidence for KLF9 involvement in E2-induced proliferation of uterine endometrial cells. Our previous studies using early pregnant mouse uteri hinted at this possibility, since maximal BrdU-labeling of endometrial epithelial cells occurred at the E-dominated uterine environment of dpc 2.5 (Velarde et al. 2005), coincident with highest uterine stromal Klf9 expression (this study). Since uterine expression of Klf9 is predominantly stromal and is lacking in luminal epithelial cells (Simmen et al., 2004), the absence of proliferative response to E2 of the latter endometrial compartment in Ovx, E2-treated Klf9 null mice suggests paracrine control by an E2-induced ST-derived growth-regulatory factor(s) whose synthesis may involve functional Klf9/ERα interactions. The latter is in agreement with previous reports that ST-localized ERα mediates the mitogenic effects of E2 on neighboring LE cells (Cooke et al. 1997) and that KLF9 can influence ERα transactivity in endometrial epithelial cells (Velarde et al. 2007). Although the identity of this putative KLF9/ERα-regulated paracrine factor(s) is currently unknown, our results suggest that this factor(s) may be involved in the inhibition of PHB2 expression in luminal epithelial cells to allow these cells, which express ERα, to optimally respond to E2. Interestingly, the negative relationship between PHB2 and KLF9 was not extended to the related Phb1 gene, whose gene product also functions as a repressor of ERα signaling (Mishra et al. 2006; He et al. 2007). However, we cannot exclude the possibility that an association between KLF9 and PHB1 expression may be manifest at the level of PHB1 protein.
To further investigate the molecular interplay between ERα and KLF9 in stromal cells, we evaluated the effect of KLF9 knockdown as a function of E2 treatment on PHB2 expression using the human stromal cell line HESC. Since loss of KLF9 expression in vivo was associated with enhanced expression of nuclear PHB2 in ST (by 40-fold), more so than in GE and LE (by 3-fold) cells, it is possible that KLF9 regulation of PHB2 levels in ST may be distinct from that in LE. Our findings that abrogation of KLF9 gene expression resulted in increased PHB2 transcript and nuclear PHB2 protein levels confirm the negative association of KLF9 and PHB2 in vivo. However, the in vitro effects were modest and did not recapitulate the 2-3-fold (pregnancy) and 40-fold (Ovx/E2 model) increases in PHB2 expression seen in vivo. Albeit this difference may be a function of the limited time of exposure to E2 in vitro, the in vivo data was also observed 24 h post-E2, raising the strong possibility of a requirement for ST and LE communication (paracrine signaling) in this E2-mediated process.
Our studies indicate that the regulation of uterine PHB2 expression involving KLF9 is complex and could occur at the levels of RNA, protein, and cellular localization. Indeed, the lack of a clear-cut association between nuclear PHB2 and KLF9 expression (this study) with LE proliferation status (Velarde et al. 2005) at dpc 3.5 and dpc 4.5, in contrast to that seen at dpc 2.5, and the loss of the inverse association of KLF9 and nuclear PHB2 levels at dpc 4.5 in LE as well as in GE and ST, suggests the additional contributions of progesterone, other KLF9-related proteins which may compensate for the loss of KLF9 function (Simmen et al. 2004) and possibly, the attaching embryo in the control of LE proliferation at early pregnancy. Since control of PHB2 levels may constitute part of a homeostatic mechanism to allow for the appropriate proliferative response of uterine cells to E2, further investigations into the function and molecular regulation of PHB2 in the uterine endometrium by KLF9 in concert with other nuclear steroid receptor co-regulators should constitute an important area for future research.
In summary, our studies define a potential mechanism whereby control of E-induced endometrial epithelial cell proliferation can be dynamically regulated by stromal KLF9 through its indirect inhibition of nuclear PHB2 expression in epithelial cells. Results suggest the participation of KLF9 in E2-dependent synthesis of an ST-derived paracrine factor(s) that mediates this epithelial mitogenic response to oestrogen. While the global significance of KLF9 regulation of ERα signaling mediated by PHB2 in luminal epithelial cells is currently unclear, the sub-fertility phenotype of Klf9 null mice (Simmen et al. 2004) suggests physiological relevance of KLF9 during the E2-dominated period of early pregnancy prior to implantation (i.e., pre-decidual stroma) and underscores the delicate control of nuclear coactivator and corepressor expression to achieve the requisite uterine response to oestrogen for successful pregnancy.
Acknowledgments
This work was supported by National Institutes of Health grant HD21961. MCV’s present address: Department of OB/GYN and Reproductive Sciences, University of California San Francisco, San Francisco, California 94143. There is no conflict of interest.
Footnotes
Publisher's Disclaimer: This is not the definitive version of record of this article. This manuscript has been accepted for publication in Journal of Endocrinology, but the version presented here has not yet been copy edited, formatted or proofed. Consequently, the Society for Endocrinology accepts no responsibility for any errors or omissions it may contain. The definitive version is now freely available at doi:10.1677/JOE-08-0383. © 2008 Society for Endocrinology.
References
- Cooke PS, Buchanan DL, Young P, Setiawan T, Brody J, Korach KS, Taylor J, Lubahn DB, Cunha GR. Stromal estrogen receptors mediate mitogenic effects of estradiol on uterine epithelium. Proc Natl Acad Sci USA. 1997;94:6535–6540. doi: 10.1073/pnas.94.12.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delage-Morroux R, Martini PG, Choi I, Kraichely DM, Hoeksema J, Katzenellenbogen BS. Analysis of estrogen receptor interaction with repressor of estrogen receptor activity (REA) and the regulation of estrogen receptor transcriptional activity by REA. J Biol Chem. 2000;275:35848–35856. doi: 10.1074/jbc.M001327200. [DOI] [PubMed] [Google Scholar]
- Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development. 2000;127:4277–4291. doi: 10.1242/dev.127.19.4277. [DOI] [PubMed] [Google Scholar]
- Dutertre M, Smith CL. Ligand-independent interactions of p160/steroid receptor coactivators and CREB-binding protein (CBP) with estrogen receptor α: regulation by phosphorylation sites in the A/B region depends on other receptor domains. Mol Endocrinol. 2003;17:1296–1314. doi: 10.1210/me.2001-0316. [DOI] [PubMed] [Google Scholar]
- Font de Mora J, Brown M. AIB1 is a conduit for kinase-mediated growth factor signaling to the estrogen receptor. Mol Cell Biol. 2000;20:5041–5047. doi: 10.1128/mcb.20.14.5041-5047.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusaro G, Dasgupta P, Rastogi S, Joshi B, Chellappan S. Prohibitin induces the transcriptional activity of p53 and is exported from the nucleus upon apoptotic signaling. J Biol Chem. 2003;278:47853–47861. doi: 10.1074/jbc.M305171200. [DOI] [PubMed] [Google Scholar]
- Gamble SC, Chotal D, Odontiadis M, Dart DA, Brooks GN, Powell SM, Reebye V, Varela-Carver A, Kawano Y, Waxman J, Bevan CL. Prohibitin, a protein down-regulated by androgens, represses androgen receptor activity. Oncogene. 2007;26:1757–1768. doi: 10.1038/sj.onc.1209967. [DOI] [PubMed] [Google Scholar]
- Green KA, Carroll JS. Oestrogen-receptor-mediated transcription and the influence of co-factors and chromatin state. Nature Rev Cancer. 2007;7:713–722. doi: 10.1038/nrc2211. [DOI] [PubMed] [Google Scholar]
- Hall JM, Couse JF, Korach KS. The multifaceted mechanisms of estradiol and estrogen receptor signaling. J Biol Chem. 2001;276:36869–36872. doi: 10.1074/jbc.R100029200. [DOI] [PubMed] [Google Scholar]
- He B, Feng Q, Mukherjee A, Lonard DM, DeMayo FJ, Katzenellenbogen BS, Lydon JP, O’Malley BW. A repressive role for prohibitin in estrogen signaling. Mol Endocrinol. 2007;22:344–360. doi: 10.1210/me.2007-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu YC, Shyr CR, Che W, Mu XM, Kim E, Chang C. Suppression of estrogen receptor-mediated transcription and cell growth by interaction with TR2 orphan receptor. J Biol Chem. 2002;277:33571–33579. doi: 10.1074/jbc.M203531200. [DOI] [PubMed] [Google Scholar]
- Imataka H, Sogawa K, Yasumoto K, Kikuchi K, Sasano K, Kobayashi A, Hayami M, Fujii-Kuriyama Y. Two regulatory proteins that bind to the basic transcription element (BTE), A GC box sequence in the promoter region of the rat P4501A1 gene. EMBO J. 1992;11:3663–3671. doi: 10.1002/j.1460-2075.1992.tb05451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krikun G, Mor G, Alvero A, Guller S, Schatz F, Sapi E, Rahman M, Caze R, Qumsiyeh M, Lockwood CJ. A novel immortalized human endometrial stromal cell line with normal progestational response. Endocrinology. 2004;145:2291–2296. doi: 10.1210/en.2003-1606. [DOI] [PubMed] [Google Scholar]
- Kurtev V, Margueron R, Kroboth K, Ogris E, Cavailles V, Seiser C. Transcriptional regulation by the repressor of estrogen receptor activity via recruitment of histone deacetylases. J Biol Chem. 2004;279:24384–24843. doi: 10.1074/jbc.M312300200. [DOI] [PubMed] [Google Scholar]
- Lonard DM, O’Malley BW. The expanding cosmos of nuclear receptor coactivators. Cell. 2006;125:411–414. doi: 10.1016/j.cell.2006.04.021. [DOI] [PubMed] [Google Scholar]
- McKenna NJ, O’Malley BW. Combinatorial control of gene expression by nuclear receptors and coregulators. Cell. 2002;108:465–474. doi: 10.1016/s0092-8674(02)00641-4. [DOI] [PubMed] [Google Scholar]
- Mishra S, Murphy LC, Murphy LJ. The prohibitins: emerging roles in diverse functions. J Cell Mol Biol Med. 2006;10:353–363. doi: 10.1111/j.1582-4934.2006.tb00404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montano MM, Ekena K, Delage-Morroux R, Chang W, Martini P, Katzenellenbogen BS. An estrogen receptor-selective coregulator that potentiates the effectiveness of antiestrogens and represses the activity of estrogens. Proc Natl Acad Sci USA. 1999;96:6947–6952. doi: 10.1073/pnas.96.12.6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita M, Kobayashi A, Yamashita T, Shimanuki T, Nakajima O, Takahasi S, Ikegami S, Inokuchi K, Yamashita K, Yamamoto M, Fujii-Kuriyama Y. Functional analysis of basic transcription element binding protein by gene targeting technology. Mol Cell Biol. 2003;23:2849–2500. doi: 10.1128/MCB.23.7.2489-2500.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussi P, Liao L, Park S-E, Ciana P, Maggi A, Katzenellenbogen BS, Xu J, O’Malley BW. Haploinsufficiency of the corepressor of estrogen receptor activity (REA) enhances estrogen receptor function in the mammary gland. Proc Natl Acad Sci USA. 2006;103:16716–16721. doi: 10.1073/pnas.0607768103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SE, Xu J, Frolova A, Liao L, O’Malley BW, Katzenellenbogen BS. Genetic deletion of the repressor of estrogen receptor activity (REA) enhances the response to estrogen in target tissues in vivo. Mol Cell Biol. 2005;25:1989–1999. doi: 10.1128/MCB.25.5.1989-1999.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmen RCM, Eason RR, McQuown JR, Linz AL, Kang T-J, Chatman L, Till SR, Fujii-Kuriyama Y, Simmen FA, Oh SP. Subfertility, uterine hypoplasia, and partial progesterone resistance in mice lacking the Krüppel-like factor 9/basic transcription element-binding protein-1 (Bteb1) gene. J Biol Chem. 2004;279:29286–29294. doi: 10.1074/jbc.M403139200. [DOI] [PubMed] [Google Scholar]
- Stenoien DL, Nye AC, Mancini MG, Patel K, Dutertre M, O’Malley BW, Smith CL, Belmont AS, Mancini A. Ligand-mediated assembly and real-time cellular dynamics of estrogen receptor α-coactivator complexes in living cells. Mol Cell Biol. 2001;21:4404–4412. doi: 10.1128/MCB.21.13.4404-4412.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suske G, Bruford E, Philipsen S. Mammalian SP/KLF transcription factors: bring in the family. Genomics. 2005;85:551–556. doi: 10.1016/j.ygeno.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Tsai MJ, O’Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- Velarde MC, Geng Y, Eason RR, Simmen FA, Simmen RCM. Null mutation of Krüppel-like factor 9/basic transcription element binding protein-1 alters peri-implantation uterine development in mice. Biol Reprod. 2005;73:472–481. doi: 10.1095/biolreprod.105.041855. [DOI] [PubMed] [Google Scholar]
- Velarde MC, Iruthayanathan M, Eason RR, Zhang D, Simmen FA, Simmen RCM. Progesterone receptor transactivation of the secretory leukocyte protease inhibitor gene in Ishikawa endometrial epithelial cells involves recruitment of Krüppel-like factor 9/Basic transcription element binding protein-1. Endocrinology. 2006;147:1969–1978. doi: 10.1210/en.2005-1419. [DOI] [PubMed] [Google Scholar]
- Velarde MC, Zeng Z, McQuown JR, Simmen FA, Simmen RCM. Krüppel-like factor 9 is a negative regulator of ligand-dependent estrogen receptor α signaling in Ishikawa endometrial adenocarcinoma cells. Mol Endocrinol. 2007;21:2988–3001. doi: 10.1210/me.2007-0242. [DOI] [PubMed] [Google Scholar]
- Wang S, Zhang B, Faller DV. BRG1/BRM and prohibitin are required for growth suppression by estrogen antagonists. EMBO J. 2004;23:2293–2303. doi: 10.1038/sj.emboj.7600231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Zhang C, Nordeen SK, Shapiro DJ. In vitro fluorescence anisotropy analysis of the interaction of full-length SRC1a with estrogen receptors α and β support an active displacement model for coregulator utilization. J Biol Chem. 2007;282:2765–2775. doi: 10.1074/jbc.M607531200. [DOI] [PubMed] [Google Scholar]
- Yahata T, Shao W, Endoh H, Hur J, Coser KR, Sun H, Ueda Y, Kato S, Isselbacher KJ, Brown M, Shioda T. Selective coactivation of estrogen-dependent transcription by CITED1 CBP/p300-binding protein. Genes Dev. 2001;15:2598–2612. doi: 10.1101/gad.906301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Zhang X-L, Michel FJ, Blum JL, Simmen FA, Simmen RCM. Direct interaction of the Krüppel-like Family (KLF) member, BTEB1 and progesterone receptor mediates progesterone-responsive gene expression in endometrial epithelial cells. Endocrinology. 2002;141:62–73. doi: 10.1210/endo.143.1.8590. [DOI] [PubMed] [Google Scholar]
- Zhang X-L, Zhang D, Michel FJ, Blum JL, Simmen FA, Simmen RCM. Selective interactions of KLF9/BTEB1 with progesterone receptor isoforms A and B determine transcriptional activity of progesterone-responsive genes in endometrial epithelial cells. J Biol Chem. 2003;278:2174–21482. doi: 10.1074/jbc.M212098200. [DOI] [PubMed] [Google Scholar]
- Zheng FF, Wu RC, Smith CL, O’Malley BW. Rapid estrogen-induced phosphorylation of the SRC-3 coactivator occurs in an extranuclear complex containing estrogen receptor. Mol Cell Biol. 2005;25:8273–8284. doi: 10.1128/MCB.25.18.8273-8284.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]