Abstract
The G-protein coupled receptor, GPR54, and its ligand, kisspeptin-54 (a KiSS-1 derived peptide) have been reported to be important players in control of LHRH-1 release. However, the role of the GPR54 signaling in primate reproductive senescence is still unclear. In the present study we investigated whether KiSS-1, GPR54, and LHRH-1 mRNA in the brain change after menopause in female rhesus monkeys using quantitative real-time PCR. Results indicate that KiSS-1, GPR54, and LHRH-1 mRNA levels in the medial basal hypothalamus (MBH) in postmenopausal females (28.3±1.1 years of age, n=5) were all significantly higher than that in eugonadal adult females (14.7±2.1 years of age, n=9), whereas KiSS-1, GPR54, and LHRH-1 mRNA levels in the preoptic area (POA) did not have any significant changes between the two age groups. To further determine the potential contribution by the absence of ovarian steroids, we compared the changes in KiSS-1, GPR54, and LHRH-1 mRNA levels in young adult ovarian intact vs. young ovariectomized females. Results indicate that KiSS-1 and LHRH-1 mRNA levels in the MBH, not POA, in ovariectomized females were significantly higher than those in ovarian intact females, whereas GPR54 mRNA levels in ovariectmized females had a tendency to be elevated in the MBH, although the values were not quite statistically significant. Collectively, in the primate the reduction in the negative feedback control by ovarian steroids appears to be responsible for the aging changes in kisspeptin-GPR54 signaling and the elevated state of the LHRH-1 neuronal system.
Keywords: LHRH-1 mRNA, KiSS-1 mRNA, GPR54 mRNA, menopause, primates
Introduction
Reproductive senescence is a consequence of aging changes in neuronal function per se and/or impaired gonadal feedback function. It has been well recognized that there is a significant species difference in the mechanism: in primates the cessation of reproductive cycles and an altered neuroendocrine change are primarily due to ovarian aging, whereas in rodents both the brain and the ovary are responsible for reproductive aging (review by 60). Elevated levels of LHRH-1 release from the hypothalamus and LH release from the pituitary gland in postmenopausal female monkeys are parallel to a decrease in circulating estrogen (16,58), similar to elevated LH levels after ovariectomy in young female monkeys (7). However, subtle changes in the neuroendocrine hypothalamus after menopause in women have also been reported (17), and therefore, it is important to examine aging changes in neuronal function in the hypothalamus in non-human primates.
Since reports in 2003 showing that mutations of GPR54 were associated with infertility or delayed puberty in human patients (5,47), a large number of studies describing the role of the G-protein coupled receptor (GPR) 54 signaling in control of the LHRH-1 system have been reported. In fact, KiSS-1 derived peptides, kisspeptins, and their cognate receptor, GPR54, appear to play a role in the mechanism initiating puberty (4,13,18,30,33,34; also see reviews: 3,22,36,46,55), negative feedback control of LHRH-1/LH release by gonadal steroid hormones (see reviews: 8,46,51), preovulatory LHRH-1/LH surge (25,42,44, see reviews:23,31), maintenance of pregnancy and lactation (6,43,59; see review:41), and sex differences in neuroendocrine function (2,24).
Despite extensive reports on kisspeptin/GPR54 in reproductive neuroendocrinology in puberty and adult life, however, the role of kisspeptin-GPR54 signaling in reproductive senescence has not been well investigated. Therefore, in the present study, we investigated whether changes in KiSS-1, GPR54, and LHRH-1 mRNA in the medial basal hypothalamus (MBH) and preoptic area (POA) occur, when female rhesus monkeys reach the postmenopausal state. In addition, to determine whether the postmenopausal changes in the GPR54 signaling system are due to the loss of ovarian function, we examined the effects of ovariectomy on KiSS-1, GPR54, and LHRH-1 mRNA in the MBH and POA.
Materials and Methods
Animals
Fourteen healthy adult female rhesus monkeys (Macaca mulatta) born and raised in the Wisconsin National Primate Research Center (WNPRC) were used in the menopause experiment. Five of 14 animals were aged postmenopausal females at 28.3±1.1 years of age and the remaining 9 females were eugonadal young females at 14.7±2.1 years of age. A second set of 12 females were used for ovariectomy experiment. Six of 12 were ovarian intact females (15.2±2.8 years of age) and the remaining 6 (14.4±5.1 years of age) were ovariectomized (OVX) for a period of 6.0±2.9 months. The monkey brains for this study were obtained through the tissue distribution program in the WNPRC, except for one female, which came from the tissue distribution in the University of Wisconsin School of Medicine and Public Health. All “eugonadal young females” and “ovarian intact female monkeys”, except for one obtained from the School of Medicine and Public Health, were at the follicular stage. The female from the School of Medicine and Public Health did not have records describing menstrual cycles. Before sacrifice, all monkeys received standard care by the WNPRC, as described previously (58). The reproductive state of all monkeys was retrospectively obtained from the WNPRC colony records. The age of menopause, defined as no menstruation for at least one year, in these 5 females was 25.8±1.1 years. The protocol for this study was reviewed and approved by the Animal Care and Use Committee, University of Wisconsin, and all experiments were conducted under the guidelines established by the NIH and USDA.
Tissue collection and RNA extraction
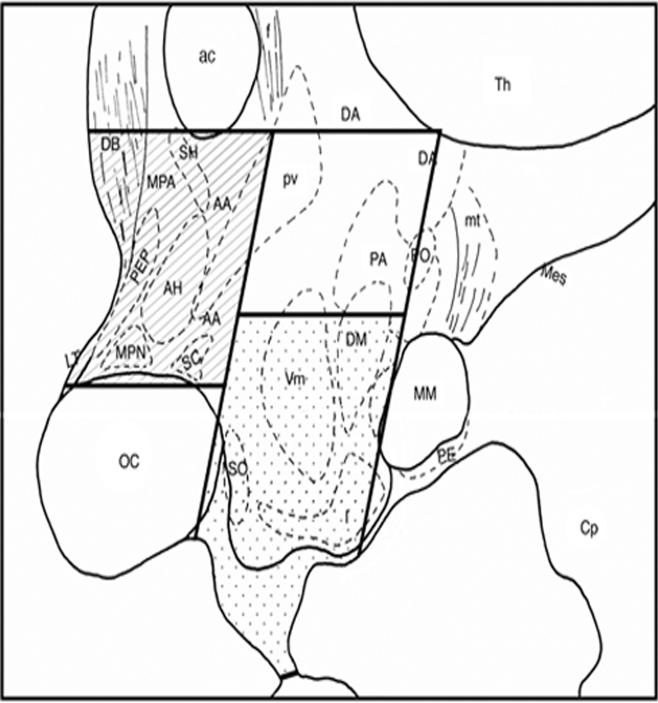
After euthanasia, the brain was removed, divided to two hemispheres, and the medial basal hypothalamus (MBH, defined as the area caudal to the optic chiasm, rostral to the mammillary body, medial to the hypothalamic sulcus, and the ventral half of the hypothalamus) and preoptic area (POA, defined as the area ventral to the anterior commissure, dorsal to the optic chiasm, rostral to the caudal edge of the optic chiasm, and caudal to the rostral edge of the optic chiasm) were rapidly dissected out (Fig. 1). The size of both tissues was approximately 4×4×2 mm3. Tissues were snap-frozen on dry ice in RNAlater (Ambion, Austin, TX), and stored at −80° C until processed for RNA extraction (up to 4 years). The time between the death of animals to tissue dissection was less than 1 h. Total RNA from MBH and POA tissues was extracted using RNA Stat-60 isolation kit (Tel-Test, Inc., Friendswood, TX), according to the manufacturer's instructions.
Figure 1.
A schematic illustration of tissue dissection from the monkey brain for quantitative RT-PCR. The mid sagittal section is shown. In this study, quantitative RT-PCR analysis was done on total RNA from POA (hatched) and MBH (dotted). Abbreviations: AA=anterior hypothalamic area, ac=anterior commissurre, AH=anterior hypothalamic nucleus, Cp=cerebral peduncle, DA=dorsal hypothalamic nucleus, DB=nucleus of diagonal band of Broca, DM=dorsomedial nucleus, f=fornix, I=infundibular nucleus (same as arcuate nucleus in rodents), LT=lamina terminalis, Mes=mesencephalic tegmentum, MM=mammillary body, MPA=medial preoptic area, MPN=medial preoptic nucleus, mt=mammillotegmental tract, OC=optic chiasm, PA=posterior hypothalamic area, PE=perimammillary nucleus, PEP=periventricular preoptic nucleus, PO=posterior nucleus, pv=paraventricular nucleus, SC=suprachiasmatic nucleus, SH=septohypothalamic nucleus, SO=supraoptic nucleus, Th=thalamus, VM=ventromedial nucleus.
Reverse transcription
Prior to conducting quantitative real-time reverse transcriptase-PCR (qRT-PCR), the total RNA was digested with RNase-free DNase I (Invitrogen, Carlsbad, CA), by which possible contamination of genomic DNA was removed. Final RNA concentration was assessed with a Beckman DU 650 (Fullerton, CA) spectrophotometer. The GeneAmp RNA PCR Core kit (Applied Biosystems, Branchburg, NJ) was used for reverse transcription with 2 μg of total RNA and random hexamer primers according to the manufacturer's specifications.
Primers and qPCR
Quantitative real-time PCR was optimized and conducted using the Stratagene Mx3000P Real-Time PCR system (Cedar Creek, TX) with Lux primers designed and synthesized by Invitrogen (Carlsbad, CA, USA) with standard purity. Primer sequences, accession numbers, and primer positions for LHRH-1, KiSS-1, and GPR54 mRNA, along with 18S rRNA are listed in Table 1. The data were obtained in duplicates and normalized with 18S ribosomal RNA values.
Table 1.
The LUX primer pairs for real-time qRT-PCR analysis, with GenBank accession numbers and primer positions.
| Gene | Accession # | Primer sequences (5′ -- 3′) | Primer Position | Efficiencies | |
|---|---|---|---|---|---|
| LHRH-1 | S75918 | Forward | CGGCTGGAGGAAAGAGAGATGCCG | 44−63 | 98.3% |
| Reverse | GCCAGTTGACCAACCTCTTTGA | 94−115 | |||
| KiSS-1 | AY823262 | Forward | TGGAATCCCTGGACCTCTCG | 37−56 | 96.2% |
| Reverse | CACCGGACGGCTTCCTCTCGG | 85−100 | |||
| GPR54 | AY823261 | Forward | ACCGTGACCAACTTCTACATCG | 76−97 | 99.6% |
| Reverse | CGTGACAGCACAGGAGGAAGGTCAG | 119−136 | |||
| 18S rRNA | X03205 | Forward | NA* | 98.2% | |
| Reverse | NA* | ||||
18S rRNA LUX™ sequences are proprietary to Invitrogen and is not made public but is known to amplify a region in the fourth quarter of the gene.
QPCR was carried out as described previously (1). The following procedure was used for amplification: An initial denaturing step at 95° C for 25 min followed by 40−45 cycles of a 95° C denaturing step for 15 sec, a 55° C annealing step for 30 sec, and a 72° C elongation step for 30 sec. After amplification, a dissociation curve analysis was performed to insure purity of PCR products. 18S rRNA was used as a reference gene in the method, as it was reported that 18S rRNA is not regulated by steroid hormones (12). Relative mRNA levels were calculated using the ΔΔCT method (30): The Δ CT for each sample was determined by obtaining the difference between the average CT of the reference gene (18S rRNA) and the average CT of the gene of interest (LHRH-1, KiSS-1, and GPR54). The Δ CT of the calibrator (the sample with the lowest count) is subtracted from the ΔCT of each of the samples to determine the ΔΔ CT. This number is then used to determine the amount of mRNA relative to the calibrator and normalized by 18S rRNA, or the n-fold difference. The n-fold difference was calculated by the equation 2−ΔΔCT.
Statistical analysis
Means (± sem) were calculated from individual n-fold difference data for each mRNA. The data from a calibrator in each mRNA run was excluded from the statistical analysis. Differences between groups were evaluated using the Student-t test. Significance was attained at p<0.05.
Results
1. Changes in LHRH-1 KiSS-1, and GPR54 mRNA with Aging
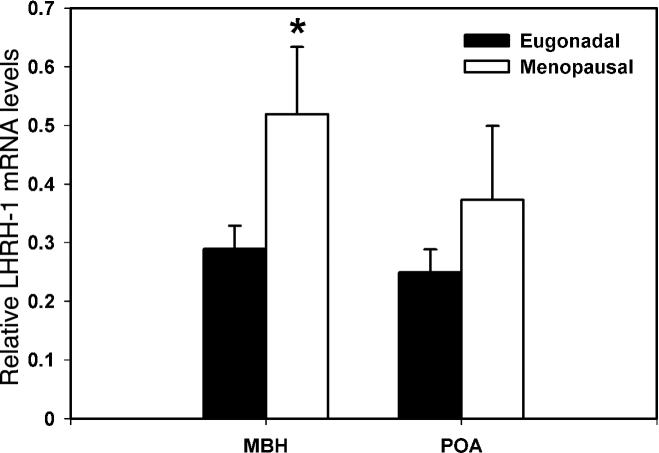
LHRH-1 mRNA levels in the MBH of postmenopausal females were significantly higher (180 % elevation, p=0.045) than those of eugonadal adult females (Fig. 2). In contrast, LHRH-1 mRNA levels in the POA did not differ between the two age groups (p=0.262, Fig. 2).
Figure 2.
Changes in LHRH-1 mRNA in the MBH (left) and POA (right) of female rhesus monkeys with aging. LHRH-1 mRNA levels in postmenopausal and eugonadal females are shown with open and closed bars, respectively. Note that LHRH-1 mRNA levels in the MBH of postmenopausal monkeys, but not in the POA, were significantly higher than those in eugonadal females. *p=0.045.
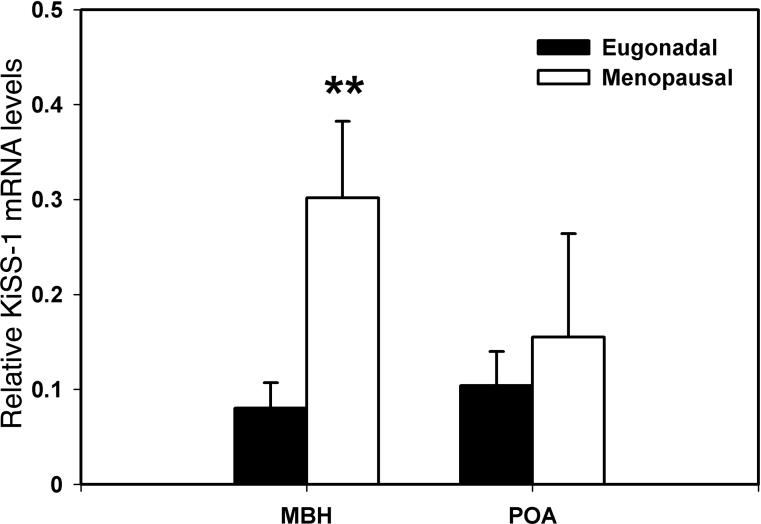
KiSS-1 mRNA levels in the MBH of postmenopausal monkeys were significantly (p=0.009) higher than those in eugonadal adult females (375% elevation, Fig. 3), whereas KiSS-1 mRNA levels in the POA were not different between the two age groups (p=0.593, Fig. 3).
Figure 3.
Changes in KiSS-1 mRNA in the MBH (left) and POA (right) of female rhesus monkeys with aging. KiSS-1 mRNA levels in postmenopausal and eugonadal females are shown with open and closed bars, respectively. Note that KiSS-1 mRNA levels in the MBH of postmenopausal monkeys, but not in the POA, were significantly higher than those in eugonadal females. **p=0.009.
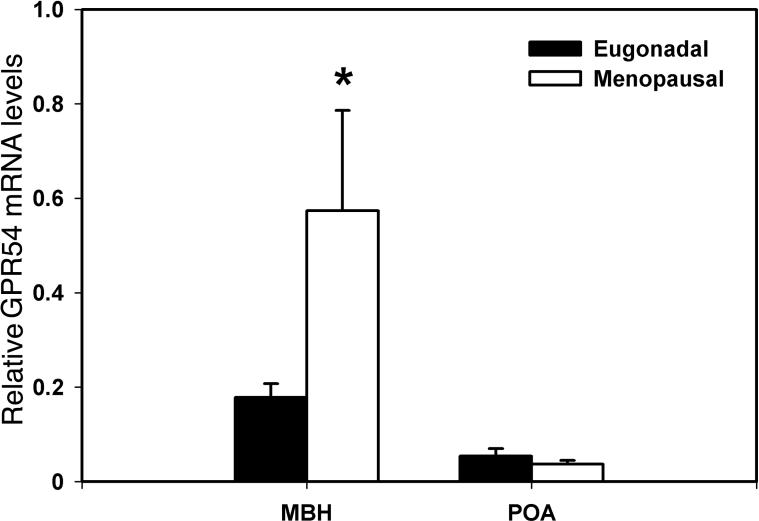
Similarly, GPR54 mRNA levels in the MBH of postmenopausal monkeys were significantly (p=0.012) higher than those in eugonadal adult females (460% elevation, Fig. 4), whereas GPR54 mRNA levels in the POA did not differ between the two age groups (p=0.502, Fig. 4).
Figure 4.
Changes in GPR54 mRNA in the MBH (left) and POA (right) of female rhesus monkeys with aging. GPR54mRNA levels in postmenopausal and eugonadal females are shown with open and closed bars, respectively. Note that GPR54 mRNA levels in the MBH of postmenopausal monkeys, but not in the POA, were significantly higher than those in eugonadal females. *p=0.012.
2. Changes in LHRH-1 KiSS-1, and GPR54 mRNA with ovariectomy
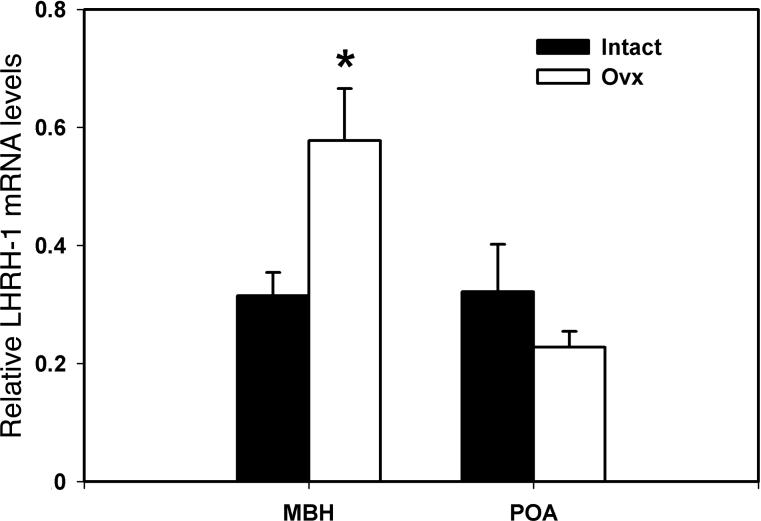
LHRH-1 mRNA levels in the MBH of OVX females were significantly higher (180 % elevation, p=0.049) than those of ovarian intact females (Fig. 5). In contrast, LHRH-1 mRNA levels in the POA did not differ between the two groups (p=0.256, Fig. 5).
Figure 5.
Effects of ovariectomy on LHRH-1 mRNA in the MBH (left) and POA (right) in female rhesus monkeys. LHRH-1 mRNA levels in OVX and ovarian intact females are shown with open and closed bars, respectively. Note that LHRH-1 mRNA levels in the MBH of OVX monkeys, but not in the POA, were significantly higher than those in ovarian intact females. *p=0.049.
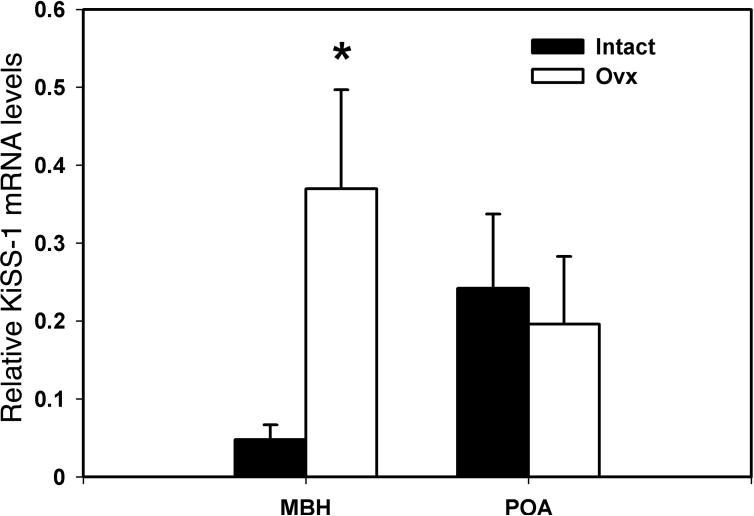
KiSS-1 mRNA levels in the MBH of OVX females were significantly (p=0.022) higher than those in those of ovarian intact females (787% elevation, Fig. 6), whereas KiSS-1 mRNA levels in the POA were not different between the two groups (p=0.733, Fig. 6).
Figure 6.
Effects of ovariectomy on KiSS-1 mRNA in the MBH (left) and POA (right) in female rhesus monkeys. KiSS-1 mRNA levels in OVX and ovarian intact females are shown with open and closed bars, respectively. Note that KiSS-1 mRNA levels in the MBH of OVX monkeys, but not in the POA, were significantly higher than those in ovarian intact females. *p=0.022.
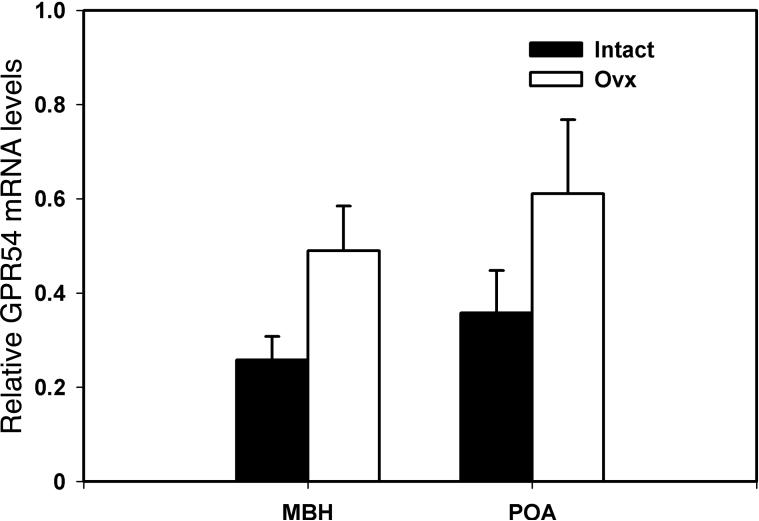
GPR54 mRNA levels in the MBH of OVX monkeys had a tendency to be higher than those in ovarian intact females (190% elevation, Fig. 7), although the values were statistically insignificant (p=0.063). GPR54 mRNA levels in the POA did not differ between the two groups (p=0.177, Fig. 7).
Figure 7.
Effects of ovariectomy on GPR54 mRNA in the MBH (left) and POA (right) in female rhesus monkeys. GPR54mRNA levels in OVX and ovarian intact females are shown with open and closed bars, respectively. Note that GPR54 mRNA levels in the MBH of OVX monkeys had a tendency to be higher than in ovarian intact females, but the p value (p=0.063) was not significant.
Discussion
The findings of this study are summarized as follows: 1) LHRH-1 mRNA levels in the MBH of postmenopausal monkeys were higher than those of eugonadal females, 2) both KiSS-1 and GPR54 mRNA levels in the MBH of postmenopausal monkeys were also higher than those of eugonadal females, 3) LHRH-1 and KiSS-1 mRNA levels in the MBH of OVX monkeys were significantly elevated as compared to those of ovarian intact young females, and 4) none of the genes in the POA were changed by aging nor ovariectomy.
Our present study showing that LHRH-1 mRNA levels in postmenopausal female monkeys were higher than those in their younger counter parts confirms a previous finding in humans by Rance and Uswandi (39) reported using in situ hybridization that LHRH-1 mRNA levels (assessed by grain density) in postmenopausal women were 50% higher than those in premenopausal women. Similarly, the present study confirms the findings by Rance and colleagues using in situ hybridization that the number of LHRH-1 mRNA expressing neurons in the MBH of young adult OVX female cynomologus monkeys was higher than that in ovarian intact counter parts (45) and by El Majdoubi et al. (10) using RNase protection assay (RPA) that orchidectomy increased LHRH-1 mRNA in the MBH in male monkeys. Because previously we also observed that circulating estradiol levels in postmenopausal monkeys were much lower than those in eugonadal females during the early follicular phase, corresponding to an elevated LHRH-1 release and LH release in the postmenopausal state (16,58), and estrogen treatment in OVX monkeys decreased the number of LHRH-1 mRNA expressing neurons (27) or LHRH-1 mRNA abundance in the MBH (9), we can conclude that the postmenopausal increase in LHRH-1 mRNA is due to the loss of ovarian estrogen.
In the present study we also found that menopause increased both KiSS-1 and GPR54 mRNA levels in the MBH. Our observation is consistent with a recent report showing that the number, as well as size, of kisspeptin mRNA expressing neurons in the infundibular nucleus (synonymous to the arcuate nucleus) increased in postmenopausal women as well as in OVX monkeys (44). These authors, however, did not examine GPR54 mRNA expressing neurons in postmenopausal women. Again, the changes in KiSS-1 and GPR54 mRNA levels with menopause are presumably due to a reduction in gonadal function, as ovariectomy resulted in higher levels of KiSS-1 and GPR54 mRNA in adult females.
It has been extensively reported that gonadal steroids modulate GPR54 and KiSS-1 mRNA expression in the brain. Gonadectomy increased and steroid replacement decreased GPR54 and KiSS-1 mRNA in the hypothalamus of rats (33). Similarly, gonadectomy increased KiSS-1 mRNA, but not GPR54 mRNA, expression in the arcuate nucleus of adult mice (52), whereas these treatments caused the opposite change in KiSS-1 mRNA in the anterior-ventral periventricular nucleus (AVPV) of adult rats (52). A similar finding regarding KiSS-1 mRNA changes in the arcuate nucleus by gonadal steroids has also been reported in sheep (50) and cynomologus female monkeys (44). The influence of estradiol on KiSS-1 mRNA appears to be direct, as a majority of kisspeptin neurons in the mouse arcuate nucleus and AVPV and sheep arcuate nucleus express estrogen receptor alpha (ERα, 11,53). Furthermore, estrogen-induced KiSS-1 mRNA expression was not observed in ERα knockout mice (52). Based on these observations Steiner and colleagues hypothesize that kisspeptin neurons in the arcuate nucleus mediate the negative feedback effects of estrogen on LHRH-1 neurons, whereas kisspeptin neurons in the AVPV mediate the positive feedback effects on LHRH-1 neurons in mice (52). In primates, despite the fact that ERα containing neurons were concentrated in the arcuate nucleus (14,29), the co-localization of ERα in kisspeptin neurons in the monkey arcuate nucleus (48) has yet to be demonstrated.
As reported in other species (20), primate LHRH-1 neurons do not express ERα (21), whereas interneurons, such as GABA, glutamate, neuropeptide Y, and kisspeptin neurons contain ERα (see review by 20). However, because LHRH-1 neurons express receptors for these neural inputs (see review by 19,56), the negative feedback action of estrogen on LHRH-1 neurons appears to be mediated by these interneurons. Moreover, LHRH-1 neurons express GPR54 (32,48,35). Therefore, it is reasonable to speculate that kisspeptin neurons in the arcuate nucleus mediate feedback effects of estrogen on LHRH-1 neurons in the primate MBH and that increased LHRH-1 mRNA levels are a consequence of the absence of the estrogen's negative feedback action on kisspeptin neurons and GPR54 containing neurons in the MBH in postmenopausal monkeys.
In contrast to a postmenopausal increase in LHRH-1, KiSS-1, and GPR54 mRNA in the MBH, we did not observe any changes in these genes in the POA. LHRH-1 neurons are found not only in the MBH, but also in the in the POA (15,49). Moreover, we as well as others (38,40,57) described the presence of a subpopulation of LHRH-1 neurons in the extra-hypothalamic regions. Interestingly, in contrast to the changes in LHRH-1 neurons found in the MBH, Rance and Uswandi (39) did not observe any changes in those LHRH-1 neurons in the dorsal POA-septal regions of postmenopausal women. As to kisspeptin neurons in the POA, our preliminary observations indicate that kisspeptin-54 immunopositive neurons with the specific antibody GQ2 (6) are present not only in the arcuate nucleus, but also in the anterior periventricular regions, but not in the AVPV, of the monkey brain, (A. Goff and E. Terasawa, unpublished observations). Nonetheless, failure to observe kisspeptin positive neurons in the primate AVPV suggests clear species difference in primates and rodents.
A series of studies by Knobil and collaborators have demonstrated that the MBH, not POA, is essential for reproductive function in primates (see 26): Isolation of the MBH by complete deafferentation did not interfere with cyclic ovulation in female monkeys (28), whereas lesions in the arcuate nucleus abolished normal reproduction in females (37). Therefore, the absence of postmenopausal changes in LHRH-1, KiSS-1, and GPR54 mRNA in the POA in this study are consistent with the hypothesis that LHRH-1 and kisspeptin neurons in the POA are not involved in control of reproductive function in primates.
Previously, we reported that pulsatile release of LHRH-1 was greatly enhanced in postmenopausal monkeys (16). In the present study, we found that LHRH-1, KiSS-1, and GPR54 mRNA levels in the MBH, but not in the POA, were all increased in postmenopausal monkeys. Our preliminary observations further indicate that the number of kisspeptin-54 expressing neurons in the arcuate nucleus increase significantly in postmenopausal monkeys (E. Hutz, and E. Terasawa, unpublished observations). Because kisspeptin neurons, but not LHRH-1 neurons, contain ERα and LHRH-1 neurons express GPR54, the absence of the negative feedback control by the ovarian steroid, estrogen, through kisspeptin neurons and GPR54 signaling appears to play a major role in postmenopausal activation of the LHRH-1 neuronal system in primates. Although it is possible that subtle changes in LHRH-1, KiSS-1, and GPR54 mRNA levels in the MBH could be due to neuronal aging per se, this is not likely, as there were no changes in these genes in the POA. Results of the present study indicate that changes in neuropeptide gene expression (LHRH-1 and KiSS-1) in the postmenopausal monkeys are strikingly similar to those in postmenopausal humans. Therefore, the findings of this study contribute to better understanding the neuroendocrine axis in postmenopausal women and the mechanism of LHRH-1 and GPR54 signaling in humans.
Acknowledgements
The authors express their sincere appreciation to Kim L. Keen for her technical assistance. This work was supported by NIH grants, HD11355 and HD15433 for ET and MH072956 for APA, and was possible to perform using the facility of Wisconsin National Primate Research Center, supported by NIH grants P51 RR000167, RR15459, and RR020141. WRK is a recipient of Neuroscience Training Program in the University of Wisconsin (T32 GM07507).
References
- 1.Auger CJ, Jessen HM, Auger AP. Microarray profiling of gene expression patterns in adult male rat brain following acute progesterone treatment. Brain Res. 2006;1067:58–66. doi: 10.1016/j.brainres.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 2.Clarkson J, Herbison AE. Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology. 2006;147:5817–25. doi: 10.1210/en.2006-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colledge WH. GPR54 and puberty. Trends Endocrinol Metab. 2004;15:448–53. doi: 10.1016/j.tem.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 4.d'Anglemont de Tassigny X, Fagg LA, Dixon JP, Day K, Leitch HG, et al. Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc Natl Acad Sci USA. 2007;104:10714–9. doi: 10.1073/pnas.0704114104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA. 2003;100:10972–6. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dhillo WS, Chaudhri OB, Patterson M, Thompson EL, Murphy KG, Badman MK, et al. Kisspeptin-54 stimulates the hypothalamic-pituitary gonadal axis in human males. J Clin Endocrinol Metab. 2005;90:6609–15. doi: 10.1210/jc.2005-1468. [DOI] [PubMed] [Google Scholar]
- 7.Dierschke DJ, Bhattacharya AN, Atkinson LE, Knobil E. Circhoral oscillations of plasma LH levels in the ovariectomized rhesus monkey. Endocrinology. 1970;87:850–3. doi: 10.1210/endo-87-5-850. [DOI] [PubMed] [Google Scholar]
- 8.Dungan HM, Clifton DK, Steiner RA. Minireview: kisspeptin neurons as central processors in the regulation of gonadotropin-releasing hormone secretion. Endocrinology. 2006;147:1154–8. doi: 10.1210/en.2005-1282. [DOI] [PubMed] [Google Scholar]
- 9.El Majdoubi M, Ramaswamy S, Sahu A, Plant TM. Effects of orchidectomy on levels of the mRNAs encoding gonadotropin-releasing hormone and other hypothalamic peptides in the adult male rhesus monkey (Macaca mulatta). J Neuroendocrinol. 2000;12:167–76. doi: 10.1046/j.1365-2826.2000.00433.x. [DOI] [PubMed] [Google Scholar]
- 10.El Majdoubi M, Sahu A, Plant TM. Effect of estrogen on hypothalamic transforming growth factor alpha and gonadotropin-releasing hormone gene expression in the female rhesus monkey. Neuroendocrinology. 1998;67:228–35. doi: 10.1159/000054318. [DOI] [PubMed] [Google Scholar]
- 11.Franceschini I, Lomet D, Cateau M, Delsol G, Tillet Y, Caraty A. Kisspeptin immunoreactive cells of the ovine preoptic area and arcuate nucleus co-express estrogen receptor alpha. Neurosci Lett. 2006;401:225–30. doi: 10.1016/j.neulet.2006.03.039. [DOI] [PubMed] [Google Scholar]
- 12.Funabashi T, Brooks PJ, Weesner GD, Pfaff DW. Luteinizing hormone-releasing hormone receptor messenger ribonucleic acid expression in the rat pituitary during lactation and the estrous cycle. J Neuroendocrinol. 1994;6:261–6. doi: 10.1111/j.1365-2826.1994.tb00581.x. [DOI] [PubMed] [Google Scholar]
- 13.Funes S, Hedrick JA, Vassileva G, Markowitz L, Abbondanzo S, Golovko A, et al. The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem Biophys Res Commun. 2003;312:1357–63. doi: 10.1016/j.bbrc.2003.11.066. [DOI] [PubMed] [Google Scholar]
- 14.Goldsmith PC, Boggan JE, Thind KK. Estrogen and progesterone receptor expression in neuroendocrine and related neurons of the pubertal female monkey hypothalamus. Neuroendocrinology. 1997;65:325–34. doi: 10.1159/000127191. [DOI] [PubMed] [Google Scholar]
- 15.Goldsmith PC, Lamberts R, Brezina LR. Gonadotropin-releasing hormone neurons and pathways in the primate Hypothalamus and forebrain. In: Norman RL, editor. Neuroendocrine Aspects of Reproduction. Academic Press; New York: 1983. pp. 7–45. [Google Scholar]
- 16.Gore AC, Windsor-Engnell BM, Terasawa E. Menopausal increases in pulsatile gonadotropin-releasing hormone release in a nonhuman primate (Macaca mulatta). Endocrinology. 2004;145:4653–9. doi: 10.1210/en.2004-0379. [DOI] [PubMed] [Google Scholar]
- 17.Hall JE. Neuroendocrine changes with reproductive aging in women. Semin Reprod Med. 2007;25:344–51. doi: 10.1055/s-2007-984740. [DOI] [PubMed] [Google Scholar]
- 18.Han S, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, et al. Activation of gonadotropin-releasing hormone (GnRH) neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25:11349–56. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herbison AE. Multimodal influence of estrogen upon gonadotropin-releasing hormone neurons. Endocr Rev. 1998;19:302–30. doi: 10.1210/edrv.19.3.0332. [DOI] [PubMed] [Google Scholar]
- 20.Herbison AE. Physiology of gonadotropin releasing hormone neural network. In: Knobil E, Neill JD, editors. Physiology of Reproduction. 3rd Edition Academic Press/Elsevier; San Diego: 2006. pp. 1415–82. [Google Scholar]
- 21.Hrabovszky E, Kalló I, Szlávik N, Keller E, Merchenthaler I, Liposits Z. Gonadotropin-releasing hormone neurons express estrogen receptor-beta. J Clin Endocrinol Metab. 2007;92:2827–30. doi: 10.1210/jc.2006-2819. [DOI] [PubMed] [Google Scholar]
- 22.Kaiser UB, Kuohung W. KiSS-1 and GPR54 as new players in gonadotropin regulation and puberty. Endocrine. 2005;26:277–84. doi: 10.1385/ENDO:26:3:277. [DOI] [PubMed] [Google Scholar]
- 23.Kauffman AS, Clifton DK, Steiner RA. Emerging ideas about kisspeptin- GPR54 signaling in the neuroendocrine regulation of reproduction. Trends Neurosci. 2007;30:504–11. doi: 10.1016/j.tins.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Kauffman AS, Gottsch ML, Roa J, Byquist AC, Crown A, Clifton DK, et al. Sexual differentiation of kiss1 gene expression in the brain of the rat. Endocrinology. 2007;148:1774–83. doi: 10.1210/en.2006-1540. [DOI] [PubMed] [Google Scholar]
- 25.Kinoshita M, Tsukamura H, Adachi S, Matsui H, Uenoyama Y, Iwata K, et al. Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology. 2005;146:4431–6. doi: 10.1210/en.2005-0195. [DOI] [PubMed] [Google Scholar]
- 26.Knobil E. The neuroendocrine control of the menstrual cycle. Recent Prog Horm Res. 1980;36:53–88. doi: 10.1016/b978-0-12-571136-4.50008-5. [DOI] [PubMed] [Google Scholar]
- 27.Krajewski SJ, Abel TW, Voytko ML, Rance NE. Ovarian steroids differentially modulate the gene expression of gonadotropin-releasing hormone neuronal subtypes in the ovariectomized cynomolgus monkey. J Clin Endocrinol Metab. 2003;88:655–62. doi: 10.1210/jc.2002-020887. [DOI] [PubMed] [Google Scholar]
- 28.Krey LC, Butler WR, Knobil E. Surgical disconnection of the medial basal hypothalamus and pituitary function in the rhesus monkey. I. Gonadotropin secretion. Endocrinology. 1975;96:1073–87. doi: 10.1210/endo-96-5-1073. [DOI] [PubMed] [Google Scholar]
- 29.Kruijver FP, Balesar R, Espila AM, Unmehopa UA, Swaab DF. Estrogen receptor-alpha distribution in the human hypothalamus in relation to sex and endocrine status. J Comp Neurol. 2003;454:115–39. doi: 10.1002/cne.10416. [DOI] [PubMed] [Google Scholar]
- 30.Lapatto R, Pallais JC, Zhang D, Chan YM, Mahan A, Cerrato F, et al. Kiss1−/− mice exhibit more variable hypogonadism than Gpr54−/− mice. Endocrinology. 2007;148:4927–36. doi: 10.1210/en.2007-0078. [DOI] [PubMed] [Google Scholar]
- 31.Maeda K, Adachi S, Inoue K, Ohkura S, Tsukamura H. Metastin/kisspeptin and control of estrous cycle in rats. Rev Endocr Metab Disord. 2007;8:21–9. doi: 10.1007/s11154-007-9032-6. [DOI] [PubMed] [Google Scholar]
- 32.Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, et al. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci USA. 2005;102:1761–6. doi: 10.1073/pnas.0409330102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Navarro VM, Castellano JM, Fernandez-Fernandez R, Barreiro ML, Roa J, Sanchez-Criado JE, et al. Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology. 2004;145:4565–74. doi: 10.1210/en.2004-0413. [DOI] [PubMed] [Google Scholar]
- 34.Navarro VM, Fernandez-Fernandez R, Castellano JM, Roa J, Mayen A, Barreiro ML, et al. Advanced vaginal opening and precocious activation of the reproductive axis by KiSS-1 peptide, the endogenous ligand of GPR54. J Physiol. 2004;561:379–86. doi: 10.1113/jphysiol.2004.072298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parhar IS, Ogawa S, Sakuma Y. Laser-captured single digoxigenin-labeled neurons of gonadotropin-releasing hormone types reveal a novel G protein-coupled receptor (GPR54) during maturation in cichlid fish. Endocrinology. 2004;145:3613–8. doi: 10.1210/en.2004-0395. [DOI] [PubMed] [Google Scholar]
- 36.Plant TM. The role of KiSS-1 in the regulation of puberty in higher primates. Eur J Endocrinol. 2006;155(S1):S11–6. doi: 10.1530/eje.1.02232. [DOI] [PubMed] [Google Scholar]
- 37.Plant TM, Krey LC, Moossy J, McCormack JT, Hess DL, Knobil E. The arcuate nucleus and the control of gonadotropin and prolactin secretion in the female rhesus monkey (Macaca mulatta). Endocrinology. 1978;102:52–62. doi: 10.1210/endo-102-1-52. [DOI] [PubMed] [Google Scholar]
- 38.Quanbeck C, Sherwood NM, Millar RP, Terasawa E. Two populations of luteinizing hormone-releasing hormone neurons in the forebrain of the rhesus macaque during embryonic development. J Comp Neurol. 1997;380:293–309. [PubMed] [Google Scholar]
- 39.Rance NE, Uswandi SV. Gonadotropin-releasing hormone gene expression is increased in the medial basal hypothalamus of postmenopausal women. J Clin Endocrinol Metab. 1996;81:3540–6. doi: 10.1210/jcem.81.10.8855798. [DOI] [PubMed] [Google Scholar]
- 40.Rance NE, Young WS, 3rd, McMullen NT. Topography of neurons expressing luteinizing hormone-releasing hormone gene transcripts in the human hypothalamus and basal forebrain. J Comp Neurol. 1994;339:573–86. doi: 10.1002/cne.903390408. [DOI] [PubMed] [Google Scholar]
- 41.Roa J, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. New frontiers in kisspeptin/GPR54 physiology as fundamental gatekeepers of reproductive function. Front Neuroendocrinol. 2008;29:48–69. doi: 10.1016/j.yfrne.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 42.Roa J, Vigo E, Castellano JM, Gaytan F, Navarro VM, Aguilar E, et al. Opposite roles of estrogen receptor (ER) {alpha} and ER{beta} in the modulation of luteinizing hormone responses to kisspeptin in the female rat: Implications for the generation of the preovulatory surge. Endocrinology. 2008;149:1627–37. doi: 10.1210/en.2007-1540. [DOI] [PubMed] [Google Scholar]
- 43.Roa J, Vigo E, Castellano JM, Navarro VM, Fernández-Fernández R, Casanueva FF, et al. Hypothalamic expression of KiSS-1 system and gonadotropin-releasing effects of kisspeptin in different reproductive states of the female Rat. Endocrinology. 2006;147:2864–78. doi: 10.1210/en.2005-1463. [DOI] [PubMed] [Google Scholar]
- 44.Rometo AM, Krajewski SJ, Voytko ML, Rance NE. Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J Clin Endocrinol Metab. 2007;92:2744–50. doi: 10.1210/jc.2007-0553. [DOI] [PubMed] [Google Scholar]
- 45.Sandoval-Guzman T, Stalcup ST, Krajewski SJ, Voytko ML, Rance NE. Effects of ovariectomy on the neuroendocrine axes regulating reproduction and energy balance in young cynomolgus macaques. J Neuroendocrinol. 2004;16:146–53. doi: 10.1111/j.0953-8194.2004.01143.x. [DOI] [PubMed] [Google Scholar]
- 46.Seminara SB. Metastin and its G protein-coupled receptor, GPR54: critical pathway modulating GnRH secretion. Front Neuroendocrinol. 2005;26:131–8. doi: 10.1016/j.yfrne.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 47.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–27. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 48.Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci USA. 2005;102:2129–34. doi: 10.1073/pnas.0409822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Silverman A-J. The gonadotropin-releasing hormone (GnRH) neuronal systems: Immunocytochemistry. In: Knobil E, Neill J, editors. The Physiology of Reproduction. Raven Press; New York: 1988. pp. 1283–304. [Google Scholar]
- 50.Smith JT, Clay CM, Caraty A, Clarke IJ. KiSS-1 messenger ribonucleic acid expression in the hypothalamus of the ewe is regulated by sex steroids and season. Endocrinology. 2007;148:1150–7. doi: 10.1210/en.2006-1435. [DOI] [PubMed] [Google Scholar]
- 51.Smith JT, Clifton DK, Steiner RA. Regulation of the neuroendocrine reproductive axis by kisspeptin-GPR54 signaling. Reproduction. 2006;131:623–30. doi: 10.1530/rep.1.00368. [DOI] [PubMed] [Google Scholar]
- 52.Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146:3686–92. doi: 10.1210/en.2005-0488. [DOI] [PubMed] [Google Scholar]
- 53.Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci. 2006;26:6687–94. doi: 10.1523/JNEUROSCI.1618-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith JT, Rao A, Pereira A, Caraty A, Millar RP, Clarke IJ. Kisspeptin is present in ovine hypophysial portal blood but does not increase during the preovulatory luteinizing hormone surge: evidence that gonadotropes are not direct targets of kisspeptin in vivo. Endocrinology. 2008;149:1951–9. doi: 10.1210/en.2007-1425. [DOI] [PubMed] [Google Scholar]
- 55.Tena-Sempere M. GPR54 and kisspeptin in reproduction. Hum Reprod Update. 2006;12:631–9. doi: 10.1093/humupd/dml023. [DOI] [PubMed] [Google Scholar]
- 56.Terasawa E. Luteinizing hormone-releasing hormone (LHRH) neurons: mechanism of pulsatile LHRH release. Vitam Horm. 2001;63:91–129. doi: 10.1016/s0083-6729(01)63004-8. [DOI] [PubMed] [Google Scholar]
- 57.Terasawa E, Busser BW, Luchansky LL, Sherwood NM, Jennes L, Millar RP, et al. Presence of luteinizing hormone-releasing hormone fragments in the rhesus monkey forebrain. J Comp Neurol. 2001;439:491–504. doi: 10.1002/cne.1364. [DOI] [PubMed] [Google Scholar]
- 58.Woller MJ, Everson-Binotto G, Nichols E, Acheson A, Keen KL, Bowers CY, Terasawa E. Aging-related changes in release of growth hormone and luteinizing hormone in female rhesus monkeys. J Clin Endocrinol Metab. 2002;87:5160–7. doi: 10.1210/jc.2002-020659. [DOI] [PubMed] [Google Scholar]
- 59.Yamada S, Uenoyama Y, Kinoshita M, Iwata K, Takase K, Matsui H, et al. Inhibition of metastin (kisspeptin-54)-GPR54 signaling in the arcuate nucleus-median eminence region during lactation in rats. Endocrinology. 2007;148:2226–32. doi: 10.1210/en.2006-1529. [DOI] [PubMed] [Google Scholar]
- 60.Yin W, Gore AC. Neuroendocrine control of reproductive aging: roles of GnRH neurons. Reproduction. 2006;131:403–14. doi: 10.1530/rep.1.00617. [DOI] [PubMed] [Google Scholar]