Abstract
Multiple myeloma (MM), a hematologic malignancy of terminally differentiated plasma cells is closely associated with induction of osteolytic bone disease, induced by stimulation of osteoclastogenesis and suppression of osteoblastogenesis. The ubiquitin-proteasome pathway regulates differentiation of bone cells and MM cell growth. The proteasome inhibitor, bortezomib, is a clinical potent antimyeloma agent. The main goal of this study was to investigate the effect of bortezomib on myeloma-induced bone resorption and tumor growth in SCID-rab mice engrafted with MM cells from 16 patients. Antimyeloma response of bortezomib, which was evident in >50% of 16 experiments and resembled clinical response, was associated with significant increased bone mineral density (BMD) and osteoblast numbers, and reduced osteoclast numbers in myelomatous bones. This bone anabolic effect, which was also visualized on X-ray radiographs and confirmed by static and dynamic histomorphometric analyses, was unique to bortezomib and was not observed in hosts responding to melphalan, a chemotherapeutic drug widely used to treat MM. Bortezomib also increased BMD and osteoblasts number and reduced osteoclasts number in nonmyelomatous implanted bones. In vitro bortezomib directly suppressed human osteoclast formation and promoted maturation of osteoblasts. We conclude that bortezomib promotes bone formation in myelomatous and nonmyelomatous bones by simultaneously inhibiting osteoclastogenesis and stimulating osteoblastogenesis. As clinical and experimental studies indicate that bone disease is both a consequence and necessity of MM progression our results suggest and that bortezomib’s effects on bone remodeling contribute to the antimyeloma efficacy of this drug.
Introduction
Multiple myeloma (MM), a hematologic malignancy of terminally differentiated plasma cells, is closely associated with induction of osteolytic bone disease and skeletal complications in >80% of patients. Myelomatous osteolysis is localized to areas adjacent to tumor growth and is often characterized by increased activity of osteoclasts and suppression of osteoblastogenesis [1-3]. Current standard management of MM bone disease is limited to the use of bisphosphonates, which deactivates osteoclasts and may induce adverse side effects such as osteonecrosis of the jaw [4] and impaired renal function [5,6]. Although bisphosphonates reduce skeletal complications, bone disease often progresses [7,8], indicating that osteoclastogenesis is only partially inhibited and that suppression of osteoblastogenesis plays a vital role in uncoupling the bone-remodeling process in MM [9].
Bortezomib is a novel pharmaceutical agent with promising efficacy, even for patients with relapsed refractory MM [10]. Some proteasome inhibitors, including bortezomib, reportedly have bone-anabolic activity in vitro and in mice [11,12]. Recent clinical observations revealed a significant correlation between the antimyeloma response elicited by bortezomib and increases in markers of bone formation in patients with MM [13]. Other clinical studies showed that bortezomib promoted osteoblast activity in patients with MM, irrespective of response to treatment [14,15]. Giuliani et al. detected an increased number of osteoblasts in bortezomib-responding patients who were previously treated with bisphosphonates [16]. Bortezomib has been shown to directly inhibit osteoclastogenesis [17] and stimulate osteoblastogenesis in vitro [16]. Oyajobi et al. reported that bortezomib enhanced new bone formation in mouse calvarial cultures by suppressing dickkopf-1 (DKK1) expression in calvarial and bone marrow (BM)-derived stromal cells [18]. Interestingly, bortezomib treatment of patients with MM has been associated with reduced levels of circulating DKK1 and RANKL [15]. DKK1 and RANKL are critically involved in MM bone disease [19,20], and experimental studies showed that blocking activity of RANKL [19,21-24] or DKK1 [25] prevented or reduced MM bone disease and that these effects were associated with reduced tumor burden.
We have recently developed the SCID-rab mouse model for human primary MM [26]. These mice are constructed by implanting mice with a nonfetal rabbit bone into which primary human myeloma cells are directly injected. This model is similar to our extensively tested and validated SCID-hu system, which utilizes a human fetal bone [21,27-29]. Myeloma cells from the majority of patients grow exclusively in the implanted bone and produce typical MM manifestations, including stimulation of osteoclastogenesis, suppression of osteoblastogenesis, and induction of severe osteolytic bone disease. In the current study, we further exploited this system to study the effects of bortezomib on myeloma bone disease and tumor growth.
Results
Effects of bortezomib on MM bone disease and tumor growth
For our studies, we exploited our SCID-rab mouse model for primary MM, which is similar to the SCID-hu model. In both systems, myeloma cells from approximately 75% of patients are successfully engrafted and grow restrictively in the implanted bone, and their growth is characterized by increased levels of human monoclonal immunoglobulins (hIg) in mice sera (indicative of tumor growth) and induction of severe osteolytic bone disease [25,26]. SCID-rab mice successfully engrafted with myeloma cells from 16 patients were used for studying the effects of bortezomib on myeloma cell growth and bone disease. As shown in Table I, myeloma cells were taken from patients with various clinical stages and bone disease. The majority of patients were newly diagnosed and were selected for the study solely based on availability of tumor cells. On establishment of MM tumor growth (indicated by hIg level >10 μg/ml), 16 hosts engrafted with myeloma cells from 16 patients were treated with bortezomib for 4-8 weeks; an additional 16 matching SCID-rab hosts served as controls and were treated with saline for 4-8 weeks (patients 1-16, Table I). As expected, myeloma cells from different patients resulted in heterogeneous myeloma growth patterns among the different mice.
TABLE I. Patients’ Characteristics and Changes in BMD of the Implanted Bone and hIg Levels in SCID-rab Mice During the Experiment.
| BMD (% of pre-Rx)d |
hIg (% of pre-Rx)e |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pt. | Stagea | Prior treatment | Isotype | MRI FLb | Bone diseasec | Cont | BOR | Cont | BOR |
| 1 | IIIb | NO | IgG λ | YES | YES | 82 | 175 | 499 | 5 |
| 2 | II | NO | IgG κ | YES | NO | 101 | 101 | 149 | 10 |
| 3 | IIIa | NO | IgA λ | YES | NA | 96 | 118 | 1255 | 25 |
| 4 | III | NO | IgG κ | YES | YES | 106 | 115 | 159 | 29 |
| 5 | IIIa | NO | IgG λ | YES | YES | 97 | 102 | 199 | 30 |
| 6 | IIIa | YES | IgA κ | NO | -f | 77 | 98 | 514 | 44 |
| 7 | IIIa | YES | IgG κ | YES | - | 89 | 114 | 389 | 40 |
| 8 | IIIb | NO | IgG λ | YES | YES | 103 | 103 | 282 | 65 |
| 9 | IIIa | YES | IgG κ | YES | YES | 79 | 99 | 679 | 77 |
| 10 | IIIb | NO | IgG κ | YES | YES | 98 | 149 | 345 | 222 |
| 11 | IIIa | NO | IgG κ | YES | YES | 67 | 57 | 320 | 252 |
| 12 | IIIa | NO | IgG κ | YES | - | 89 | 90 | 315 | 297 |
| 13 | IIIa | NO | IgG κ | NO | NO | 61 | 70 | 442 | 411 |
| 14 | IIIa | NAg | IgG κ | NA | NO | 85 | 94 | 848 | 426 |
| 15 | III | NO | IgG λ | YES | NO | 54 | 84 | 1028 | 930 |
| 16 | IIIa | NO | IgG κ | YES | YES | 79 | 92 | 170 | 156 |
Stage at diagnosis, according to the Durie-Salmon staging system.
Existence of focal lesions by magnetic resonance imaging (MRI).
Existence of lytic bone lesions examined by standard X-rays.
BMD of the implanted bone determined by DEXA and calculated as percent of pretreatment (pre-Rx) level.
Circulating human Ig in mice sera determined by ELISA and calculated as percent of pretreatment level.
Not done.
Data not available.
When assessing antimyeloma response to treatment, “response” was defined as hIg levels lower than pretreatment levels, and “partial response” as hIg levels at least 25% lower than saline-treated hosts [25,30].
Saline treatment resulted in increased tumor burden in all experiments, but bortezomib treatment resulted in a response in nine experiments (patients 1-9), partial response in two experiments (patients 10 and 14), and no response in five experiments (patients 11, 12, 13, 15, and 16) (Table I). Overall, tumor burden in saline- and bortezomib-treated hosts increased from pretreatment levels by 475 ± 81% and 188 ± 60%, respectively (p < 0.005, Fig. 1A). We further analyzed antimyeloma effects of bortezomib in responders (responsive and partially responsive hosts, n = 11) and nonresponders (n = 5). In the responders, tumor burden was reduced from pretreatment levels (p < 0.003), but in the nonresponders, tumor burden levels similarly increased in saline- and bortezomib-treated hosts (Fig. 1A).
Figure 1.
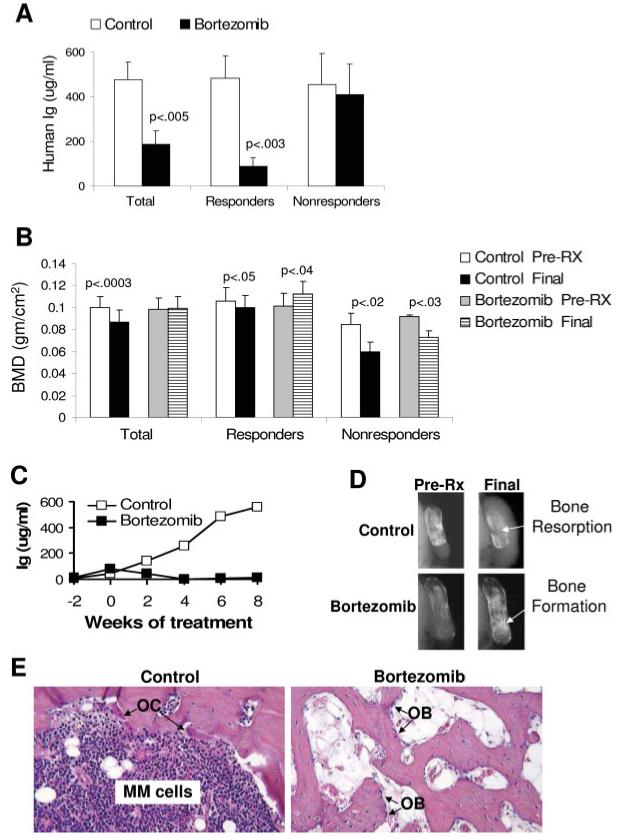
In vivo antimyeloma response of bortezomib (BOR) is associated with increased bone mass. SCID-rab mice engrafted with myeloma cells from 16 patients were treated with saline (control) or bortezomib. (A, B). Changes from pretreatment levels (pre-Rx) of MM burden (A) and levels BMD of the implanted bone (B) at pretreatment (pre-Rx) and experiment’s end (final) in all mice (total), responders/partial responders (n = 11) and nonresponders (n = 5, see Results for definition of response). (C) A representative experiment demonstrating the in vivo inhibitory effect of bortezomib on growth of myeloma cells from patient 1. (D) X-ray radiographs of myelomatous bones engrafted with myeloma cells from patient 1 and taken pre-Rx and at experiment’s end. Note that treatment began when bone disease was evident and that, whereas bone loss continued in the control bone, bone mass markedly increased following bortezomib treatment. (E) H&E staining of histological sections of myelomatous bones engrafted with myeloma cells from patient 1 (×10 original magnification). Note increased myeloma cell infiltration and osteoclast (OC) activity in control bone. In contrast, myelomatous bone from a host treated with bortezomib had no apparent myeloma cells but possessed increased trabecular bone and osteoblast (OB) numbers. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Treatment effects on MM-induced bone disease were analyzed by X-ray radiography, comparing measurements of bone mineral density (BMD) of implanted myelomatous bones before initiation of treatment and at the end of each experiment. In control hosts, BMD of implanted bones was 1%-46% lower than pretreatment levels in 13 experiments and was slightly elevated in 3 experiments (Table I). In contrast, in bortezomib-treated hosts, BMD of implanted bones was by 1%-75% higher than pretreatment levels in 8 experiments and was 1%-43% lower in 8 experiments (Table I). Overall, in control hosts, BMD of implanted bones declined by 14.6 ± 3.8% (p < 0.003), whereas in bortezomib-treated hosts BMD of implanted bones increased by 3.8 ± 7.0% from pretreatment levels. Importantly, however, bortezomib treatment in the responders (showing antimyeloma responses and partial responses) resulted in 15.3 ± 7.5% (p < 0.04) higher BMD of implanted bones than pretreatment levels. In the nonresponders group BMD of implanted bones was markedly lower than pretreatment levels by 29 ± 6% (p < 0.02) and 21 ± 6% (p < 0.03) in control- and bortezomib-treated hosts, respectively (Fig. 1B), indicating that bortezomib treatment reduced bone loss in nonresponding hosts.
Although our study revealed that bortezomib increased bone mass more profoundly in a subset of hosts, changes in BMD of implanted bones was not appreciably correlated with changes in tumor burden. For instance, hosts engrafted with myeloma cells from patients 2, 5, 6, and 8 showed antimyeloma responses to bortezomib, but the BMD of the implanted bones was slightly lower or higher than pretreatment levels (Table I). Conversely, hosts engrafted with myeloma cells from patient 10 partially responded to bortezomib, but BMD of the implanted bone was markedly higher (49%) than pretreatment levels. These observations emphasize the heterogeneity of bone anabolic activities of bortezomib in treating MM [15,31]. The association between antimyeloma response and bone anabolic effect of bortezomib was also visualized on X-radiographs (Fig. 1D). Representative results demonstrating antimyeloma effect of bortezomib in the SCID-rab system, as assessed by hIg changes and histologically, are presented (Fig. 1C,E).
To test whether the in vivo effects of bortezomib on bone remodeling resulted from direct effects on bone cells, rather than an indirect consequence of reduced tumor burden, we compared its effects on MM bone disease with those of melphalan, a drug widely used to treat MM. Our in vitro data showed that melphalan had no cytotoxic effects on osteoblasts and mesenchymal stem cells (MSCs) at doses that inhibit myeloma cell growth (Fig. 2E). Myeloma cells recovered from engrafted hosts (cells from patient 1) were passaged into newly constructed SCID-rab mice, subsequently established as a stroma-dependent cell line, labeled a MM cell line (BN), and infected with luciferase-expressing lentivirus [32]. The use of BN cells enabled engraftment of a larger number of SCID-rab mice for this study.
Figure 2.
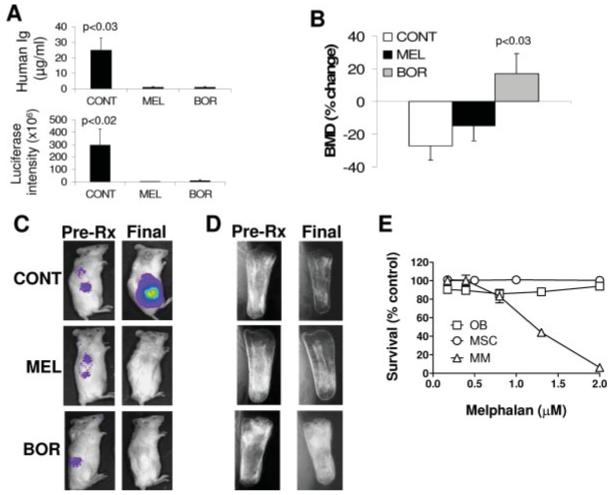
Bone anabolism is unique to bortezomib. Myeloma cells recovered from hosts engrafted with cells from patient 1 were passaged into newly constructed SCID-rab mice, subsequently established as a stroma-dependent BN cell line, infected with luciferase-expressing lentivirus, and then injected into newly constructed SCID-rab mice. On establishment of myeloma growth, hosts were treated with saline (CONT, n = 5), melphalan (MEL, n = 3), or bortezomib (BOR, n = 5). A: Human Ig levels (top panel) and live animal imaging of luciferase expression (lower panel) at the end of the experiment. B: Percent change of BMD of the myelomatous bones from pretreatment levels. C: Representative live animal imaging demonstrating luciferase expression before initiation of treatment and at the end of the experiment. D: X-ray radiographs visualizing changes in bone mass during the experimental period. Note that, while bortezomib and melphalan inhibited tumor growth, increased bone mass of myelomatous bones was evident only in hosts treated with bortezomib. E: In vitro melphalan had no cytotoxic effect on osteoblasts and MSCs at doses that inhibit growth of BN myeloma cells. These data suggest that melphalan’s lack of bone-anabolic effect in vivo was not a consequence of cytotoxic effect on osteoblasts. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
On establishment of myeloma growth (based on hIg levels), SCID-rab mice were treated for 5 weeks with bortezomib (n = 5), melphalan (n = 3), or saline (n = 5). The two drugs effectively eradicated myeloma growth, as demonstrated by live animal imaging and changes in circulating hIg (Fig. 2A); however, bortezomib treatment resulted in BMD of myelomatous bones that was markedly higher (17 ± 9%) than pretreatment levels, whereas saline- and melphalan-treatments resulted in BMD of myelomatous bones that was markedly lower than pretreatment levels (27 ± 4% and 15 ± 9%, respectively) (p < 0.03 vs. saline; Fig. 2B). The effects of bortezomib and melphalan on myeloma growth and bone mass, as visualized by live animal imaging (Fig. 2C) and radiography (Fig. 2D) confirmed our findings with primary myeloma cells and suggest that bortezomib’s effects on bone remodeling are not a consequence of reduced myeloma tumor burden but rather are a direct effect on bone cells.
Effects of bortezomib on osteoblastogenesis, osteoclastogenesis, and bone remodeling
To more closely examine the effects of bortezomib on bone remodeling, we performed static histomorphometric analyses and tetracycline labeling assays in myelomatous bones engrafted with myeloma cells and treated with saline or bortezomib. The histomorphometric analyses revealed significant increases in bone volume/total volume (BV/TV, p < 0.03), trabecular thickness (Tb.Th, p < 0.04), and trabecular number (Tb.N, p < 0.02) in myelomatous bones from hosts treated with bortezomib (Fig. 3A). Furthermore, dynamic histomorphometry in these bones revealed marked increased in double-labeled surface (dl.s/BS, p < 0.0004), mineral apposition rate [MAR, p < 0.004)], and bone formation rates [(BFRs), p < 0.0007] by bortezomib treatment (Fig. 3B-D).
Figure 3.
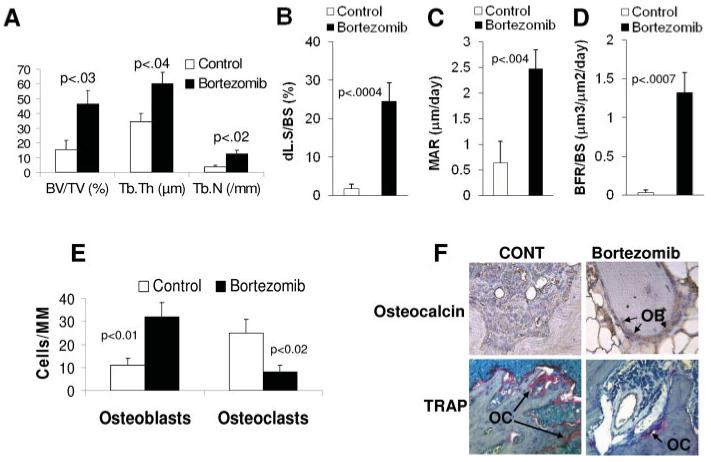
Bortezomib promotes bone formation in myelomatous bones through simultaneous stimulation of osteoblastogenesis and inhibition of osteoclastogenesis. (A-D) Static (A) and dynamic (B-D) histomorphometry demonstrating increased bone mass and bone formation by bortezomib. (E) Quantification of numbers of osteocalcin-expressing osteoblasts and TRAP-expression in multinucleated osteoclasts in myelomatous bones from control and bortezomib-treated hosts. (F) Myelomatous bone sections from control and bortezomib-treated hosts immunohistochemically stained for osteocalcin and histochemically stained for TRAP. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
To further analyze the effects of bortezomib on bone cells in vivo, myelomatous bone sections from saline- and bortezomib-treated SCID-rab mice were stained for tartrate-resistant acid phosphatase (TRAP) and immunohistochemically stained for osteocalcin, and the numbers of TRAP-expressing multinucleated osteoclasts and osteocalcin-expressing osteogenic cells were counted (Fig. 3E) [25]. Treatment with bortezomib resulted in increased numbers of osteoblasts (32 ± 6 vs. 11 ± 3 osteoblasts/mm bone in control hosts, p < 0.02) and reduced numbers of multinucleated osteoclasts (8 ± 3 vs. 25 ± 6 osteoclasts/mm bone in control hosts, p < 0.008). Examples of TRAP and osteocalcin staining in bone sections are presented in Fig. 3F. These in vivo data suggest that bortezomib increases bone mass by affecting osteoblasts and osteoclasts.
Effects of bortezomib on bone remodeling in nonmyelomatous bones and on osteoclastogenesis and osteoblastogenesis in vitro
We also examined the effects of bortezomib treatment on BMD of implanted bones in nonmyelomatous SCID-rab mice (n = 10). Whereas BMD of implanted bones was slightly reduced from pretreatment levels in saline-treated hosts, it was increased by 6 ± 2% (p < 0.02) from pretreatment levels in bortezomib-treated hosts (Fig. 4A). In contrast, BMD levels of the murine femurs were not affected by bortezomib treatment in these mice (Fig. 4B). Analyses of osteocalcin and TRAP staining of implanted bone sections from these mice revealed an increased number of osteoblasts (osteocalcin positive; p < 0.0003) and a reduced number of osteoclasts (TRAP positive; p < 0.03) following bortezomib treatment (Fig. 4C). The static (Fig. 4D) and dynamic (Fig. 4E-G) histomorphometric analyses revealed that bortezomib significantly increased BV/TV (p < 0.0005), Tb.Th (p < 0.005), Tb.N (p < 0.03), dl.s/BS (p < 0.0003), MAR (0.0001), and BFR (p < 0.0001) in nonmyelomatous implanted rabbit bones from hosts treated with bortezomib. These data suggest that bortezomib is bone anabolic, even in nonmyelomatous rabbit bones in SCID-rab mice.
Figure 4.
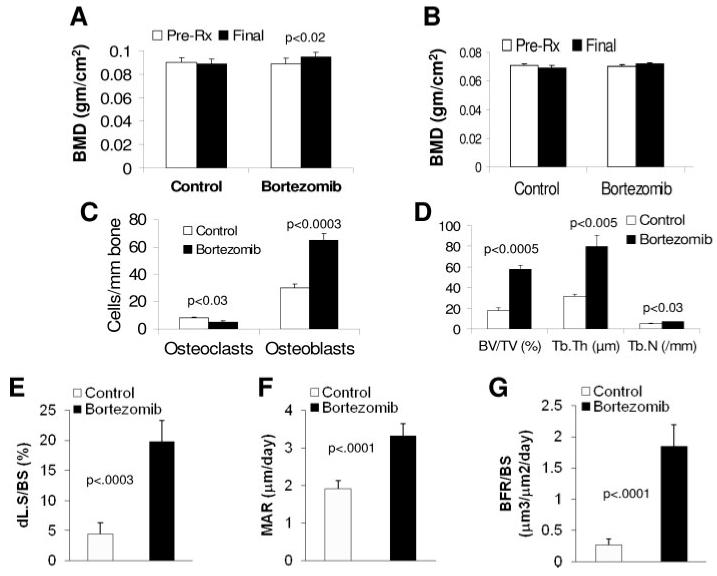
Bortezomib stimulates osteoblastogenesis and inhibits osteoclastogenesis in nonmyelomatous implanted bones and in vitro. Nonmyelomatous SCID-hu mice were treated with saline (control) or bortezomib (n = 10) for 4 weeks. (A) Levels of BMD of the implanted bones at pretreatment (pre-Rx) and experiment’s end. Bortezomib significantly increased BMD of the implanted bone. (B) Bortezomib had no effect on BMD of the murine femur in these mice. (C) Bortezomib reduced the numbers of TRAP-expressing osteoclasts and increased the numbers of osteocalcin-expressing osteoblasts in the implanted bones. (D-G) Static (D) and dynamic (E-G) histomorphometry demonstrating increased bone mass and bone formation by bortezomib.
To determine whether bortezomib directly acted on bone cells, we generated cultures of MSCs [29,33] and osteoclast precursors [34], and tested the drug’s effect on their differentiation into mature osteoblasts and multinucleated osteoclasts, respectively (see “Methods”). At doses (2.5-5 nM) that inhibited growth of BN myeloma cells (Fig. 5A) [32], bortezomib markedly prevented differentiation of osteoclast precursors into multinucleate, TRAP-expressing osteoclasts (Fig. 5B). Culture of fetal human MSCs in osteoblastic medium in the presence of bortezomib resulted in enhanced differentiation into bone-building osteoblasts, as indicated by increased mineral deposition detected by Alizarin Red S staining (Fig. 5C). These experiments indicate that bortezomib directly inhibits osteoclastogenesis and promotes osteoblastogenesis.
Figure 5.
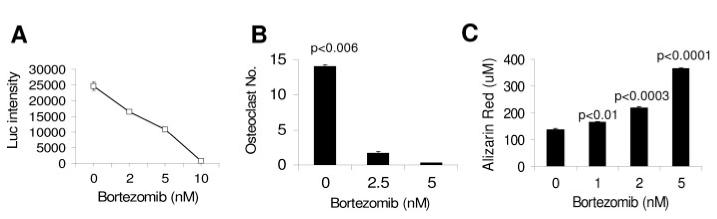
Bortezomib directly inhibits osteoclastogenesis and promotes osteoblastogenesis in vitro. (A) Bortezomib inhibited growth of BN myeloma cells in vitro in a dose-dependent manner. (B) Effects of bortezomib on differentiation of osteoclast precursors into TRAP-expressing multinucleated cells in vitro. (C) Effects of bortezomib on differentiation of MSCs into osteoblasts, as assessed by quantification of Alizarin Red deposition in vitro. Note that bortezomib inhibited osteoclast formation and stimulated osteoblast differentiation at doses (2.5-5 nM) that inhibited BN myeloma cell growth.
Discussion
In this study, we demonstrate that bortezomib is capable of increasing bone mass in myelomatous and nonmyelomatous bones in vivo and that increased bone formation stimulated by this agent is associated with a concomitant reduction in primary myeloma tumor burden in our experimental model. Using stroma-dependent BN myeloma cells and live-animal imaging in SCID-rab mice, we further validate our findings and demonstrate that the antimyeloma effects of bortezomib, but not melphalan, are associated with increased BMD. Bortezomib significantly reduced the number of osteoclasts and increased the number of differentiating osteoblasts in implanted bones in SCID-rab mice. The in vitro data further indicate that bortezomib affects bone remodeling by simultaneous inhibition of osteoclastogenesis and stimulation of osteoblastogenesis. The results of this study support our and others’ previous findings demonstrating that increased bone mass through exogenous MSC cytotherapy [29] or treatment with DKK1-neutralizing antibody [29], Wnt3a [35], or lithium chloride [36] have inhibitory effects on myeloma tumor burden in bone.
The mechanisms by which proteasome inhibitors promote bone formation are gradually being revealed. In our work, bortezomib acted directly on osteoclast and osteoblast precursors, confirming previous in vitro findings [16-18]. The ubiquitin-proteasome pathway may modulate osteoblast differentiation through upregulating bone morphogenetic protein 2 (BMP-2) expression [11] and preventing proteolytic degradation of the osteoblast transcription factor Runx2/Cbfa1 [16,37]. Bortezomib reduces circulating levels of DKK1 and RANKL in patients with MM [15]. DKK1 plays a critical role in regulating osteoblastogenesis in physiological and pathological conditions, including MM [9]. The inhibitory effects of bortezomib on DKK1 levels in patients with MM is probably a consequence of reduced DKK1 expression in osteoblast precursors [18] and myeloma cells themselves [9]. RANKL expression is upregulated in myelomatous bones and is critically involved in stimulating osteoclast activity [19]. In addition to inhibiting RANKL, bortezomib inhibits osteoclast differentiation through a mechanism associated with inhibition of NF-κB activity in osteoclast precursors [17]. Collectively, these studies suggest that bortezomib increases bone mass by simultaneously stimulating bone formation and suppressing bone resorption.
Despite direct effects on bone cells, bortezomib seems to have more profound bone-anabolic efficacy in myelomatous bones of SCID-rab mice and patients with MM who also show an antimyeloma response to the drug [13,16]. Although other clinical studies showed that bortezomib promotes osteoblast activity in patients with MM irrespective of response to treatment, the effects are significantly higher in responding patients than nonresponding patients [14,15]. It should also be noted that these previous studies were based on retrospective observations in patients who were previously treated with a bisphosphonate. The antimyeloma responses of other potent drugs, such as melphalan, were not associated with bone anabolism, suggesting that bortezomib’s effect on skeletal homeostasis is not a consequence of reduced tumor burden. The failure of bortezomib to efficiently prevent bone loss in nonresponding patients, as well as an understanding of its transient effects on bone formation markers in certain responding patients [13], requires further investigation.
The majority of patients with MM are elderly individuals whose bone remodeling is impaired by age and MM. In these patients, bortezomib may initially effectively lower MM burden, inhibit osteoclast differentiation, and reduce levels of restraint factors, such as DKK1, thus accelerating osteogenic differentiation of the existing pool of MSCs. However, this effect is transient, perhaps due to the limited pool of MSCs or an inability of these cells to retain their proliferative and differentiation potential over time. In contrast, MSCs in implanted rabbit bones in SCID-rab mice may retain their potential to differentiate into osteoblasts for lengthy periods of time, resulting in marked increases in bone formation.
As in the clinical setting, bortezomib heterogeneously affected myeloma tumor growth in our animal model. Although an exact comparison cannot be done, paraprotein levels were reduced >50% from pretreatment levels in 7 (43%) of 16 experiments (Table I). Similar effects were observed in various clinical trials with bortezomib as a single agent [10,38]. Our study also revealed that the BMD of uninvolved murine bones was not affected by growth of MM in SCID-rab mice. This observation supports the notion that, in contrast to bone manifestations, such as breast cancer, clinical and experimental MM bone disease mainly occurs in bone areas adjacent to tumor cells [2].
Bortezomib also slightly increases BMD of nonmyelomatous rabbit bones; however, interestingly, bortezomib had no effect on BMD of mouse femurs in our experimental model.
In previous studies, Garrett et al. [11] used 5-week-old Swiss ICR white mice and Mukherjee et al. [12] used 7-week-old C57BL/6 mice to demonstrate the bone-anabolic response of proteasome inhibitors PS1, epoxomicin, and bortezomib in mouse bones in vivo. In the current study, we used CB.17/Icr-SCID mice to construct the SCID-rab model; treatment of SCID-rab mice with bortezomib was initiated at least at 15 weeks of age. Our conflicting results may suggest that regulation of mouse bone remodeling by the ubiquitin-proteasome pathway is influenced by age, strain, and/or immune function.
Interestingly, recent studies indicated that restoring or increasing Wnt signaling in myelomatous bones by using DKK1-neutralizing antibody [25], Wnt3a [35], or lithium chloride [36] prevented bone disease, stimulated bone formation, and inhibited myeloma growth. Furthermore, in their in vitro study, Oyajobi et al. [18] demonstrated that bortezomib downregulated DKK1 expression in calvarial cultures and stromal cells and that DKK1 blocked bortezomib’s stimulatory effects on bone formation. These studies suggest that proteasome inhibitors also promote bone formation through regulation of Wnt signaling, including reduction in DKK1 levels and upregulation of BMP-2 in bone.
Bortezomib has been shown to directly inhibit myeloma cell growth at low nanomolar concentrations [39]. Although we were unable to prove that the antimyeloma response of bortezomib could be entirely mediated through effects on bone remodeling, previous experimental studies showing that prevention of osteolysis [19,21-24] or promotion of bone formation [25,29] has a negative impact on progression of MM. Our results are also supported by the in vitro findings that culture of osteoclasts alone supports longterm survival of primary myeloma cells [34] and that mature osteoblasts attenuate the growth of myeloma cells in a subset of patients [29]. Intriguingly, we have recently discovered that decorin, a main small leucine-rich proteoglycan highly expressed and produced by osteoblasts, is involved in the anti-MM effects of osteoblasts and that bortezomib had a tendency to increase decorin expression in osteoblasts, particularly those generated from patients with MM [40].
Collectively, we have shown that bortezomib can induce osteoblastogenesis and suppress osteoclastogenesis in vivo and in vitro. The increased BMD of myelomatous bones by bortezomib is accompanied by a reduction in tumor burden. Our study suggests that in addition to direct effect on MM cells bortezomib controls MM also through regulation of osteoblast and osteoclast activity, resulting in an inhospitable BM environment for tumor cells.
Materials and Methods
Myeloma cells
Myeloma cells were obtained from heparinized BM aspirates from 16 patients with active myeloma during scheduled clinic visits. Signed Institutional Review Board-approved informed consent forms are kept on record. Pertinent patient information is provided in Table I. BM samples were separated by density centrifugation using Ficoll-Paque (specific gravity, 1.077 g/ml; Amersham Biosciences Corp., Piscataway, NJ), and the proportion of myeloma plasma cells in the light-density cell fractions was determined by CD38/CD45 flow cytometry.
Construction of primary myelomatous SCID-rab mice
SCID-rab mice were constructed as previously described [26]. Briefly, 6- to 8-week-old CB.17/Icr-SCID mice were obtained from Harlan Sprague Dawley (Indianapolis, IN) and approximately 4-week-old New Zealand rabbits from Myrtle Rabbitry (Thompson Station, TN). The mice and rabbits were housed and monitored in the Department of Laboratory Animal Medicine facility at the University of Arkansas for Medical Sciences. The IACUC approved all experimental procedures and protocols. The rabbits were deeply anesthetized with a high dose of phentobarbital sodium and euthanized by cervical dislocation. Femora and tibiae were cut into two pieces, with the proximal and distal ends kept closed. A rabbit bone was subcutaneously inserted into the mouse through a small (5 mm) incision. The incision was then closed with sterile surgical staples, and bone engraftment was allowed to take place for 6-8 weeks. For each experiment, 3-10 × 106 unseparated myeloma BM cells containing >15% plasma cells in 100 ll of phosphate-buffered saline (PBS) were injected directly into the implanted rabbit bone. Mice were periodically bled from the tail veins and changes in levels of circulating hIg of the M-protein isotype were used as an indicator of myeloma growth. When hIg level reached 10 μg/ml or higher, two mice injected with cells from the same patient were used for study. Usually, the hosts with the higher hIg levels (indicative of higher tumor burden) were selected for treatment while the others served as controls.
Drug treatment
Bortezomib (Millennium Pharmaceuticals, Boston, MA) was solubilized in 0.9% saline. Myelomatous or nonmyelomatous SCID-rab mice were treated subcutaneously with saline, bortezomib (0.5 mg/kg body weight twice a week) [41] or melphalan (2.5 mg/kg body weight twice a week) for 4-8 weeks. Nonmyelomatous SCID-rab mice (n = 10) were similarly treated for 4-5 weeks. This examined period of time was sufficient to induce changes in bone remodeling.
Determination of hIg levels
Levels of human κ and λ light chains were determined by ELISA as previously described [2,28]. At the end of each experiment, all samples were analyzed in the same assay to preclude interassay variability.
Lentiviral vector and infection, and live animal imaging
Construction of lentiviral vectors, cell infection, and selection were performed as previously described [32]. For live imaging, mice were anesthetized with ketamine plus xylazine and injected intraperitoneally with d-luciferin firefly (150 mg/kg, Xenogen Corp., Alameda, CA). Luciferase activity was detected with the IVIS 200 imaging system (Xenogen) as previously described [32].
Immunohistochemisry and histochemistry
Rabbit bones were fixed in 10% phosphate-buffered formalin for 24 hr. Rabbit bones were further decalcified with 10% (wt/vol) EDTA, pH 7.0, and embedded in paraffin for sectioning. Sections (5-μm) were deparafinized in xylene, rehydrated with ethanol, and rinsed in saline and then underwent antigen retrieval by microwave. After peroxidase quenching with 3% hydrogen peroxide for 5 min, sections were reacted with 5 μg/ml of mouse antibovine osteocalcin monoclonal antibody and mouse IgG control antibody (QED Bioscience, San Diego, CA); the assay was completed with the use of Dako’s immunoperoxidase kit (Carpinteria, CA). Sections were lightly counterstained with hematoxylin [26,29]. According to the manufacturer, the osteocalcin antibody cross-reacts with human and rabbit tissues but not with mouse tissue. Deparaffinized bone sections were stained with tartrate-resistant acid phosphatase (TRAP) with the use of an acid phosphatase kit (Sigma-Aldrich, St. Louis, MO) [21]. Osteocalcin-expressing osteoblasts and TRAP-positive multinucleate osteoclasts in four nonoverlapping, millimeter-square areas were counted.
Histomorphometric analyses
For static histomorphometry, in most experiments decalcified implanted bone sections were stained with H&E whereas in certain experiments undecalcified bone sections were stained with Masson’s trichrome and used for analysis. Images of the trabecular area were obtained with a 10× objective using an Olympus BH2 microscope (Olympus, Melville, NY). Images were acquired using a SPOT2 digital camera (Diagnostic Instruments, Sterling Heights, MI) and were processed; and a total of five images were obtained per section. BV/TV, Tb.Th, and Tn.N were measured using Osteometrics software (Osteometric, Atlanta, GA). For dynamic histomorphometry, indicated mice treated with saline or bortezomib for 3 weeks were intraperitoneally injected with tetracycline (30 μg/kg, Sigma-Aldrich) at 10 days and 3 days before sacrificing. Undecalcifed implanted bones were processed as previously described [42]. Images demonstrating typical single- and double-labeled areas of the whole implanted bone were obtained with a 20× objective and were analyzed using Osteometrics software.
Effects of bortezomib on osteoblast differentiation
MSCs were prepared as previously described [33,43]. Briefly, BM mononucleate cells (2 × 106 cells/ml) from patients with MM and human fetal bone fragments (Advanced Bioscience Resources, Alameda, CA) were cultured in DMEM, low glucose (DMEM-LG), supplemented with 10% FBS, and antibiotics (MSC medium). One-half of the medium was replaced every 4-6 days, and adherent cells were allowed to reach 80% confluency before they were subcultured with trypsin-EDTA. Adherent MSCs expressed CD166, but not CD45 or CD34. For differentiation into osteoblasts, MSCs were incubated with DMEM-LG supplemented with 10% FBS, dexamethasone (100 nM), b(glycerophosphate (10 mM), and ascorbate (0.05 mM). MSCs formed aggregates or nodules, had increased expression of alkaline phosphatase within 1-2 weeks followed by calcium deposition, lost expression of CD166, and increased expression of osteocalcin and BMP-2 [29].
For the study, MSCs were differentiated into osteoblasts in the presence or absence of bortezomib (1-5 nM) for 4 weeks at the indicated doses. The cultures were fixed with a 1:1 mixture (vol/vol) of 37% formaldehyde and ethanol for 5 min, washed three times with PBS, and stained for 10 min with a solution of 2% of Alizarin Red S (Sigma-Aldrich) at pH 4.2. Alizarin Red S deposition was quantified with the use of the Osteogenesis Quantitation Kit (Chemicon, Temecula, CA). The stain was extracted according to the manufacturer’s recommended procedures, and the OD405 of the samples were compared with an Alizarin Red S standard curve.
Effects of bortezomib on osteoclast differentiation
Osteoclast precursors and bone-resorbing osteoclasts were prepared as previously described [34]. Briefly, mononuclear cells were obtained from peripheral blood of patients with MM and healthy subjects and from BM of patients. Before stimulation to generate osteoclast precursors, the BM samples were depleted of plasma cells by CD138 immunomagnetic bead selection and then cultured for 5 days to deplete adherent stromal cells. The nonadherent mononuclear blood and BM cells were cultured at 2.5 × 106 cells/ml in α-MEM supplemented with 10% FBS, RANKL (50 ng/ml, PeproTech, Rocky Hill, NJ), M-CSF (25 ng/ml, PeproTech), and antibiotic cocktail containing penicillin, streptomycin, and neomycin (Gibco, Grand Island, NY) (osteoclast medium) for 3-5 days, at which time nonadherent cells were removed and the remaining adherent cells served as osteoclast precursors [34].
For the study, osteoclast precursors were incubated in osteoclast medium in the absence and presence of bortezomib (2.5-5 nM) for 5-7 days. The cultures were then fixed and stained for TRAP, and the number of multinucleate TRAP-positive osteoclasts was counted as described previously [34].
Statistical analyses
All values are expressed as mean ± SEM. Unless specifically indicated, Student’s paired t-test was used to test the effect of treatment on BMD, myeloma tumor burden, and osteoblast and osteoclast numbers.
Acknowledgments
The authors also wish to thank the faculty, staff, and patients of the Myeloma Institute for Research and Therapy for their support.
Contract grant sponsor: National Cancer Institute; Contract grant number: CA-93897; Multiple Myeloma Research Foundation, Senior and Translational Research Awards; Millennium Pharmaceuticles; Fondazione Catanese per lo Studio e la Cura delle Malattie Neoplastiche del Sangue (FON.CA.NE.SA), Italy.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1.Bataille R, Chappard D, Marcelli C, et al. Recruitment of new osteoblasts and osteoclasts is the earliest critical event in the pathogenesis of human multiple myeloma. J Clin Invest. 1991;88:62–66. doi: 10.1172/JCI115305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350:1655–1664. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 3.Barille-Nion S, Bataille R. New insights in myeloma-induced osteolysis. Leuk Lymphoma. 2003;44:1463–1467. doi: 10.3109/10428190309178765. [DOI] [PubMed] [Google Scholar]
- 4.Bamias A, Kastritis E, Bamia C, et al. Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: Incidence and risk factors. J Clin Oncol. 2005;23:8580–8587. doi: 10.1200/JCO.2005.02.8670. [DOI] [PubMed] [Google Scholar]
- 5.Rosen LS, Gordon D, Kaminski M, et al. Zoledronic acid versus pamidronate in the treatment of skeletal metastases in patients with breast cancer or osteolytic lesions of multiple myeloma: A phase III, double-blind, comparative trial. Cancer J. 2001;7:377–387. [PubMed] [Google Scholar]
- 6.Markowitz GS, Fine PL, Stack JI, et al. Toxic acute tubular necrosis following treatment with zoledronate (Zometa) Kidney Int. 2003;64:281–289. doi: 10.1046/j.1523-1755.2003.00071.x. [DOI] [PubMed] [Google Scholar]
- 7.Coleman RE, Major P, Lipton A, et al. Predictive value of bone resorption and formation markers in cancer patients with bone metastases receiving the bisphosphonate zoledronic acid. J Clin Oncol. 2005;23:4925–4935. doi: 10.1200/JCO.2005.06.091. [DOI] [PubMed] [Google Scholar]
- 8.Attal M, Harousseau JL, Leyvraz S, et al. Maintenance therapy with thalidomide improves survival in patients with multiple myeloma. Blood. 2006;108:3289–3294. doi: 10.1182/blood-2006-05-022962. [DOI] [PubMed] [Google Scholar]
- 9.Stewart JP, Shaughnessy JD., Jr. Role of osteoblast suppression in multiple myeloma. J Cell Biochem. 2006;98:1–13. doi: 10.1002/jcb.20774. [DOI] [PubMed] [Google Scholar]
- 10.Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348:2609–2617. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- 11.Garrett IR, Chen D, Gutierrez G, et al. Selective inhibitors of the osteoblast proteasome stimulate bone formation in vivo and in vitro. J Clin Invest. 2003;111:1771–1782. doi: 10.1172/JCI16198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mukherjee S, Raje N, Schoonmaker JA, et al. Pharmacologic targeting of a stem/progenitor population in vivo is associated with enhanced bone regeneration in mice. J Clin Invest. 2008;118:491–504. doi: 10.1172/JCI33102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zangari M, Esseltine D, Lee CK, et al. Response to bortezomib is associated to osteoblastic activation in patients with multiple myeloma. Br J Haematol. 2005;131:71–73. doi: 10.1111/j.1365-2141.2005.05733.x. [DOI] [PubMed] [Google Scholar]
- 14.Heider U, Kaiser M, Muller C, et al. Bortezomib increases osteoblast activity in myeloma patients irrespective of response to treatment. Eur J Haematol. 2006;77:233–238. doi: 10.1111/j.1600-0609.2006.00692.x. [DOI] [PubMed] [Google Scholar]
- 15.Terpos E, Heath DJ, Rahemtulla A, et al. Bortezomib reduces serum dickkopf-1 and receptor activator of nuclear factor-κB ligand concentrations and normalises indices of bone remodelling in patients with relapsed multiple myeloma. Br J Haematol. 2006;135:688–692. doi: 10.1111/j.1365-2141.2006.06356.x. [DOI] [PubMed] [Google Scholar]
- 16.Giuliani N, Morandi F, Tagliaferri S, et al. The proteasome inhibitor bortezomib affects osteoblast differentiation in vitro and in vivo in multiple myeloma patients. Blood. 2007;110:334–338. doi: 10.1182/blood-2006-11-059188. [DOI] [PubMed] [Google Scholar]
- 17.Zavrski I, Krebbel H, Wildemann B, et al. Proteasome inhibitors abrogate osteoclast differentiation and osteoclast function. Biochem Biophys Res Commun. 2005;333:200–205. doi: 10.1016/j.bbrc.2005.05.098. [DOI] [PubMed] [Google Scholar]
- 18.Oyajobi BO, Garrett IR, Gupta A, et al. Stimulation of new bone formation by the proteasome inhibitor, bortezomib: Implications for myeloma bone disease. Br J Haematol. 2007;139:434–438. doi: 10.1111/j.1365-2141.2007.06829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pearse RN, Sordillo EM, Yaccoby S, et al. Multiple myeloma disrupts the TRANCE/ osteoprotegerin cytokine axis to trigger bone destruction and promote tumor progression. Proc Natl Acad Sci USA. 2001;98:11581–11586. doi: 10.1073/pnas.201394498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tian E, Zhan F, Walker R, et al. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med. 2003;349:2483–2494. doi: 10.1056/NEJMoa030847. [DOI] [PubMed] [Google Scholar]
- 21.Yaccoby S, Pearse RN, Johnson CL, et al. Myeloma interacts with the bone marrow microenvironment to induce osteoclastogenesis and is dependent on osteoclast activity. Br J Haematol. 2002;116:278–290. doi: 10.1046/j.1365-2141.2002.03257.x. [DOI] [PubMed] [Google Scholar]
- 22.Croucher PI, Shipman CM, Lippitt J, et al. Osteoprotegerin inhibits the development of osteolytic bone disease in multiple myeloma. Blood. 2001;98:3534–3540. doi: 10.1182/blood.v98.13.3534. [DOI] [PubMed] [Google Scholar]
- 23.Vanderkerken K, De Leenheer E, Shipman C, et al. Recombinant osteoprotegerin decreases tumor burden and increases survival in a murine model of multiple myeloma. Cancer Res. 2003;63:287–289. [PubMed] [Google Scholar]
- 24.Croucher PI, De Hendrik R, Perry MJ, et al. Zoledronic acid treatment of 5T2MM-bearing mice inhibits the development of myeloma bone disease: Evidence for decreased osteolysis, tumor burden and angiogenesis, and increased survival. J Bone Miner Res. 2003;18:482–492. doi: 10.1359/jbmr.2003.18.3.482. [DOI] [PubMed] [Google Scholar]
- 25.Yaccoby S, Ling W, Zhan F, et al. Antibody-based inhibition of DKK1 suppresses tumor-induced bone resorption and multiple myeloma growth in vivo. Blood. 2007;109:2106–2111. doi: 10.1182/blood-2006-09-047712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yata K, Yaccoby S. The SCID-rab model: A novel in vivo system for primary human myeloma demonstrating growth of CD138-expressing malignant cells. Leukemia. 2004;18:1891–1897. doi: 10.1038/sj.leu.2403513. [DOI] [PubMed] [Google Scholar]
- 27.Yaccoby S, Barlogie B, Epstein J. Primary myeloma cells growing in SCID-hu mice: A model for studying the biology and treatment of myeloma and its manifestations. Blood. 1998;92:2908–2913. [PubMed] [Google Scholar]
- 28.Yaccoby S, Epstein J. The proliferative potential of myeloma plasma cells manifest in the SCID-hu host. Blood. 1999;94:3576–3582. [PubMed] [Google Scholar]
- 29.Yaccoby S, Wezeman MJ, Zangari M, et al. Inhibitory effects of osteoblasts and increased bone formation on myeloma in novel culture systems and a myelomatous mouse model. Haematologica. 2006;91:192–199. [PMC free article] [PubMed] [Google Scholar]
- 30.Yaccoby S, Pennisi A, Li X, et al. Atacicept (TACI-Ig) inhibits growth of TACI (high) primary myeloma cells in SCID-hu mice and in coculture with osteoclasts. Leukemia. 2008;22:406–413. doi: 10.1038/sj.leu.2405048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zangari M, Yaccoby S, Cavallo F, Esseltine D, Tricot G. Response to bortezomib and activation of osteoblasts in multiple myeloma. Clin Lymphoma Myeloma. 2006;7:109–114. doi: 10.3816/CLM.2006.n.047. [DOI] [PubMed] [Google Scholar]
- 32.Li X, Pennisi A, Zhan F, et al. Establishment and exploitation of hyperdiploid and non-hyperdiploid human myeloma cell lines. Br J Haematol. 2007;138:802–811. doi: 10.1111/j.1365-2141.2007.06742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ge Y, Zhan F, Barlogie B, et al. Fibroblast activation protein (FAP) is upregulated in myelomatous bone and supports myeloma cell survival. Br J Haematol. 2006;133:83–92. doi: 10.1111/j.1365-2141.2006.05976.x. [DOI] [PubMed] [Google Scholar]
- 34.Yaccoby S, Wezeman MJ, Henderson A, et al. Cancer and the microenvironment: Myeloma-osteoclast interactions as a model. Cancer Res. 2004;64:2016–2023. doi: 10.1158/0008-5472.can-03-1131. [DOI] [PubMed] [Google Scholar]
- 35.Qiang YW, Shaughnessy JD, Jr., Yaccoby S. Wnt3a signaling within bone inhibits multiple myeloma bone disease and tumor growth. Blood. 2008;112:374–382. doi: 10.1182/blood-2007-10-120253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edwards CM, Edwards JR, Lwin ST, et al. Increasing Wnt signaling in the bone marrow microenvironment inhibits the development of myeloma bone disease and reduces tumor burden in bone in vivo. Blood. 2008;111:2833–2842. doi: 10.1182/blood-2007-03-077685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bellido T, Ali AA, Plotkin LI, et al. Proteasomal degradation of Runx2 shortens parathyroid hormone-induced anti-apoptotic signaling in osteoblasts. A putative explanation for why intermittent administration is needed for bone anabolism. J Biol Chem. 2003;278:50259–50272. doi: 10.1074/jbc.M307444200. [DOI] [PubMed] [Google Scholar]
- 38.Richardson PG, Mitsiades C, Schlossman R, Munshi N, Anderson K. New drugs for myeloma. Oncologist. 2007;12:664–689. doi: 10.1634/theoncologist.12-6-664. [DOI] [PubMed] [Google Scholar]
- 39.Hideshima T, Richardson P, Chauhan D, et al. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 2001;61:3071–3076. [PubMed] [Google Scholar]
- 40.Li X, Pennisi A, Yaccoby S. Role of decorin in the antimyeloma effects of osteoblasts. Blood. 2008;112:159–168. doi: 10.1182/blood-2007-11-124164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.LeBlanc R, Catley LP, Hideshima T, et al. Proteasome inhibitor PS-341 inhibits human myeloma cell growth in vivo and prolongs survival in a murine model. Cancer Res. 2002;62:4996–5000. [PubMed] [Google Scholar]
- 42.Perrien DS, Akel NS, Edwards PK, et al. Inhibin A is an endocrine stimulator of bone mass and strength. Endocrinology. 2007;148:1654–1665. doi: 10.1210/en.2006-0848. [DOI] [PubMed] [Google Scholar]
- 43.Yaccoby S. The phenotypic plasticity of myeloma plasma cells as expressed by dedifferentiation into an immature, resilient, and apoptosis-resistant phenotype. Clin Cancer Res. 2005;11:7599–7606. doi: 10.1158/1078-0432.CCR-05-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]


