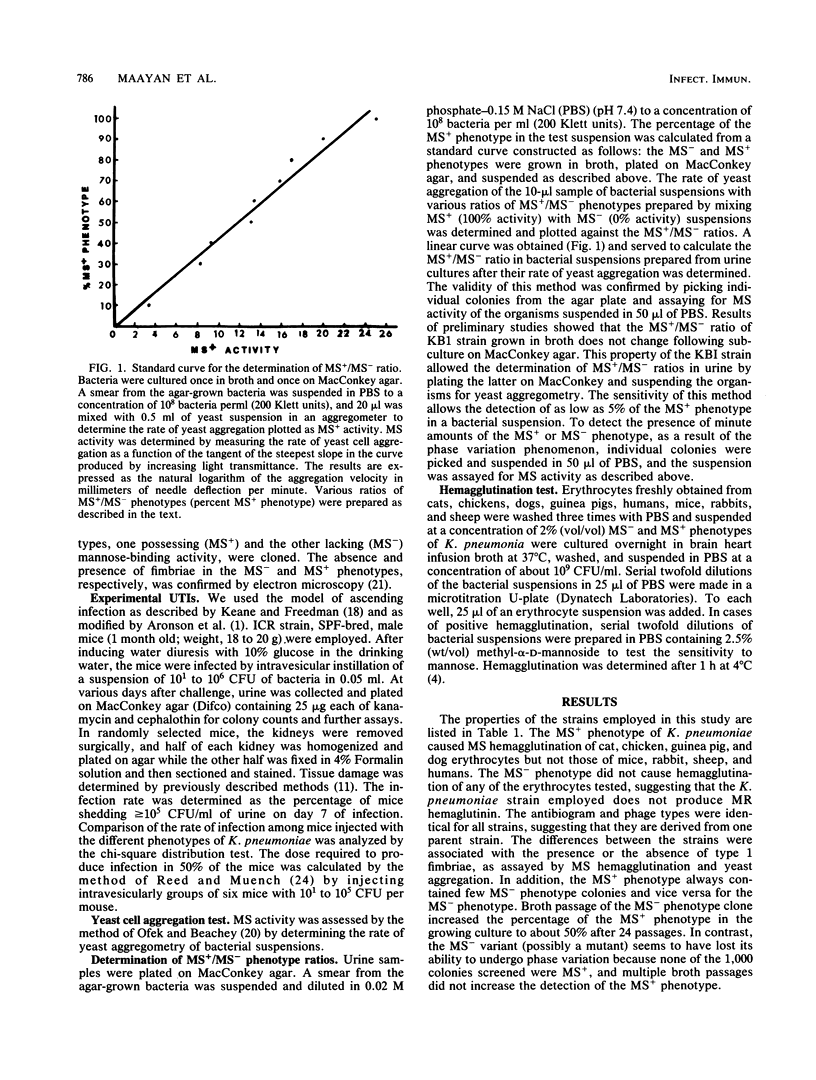
Abstract
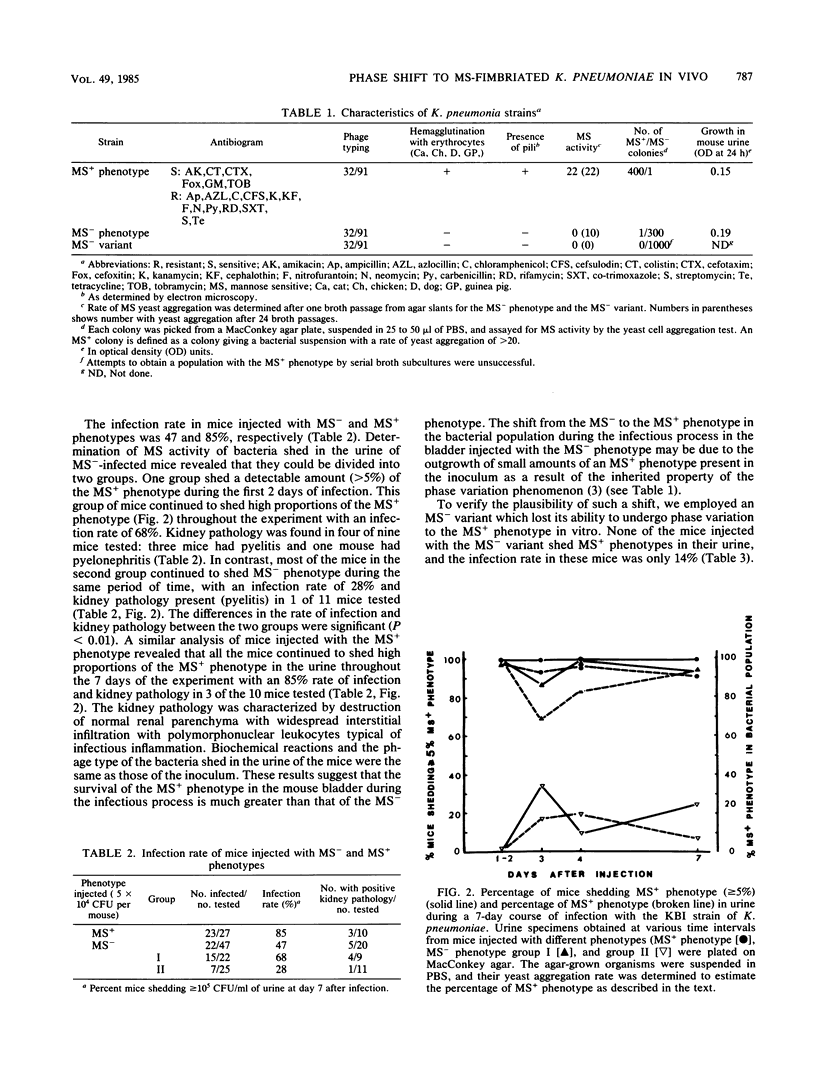
The infection rate (percentage of mice shedding 10(5) organisms per ml of urine) in 27 mice infected intravesicularly with a mannose-specific (MS+) phenotype of Klebsiella pneumoniae was 85% at day 7, and all the bacteria shed during the 7 days exhibited strong MS activity as estimated by a yeast aggregation assay. In contrast, the outcome of infection with an MS- phenotype of the same strain in 47 mice was heterogeneous: one group of 25 mice continued to shed the originally injected phenotype (MS-) throughout the investigation period, whereas the second group (22 mice) shed bacteria with various degrees of phenotypic conversion to MS+. In the first group, the rate of infection at day 7 was significantly reduced (28%) compared with that of the second group (68%). Mice infected with a mixture of 5% MS+ bacteria and 95% of an MS- variant which lost its ability to undergo phase variation had an infection rate of 89%, but at day 7 95% of the excreted bacteria were MS+. The infection rate of mice injected with the MS- variant was 14%, and none of the mice shed MS+ bacteria. The incidence of kidney pathology was higher in mice inoculated with the MS+ phenotype (3 of 10) or in the group in which the MS+ overgrew the MS- phenotype (4 of 10) as compared with the group of mice in which no such shift occurred (1 of 11). The kidneys of four mice which excreted mostly MS+ organisms harbored a population predominantly of the MS- phenotype. These results suggest that the MS adhesin confers an advantage in the initial steps of the infectious process in the bladder but not in later stages of infection in the kidney, emphasizing the importance of phase variation in the survival of bacteria at the various stages of the infectious process.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson M., Medalia O., Schori L., Mirelman D., Sharon N., Ofek I. Prevention of colonization of the urinary tract of mice with Escherichia coli by blocking of bacterial adherence with methyl alpha-D-mannopyranoside. J Infect Dis. 1979 Mar;139(3):329–332. doi: 10.1093/infdis/139.3.329. [DOI] [PubMed] [Google Scholar]
- Bar-Shavit Z., Ofek I., Goldman R., Mirelman D., Sharon N. Mannose residues on phagocytes as receptors for the attachment of Escherichia coli and Salmonella typhi. Biochem Biophys Res Commun. 1977 Sep 9;78(1):455–460. doi: 10.1016/0006-291x(77)91276-1. [DOI] [PubMed] [Google Scholar]
- DUGUID J. P. Fimbriae and adhesive properties in Klebsiella strains. J Gen Microbiol. 1959 Aug;21:271–286. doi: 10.1099/00221287-21-1-271. [DOI] [PubMed] [Google Scholar]
- Duguid J. P., Clegg S., Wilson M. I. The fimbrial and non-fimbrial haemagglutinins of Escherichia coli. J Med Microbiol. 1979 May;12(2):213–227. doi: 10.1099/00222615-12-2-213. [DOI] [PubMed] [Google Scholar]
- Eisenstein B. I., Dodd D. C. Pseudocatabolite repression of type 1 fimbriae of Escherichia coli. J Bacteriol. 1982 Sep;151(3):1560–1567. doi: 10.1128/jb.151.3.1560-1567.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein B. I. Phase variation of type 1 fimbriae in Escherichia coli is under transcriptional control. Science. 1981 Oct 16;214(4518):337–339. doi: 10.1126/science.6116279. [DOI] [PubMed] [Google Scholar]
- Fader R. C., Davis C. P. Effect of piliation on Klebsiella pneumoniae infection in rat bladders. Infect Immun. 1980 Nov;30(2):554–561. doi: 10.1128/iai.30.2.554-561.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fader R. C., Davis C. P. Klebsiella pneumoniae-induced experimental pyelitis: the effect of piliation on infectivity. J Urol. 1982 Jul;128(1):197–201. doi: 10.1016/s0022-5347(17)52817-7. [DOI] [PubMed] [Google Scholar]
- Guerina N. G., Kessler T. W., Guerina V. J., Neutra M. R., Clegg H. W., Langermann S., Scannapieco F. A., Goldmann D. A. The role of pili and capsule in the pathogenesis of neonatal infection with Escherichia coli K1. J Infect Dis. 1983 Sep;148(3):395–405. doi: 10.1093/infdis/148.3.395. [DOI] [PubMed] [Google Scholar]
- Hagberg L., Hull R., Hull S., Falkow S., Freter R., Svanborg Edén C. Contribution of adhesion to bacterial persistence in the mouse urinary tract. Infect Immun. 1983 Apr;40(1):265–272. doi: 10.1128/iai.40.1.265-272.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harber M. J., Chick S., Mackenzie R., Asscher A. W. Lack of adherence to epithelial cells by freshly isolated urinary pathogens. Lancet. 1982 Mar 13;1(8272):586–588. doi: 10.1016/s0140-6736(82)91749-4. [DOI] [PubMed] [Google Scholar]
- Iwahi T., Abe Y., Nakao M., Imada A., Tsuchiya K. Role of type 1 fimbriae in the pathogenesis of ascending urinary tract infection induced by escherichia coli in mice. Infect Immun. 1983 Mar;39(3):1307–1315. doi: 10.1128/iai.39.3.1307-1315.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwahi T., Abe Y., Tsuchiya K. Virulence of Escherichia coli in ascending urinary-tract infection in mice. J Med Microbiol. 1982 Aug;15(3):303–316. doi: 10.1099/00222615-15-3-303. [DOI] [PubMed] [Google Scholar]
- Jones G. W., Isaacson R. E. Proteinaceous bacterial adhesins and their receptors. Crit Rev Microbiol. 1983;10(3):229–260. doi: 10.3109/10408418209113564. [DOI] [PubMed] [Google Scholar]
- Keane W. F., Freedman L. R. Experimental pyelonephritis. XIV. Pyelonephritis in normal mice produced by inoculation of E. coli into the bladder lumen during water diuresis. Yale J Biol Med. 1967 Dec;40(3):231–237. [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H., Bisno A. L. Resistance of Neisseria gonorrhoeae to phagocytosis: relationship to colonial morphology and surface pili. J Infect Dis. 1974 Mar;129(3):310–316. doi: 10.1093/infdis/129.3.310. [DOI] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H. Mannose binding and epithelial cell adherence of Escherichia coli. Infect Immun. 1978 Oct;22(1):247–254. doi: 10.1128/iai.22.1.247-254.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Mosek A., Sharon N. Mannose-specific adherence of Escherichia coli freshly excreted in the urine of patients with urinary tract infections, and of isolates subcultured from the infected urine. Infect Immun. 1981 Dec;34(3):708–711. doi: 10.1128/iai.34.3.708-711.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon N., Eshdat Y., Silverblatt F. J., Ofek I. Bacterial adherence to cell surface sugars. Ciba Found Symp. 1981;80:119–141. doi: 10.1002/9780470720639.ch9. [DOI] [PubMed] [Google Scholar]
- Silverblatt F. J., Cohen L. S. Antipili antibody affords protection against experimental ascending pyelonephritis. J Clin Invest. 1979 Jul;64(1):333–336. doi: 10.1172/JCI109458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverblatt F. J., Ofek I. Interaction of bacterial pili and leukocytes. Infection. 1983 Jul-Aug;11(4):235–238. doi: 10.1007/BF01641208. [DOI] [PubMed] [Google Scholar]
- Svanborg Edén C., Hagberg L., Hanson L. A., Hull S., Hull R., Jodal U., Leffler H., Lomberg H., Straube E. Bacterial adherence--a pathogenetic mechanism in urinary tract infections caused by Escherichia coli. Prog Allergy. 1983;33:175–188. [PubMed] [Google Scholar]


