Summary
Rho kinase (ROCK) belongs to a family of serine/threonine kinases that are activated via interaction with Rho GTPases. ROCK is involved in a wide range of fundamental cellular functions such as contraction, adhesion, migration, and proliferation. Recent studies have shown that ROCK plays an important role in the regulation of apoptosis in various cell types and animal disease models. Two ROCK isoforms, ROCK1 and ROCK2, are assumed to be function redundant, based largely on kinase construct overexpression, and chemical inhibitors (Y27632 and fasudil), which inhibit both ROCK1 and ROCK2. Gene targeting and RNA interference approaches allow further dissection of distinct cellular, physiological and patho-physiological functions of the two ROCK isoforms. This review, based on recent molecular, cellular and animal studies, focuses on the current understanding of ROCK signaling in the regulation of apoptosis and highlights the new findings from recently generated ROCK-deficient mice.
Keywords: ROCK1, ROCK2, apoptosis, survival, gene targeting
Introduction
Rho GTPase family proteins, which include Rho, Rac1 and Cdc42, control a wide variety of cellular processes such as cell adhesion, motility, proliferation, differentiation and apoptosis [27, 44, 143]. One of the best-characterized effectors of Rho is Rho-associated coiled-coil-containing protein kinase (hereafter referred to as ROCK), which was discovered in 1996 [54, 73, 82, 95]. During the last decade, the Rho/ROCK signaling pathway has attracted much attention in various research fields, and more than 2,000 articles have been published; many of which focus on ROCK function in the cardiovascular system, central nervous system, cancers and embryonic development. Several excellent recent reviews have covered large aspects [11, 33, 51, 78, 94, 97, 113, 123]. The up to date progress in the translational research has demonstrated that ROCK is an important therapeutic target for the treatment of various cardiovascular diseases and neurological disorders, cancers, etc. In these studies, two relatively selective ROCK inhibitors, Y27632 [141] and fasudil [5], have been extensively used to dissect the roles of ROCK in cellular signaling and in animal disease models and these studies suggest that inhibition of ROCK has great therapeutic potential.
During the last decade, we have also witnessed dramatic progress in the understanding of physiological and patho-physiological roles as well as molecular mechanisms of apoptosis. The involvements of ROCK in apoptosis have recently been extensively examined. In this review, we summarize the current understanding of ROCK signaling in the regulation of apoptosis and highlight the new findings from recently generated ROCK-deficient mice.
Apoptosis
Three types of cell death are known: apoptosis, necrosis and autophagy. Apoptosis is a controlled, energy-dependent active form of cell death characterized by morphological changes such as shrinkage of the cell, condensation of chromatin and disintegration of the cell into small fragments that can be removed by phagocytosis [19]. Necrosis is an uncontrolled, energy-independent process characterized by cellular edema and disruption of the plasma membrane, leading to release of the cellular components and inflammatory tissue response [57]. Autophagy is characterized by sequestration of bulk cytoplasm and organelles in double or multi-membrane autophagic vesicles and their delivery to and subsequent degradation by cell’s own lysosomal system [124]. A role for ROCK in cell autophagy has not been reported.
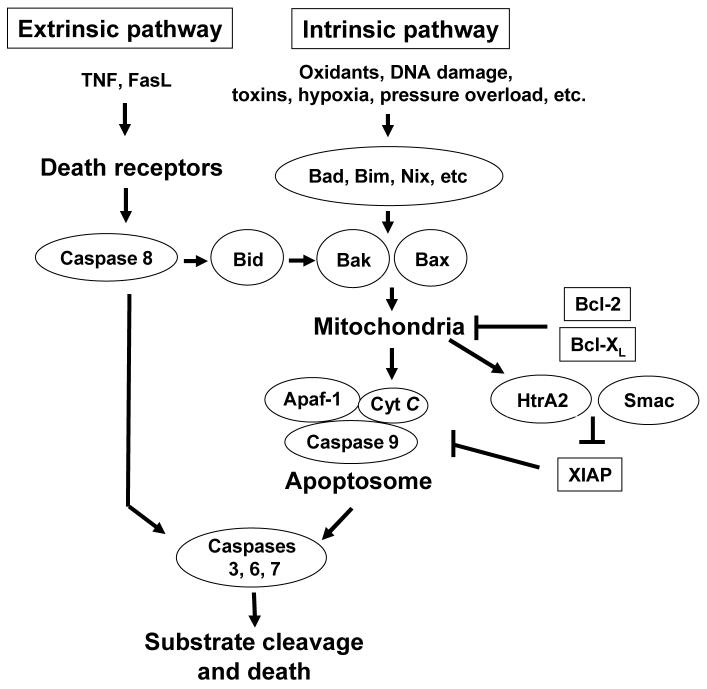
Apoptosis occurs during development and throughout life and plays an important role in normal tissue homeostasis [19]. The deregulation of apoptosis has been associated with cancer, neurodegeneration, autoimmunity and cardiovascular diseases. Apoptosis results from the activation of caspases, which are a family of cysteine proteases that cleave target proteins at specific aspartate residues. Apoptosis can be initiated by two evolutionarily conserved pathways (Fig. 1). The extrinsic pathway, which utilizes cell surface death receptors, is responsive to a highly specialized subset of death signals (e.g., inflammatory signals). In contrast, the intrinsic pathway relies on signaling through the mitochondria and the endoplasmic reticulum (ER) [19, 31]. Both types of triggering pathways converge in the activation of downstream executioner caspases, including caspase 3, 6 and 7.
Figure 1. The extrinsic and intrinsic pathways of caspase activation.
In the extrinsic pathway, death ligands, such as FasL and TNFα, bind to their respective receptors, leading to caspase 8 activation. Activated caspase 8 directly activates procaspase 3, which then starts death cascade. Caspase 8 can also signal indirectly through the mitochondria via activation of Bid which translocates to mitochondria and activates Bax and Bak to trigger release of cytochrome c (Cyt C). In the intrinsic pathway, many stress stimuli activate caspases through the activation of BH3-only proteins of the Bcl-2 family, such as Bid, Bad, Bim, Nix, etc. The activated BH3-only proteins then activate Bax and Bak, leading to the release of cytochrome c as well as the release of Smac and HtrA2 from mitochondria. This process is antagonized by Bcl-2 and Bcl-XL. Cytochrome c forms a complex with the adaptor protein Apaf-1, and procaspase 9, resulting in the formation of apoptosome, which leads to the proteolytic cleavage and concomitant activation of caspase 9. Active caspase 9 directly cleaves and activates procaspase 3. This process is antagonized by XIAP, which can be inhibited by Smac and HtrA2 following their release from mitochondria.
In the extrinsic pathway, death ligands, such as FasL and TNFα, bind to their respective receptors and stimulate recruitment of the adaptor proteins Fas-associated via death domain (FADD) [6] and TNFR associated death domain (TRADD) [21], respectively. FADD and TRADD recruit procaspase 8 into a complex named death-inducing signaling complex (DISC), where it undergoes dimerization and concomitant activation. Activated caspase 8 then activates procaspase 3 directly, which then cleaves various subcellular cytoplasmic proteins and fragments nuclear DNA. Caspase 8 can also signal indirectly through the mitochondria via activation of Bid which translocates to mitochondria and activates Bax and Bak to trigger release of cytochrome c [42, 75, 79].
In many cell types, cytochrome c-dependent apoptosome formation, and the resulting activation of caspase 9, is the principal mechanism of caspase 3 activation via the intrinsic apoptosis pathway. Mitochondria play an important role in transmitting and amplifying death signals in the intrinsic pathway. The precise connections between apoptotic stimuli and the activation of the intrinsic pathway are not clear in many instances. However, it is clear that many stress stimuli activate caspases through the activation of BH3-only proteins of the Bcl-2 family, such as Bid, Bad, Bim, Nix, etc. The activated BH3-only proteins initiate mitochondrial outer membrane permeabilization (MOMP) by activating proapoptotic Bcl-2 family proteins, Bax and Bak, in mitochondria, leading to the release of cytochrome c as well as the release of Smac/DIABLO and Omi/HtrA2. This process is antagonized by anti-apoptotic Bcl-2 family proteins, such as Bcl-2 and Bcl-XL. After releasing from mitochondria, cytochrome c forms a complex with the adaptor protein Apaf-1, dATP, and procaspase 9, resulting in the formation of a structure known as the apoptosome, which leads to the proteolytic cleavage and concomitant activation of caspase 9. Active caspase 9 directly cleaves and activates procaspase 3. When a critical amount of activated caspase 3 is present within a cell, apoptosis is triggered. This process is antagonized by XIAP (X-linked inhibitor of apoptosis), which can be inhibited by Smac/DIABLO and Omi/HtrA2 following their release from mitochondria.
Mitochondrial cytochrome c release can also follow mitochondrial permeability transition (mPT), an event triggered by changes in the permeability of the inner mitochondrial membrane. mPT can be induced by increased cytosolic Ca2+ levels, as well as moderate oxidative stress (resulting from, for example, exposure to toxins). mPT results in a loss of mitochondrial membrane potential (ΔΨm), mitochondrial swelling, and outer membrane rupture, which in turn results in release of cytochrome c [7]. mPT has also been implicated in necrosis [7, 96]. Caspase 3 activation resulting from cytochrome c release can stimulate mPT [69, 111], which can subsequently act as a feed-forward mechanism for further cytochrome c release.
Most knowledge about apoptotic cell death has come from the study of dividing or undifferentiated cells. The mechanisms of cell death in terminally differentiated, nondividing cells such as cardiomyocytes and neurons are less well defined. Recent studies have shown that Apaf-1 levels in post-mitotic cells such as cardiomyocytes and sympathetic neurons are markedly reduced (as compared to mitotic cells) [108, 109, 116], resulting in a significant decrease in apoptosome activity. The reduced activity of E2F1 [30], which is a transcriptional regulator of Apaf-1 [93] in terminally differentiated cardiomyocytes, is likely to contribute to the marked reduction of Apaf-1 in these cells. Given the reduced apoptosome activity (resulting from low levels of Apaf-1), endogenous XIAP has a greater inhibitory impact on apoptosis in post-mitotic cells. These results suggest that the inhibition of apoptosome activity by endogenous XIAP may be a rate-limiting step for cardiomyocyte and neuron apoptosis induced by pathological stimuli.
Structure and expression of ROCK isoforms
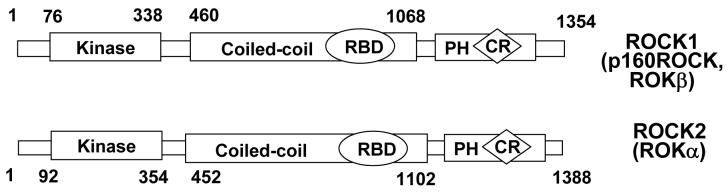
ROCK was initially identified as a Rho-binding protein with serine/threonine protein kinase activity and with a molecular mass of about 160 kDa [54, 73, 82, 95]. The protein contains a catalytic kinase domain at the amino terminus, followed by a central coiled-coil domain including a Rho-binding domain (RBD), and a carboxyl-terminal pleckstrin-homology (PH) domain with an internal cysteine-rich (CR) domain (Fig. 2). The ROCK family contains two members: ROCK1 (also called ROKβ or p160ROCK) and ROCK2 (also known as ROKα), which share 65% overall identity and 92% identity in the kinase domain [54, 82, 95]. ROCK shares 45-50% homology with myotonic dystrophy kinase (DMPK), DMPK-related cdc42-binding kinase (MRCK) and citron kinase [113].
Figure 2. Molecular structure of ROCK1 and ROCK2.
ROCK sequences comprise a serine/threonine kinase domain located at the amino terminus of the protein, followed by a central coiled-coil domain containing the Rho-binding domain (RBD), and a carboxyl terminal pleckstrin homology (PH) domain with an internal cysteine-rich (CR) domain.
ROCK has auto-inhibitory activity[3]. In the inactive form, the carboxyl terminal PH domain and RBD of ROCK interact with the kinase domain, which forms an auto-inhibitory loop. The kinase domain is about 300 amino acids in length and contains the conserved motifs associated with serine/threonine protein kinases [46]. ROCK inhibitors such as Y27632 and fasudil bind to the kinase domain and inhibit ROCK1 and ROCK2 with similar potency [10, 56, 141]. The coiled-coil domain is thought to interact with other α-helical proteins. The RBD localized in the coiled-coil domain is about 80 amino acids in length and interacts only with activated Rho GTPases including RhoA, RhoB and RhoC [36]. The PH domain is believed to interact with lipid mediators such as arachidonic acid (AA) [29, 35] and sphingosylphosphorylcholine (SPC) [29, 35, 126], and might also participate in protein localization [14, 60, 151]. The amino-terminal regions, upstream of the kinase domain of ROCK, can be involved in the interaction with the substrates (e.g. RhoE) [112] and can also be important for the kinase activity [73].
Both ROCK1 and ROCK2 are ubiquitously expressed across human, rat and mouse tissues [54, 73, 95, 147]. ROCK2 is more prominent in brain and skeletal muscle, whereas ROCK1 is more pronounced in liver, testes and kidney. Both ROCK1 and ROCK2 are expressed in vascular smooth muscle and heart. In early mouse embryos, both ROCK1 and ROCK2 are expressed, with ROCK1 highly enriched in the developing heart and ROCK2 ubiquitously expressed [146].
ROCK1 and ROCK2 are essentially cytosolic in the resting state, but are translocated to membrane upon Rho activation [74, 82]. In addition, ROCK2 has been found to be located at cleavage furrows during cytokinesis [65], at stress fiber [14] and vimentin intermediate filament network [128], whereas ROCK1 has been shown to be co-localized with the centrosomes [15]. Two recent reports, using siRNA approach, have shown that ROCK1 and ROCK2 have distinct distributions in primary rat embryonic fibroblasts [151] and are involved with different myosin compartments [151, 152]. In these fibroblasts, ROCK1 is mainly diffuse and perinuclear, and is required for stress fiber and focal adhesion formation whereas ROCK2 exhibits both cell membrane and intense perinuclear distributions and is required for phagocytosis [151].
Substrates of ROCK
Both ROCK1 and ROCK2 phosphorylate a variety of protein substrates at serine or threonine residues. The consensus sequence of ROCK substrates is R/K-X-S/T or R/K-X-X-S/T (R, arginine; K, lysine; S, serine; T, threonine) [4, 37, 41, 61, 83]. More than 20 ROCK substrates have been identified (reviewed in refs [51, 78, 113]).
The first characterized targets of ROCK are myosin light chain (MLC) [4, 66] and the myosin binding subunit of MLC phosphatase (MYPT1) [4, 61, 62]. ROCK can increase MLC phosphorylation through direct effect on MLC or indirectly by inactivating MLC phosphatase. The increased MLC phosphorylation results in stimulation of actomyosin contractility [2, 55, 73]. Inactivation of MLC phosphatase by ROCK plays an important physiological role such as agonist-induced Ca2+ sensitization in smooth muscle contraction [28, 29, 141]. Most of the ROCK substrates are cellular proteins associated with the regulation of actin cytoskeleton. Besides MLC and MYPT1, this important subset of ROCK substrates include CPI-17 [63], calponin [58], LIM kinases [80, 99, 132], ezrin/radixin/moesin (ERM) [83], adducin [37], sodium-hydrogen exchanger (NHE1) [137] and ZIP kinase [43].
Several ROCK substrates are involved in the regulation of cell death and survival (Fig. 3). Increased MLC phosphorylation and actomyosin contraction in apoptotic cells regulate plasma membrane blebbing[88]. Caspase 3-mediated ROCK1 activation is responsible for the increased MLC phosphorylation in a variety of cell types [16, 119]. Phosphatase and tensin homologue (PTEN) is a newly identified ROCK substrate [77]. The phosphorylation of PTEN by ROCK stimulates its phosphatase activity. PTEN dephosphorylates both proteins and phosphoinositides and is a negative regulator of phosphatidylionositol (PI)3-kinase/Akt pathway, which has important roles in a diverse range of biological processes including cell survival [22]. PTEN mediates reduction of Akt phosphorylation induced by ROCK activation in HEK cells [13, 77]. Reduced PTEN activity may contribute to the protective effect of ROCK inhibition in endothelial cells [148] and to the protective effects of suppression of ROCK1 in cardiomyocytes [13]. In addition, ROCK has been shown to interact with and negatively regulate insulin receptor substrate 1 (IRS1) signaling and PI3-kinase activation in vascular smooth muscle cells [9] and in fibroblasts derived from p190B RhoGAP null mice [131]. In contrast, a recent study has shown that ROCK interacts with and phosphorylates IRS1 at Ser632/635 sites thereby enhancing PI3-kinase activation in adipocytes and muscle cell lines and in isolated soleus muscle ex vivo [39]. ROCK appears to be involved in both positive and negative regulation of PI3-kinase/Akt signaling and the outcome may be cell type-dependent and stimulus-dependent. Finally, ROCK2 activation was found to promote apoptosis through increasing ezrin phosphorylation, which then leads to Fas clustering and membrane expression in Raf-1 deficient embryonic fibroblasts [107]. Fas activation stimulates the formation of Raf-1-ROCK2 complexes thereby down-regulating ROCK2 activity. This mechanism may contribute to the phenotype of Raf-1 deficient mice such as fetal liver apoptosis, embryonic lethality, and selective hypersensitivity to Fas-induced cell death [52, 87].
Figure 3. Potential ROCK targets in apoptotic signaling.
Several ROCK substrates are involved in the regulation of apoptosis. ROCK can increase MLC phosphorylation through direct effect on MLC or indirectly by inactivating MLC phosphatase (MYPT1). The increased MLC phosphorylation results in stimulation of actomyosin contractility, which regulates morphological apoptotic events during the execution phase of apoptosis including plasma membrane blebbing, nuclear disintegration and fragmentation of apoptotic cells. Under some conditions, the actin cytoskeleton rearrangement induced by ROCK activation can also be involved in the intracellular signaling involved in the initiating stages of apoptosis through the regulation of assembly of death receptor complex or loss of cell adhesion. In addition, ROCK has been shown to stimulate phosphatase and tensin homologue (PTEN) and inhibit insulin receptor substrate 1 (IRS1) signaling (broken line indicates that both positive and negative regulations of IRS1 signaling by ROCK have been reported), resulting in inactivation of Akt. Akt has important roles in promoting cell survival, possibly through inhibition of both extrinsic and intrinsic pathways. Finally, ROCK activation was found to promote apoptosis through increasing ezrin phosphorylation, which in turn led to Fas clustering and membrane expression.
The majority of ROCK substrates have been identified from cell culture experiments. In most cases, only one ROCK isoform (more generally ROCK2) has been tested. Because ROCK1 and ROCK2 share 92% identity in the kinase domain, it has been assumed that they share the same substrates. However, ROCK1 and ROCK2 may have different targets as only ROCK1, but not ROCK2, binds to and phosphorylates RhoE [114].
Regulation of ROCK activity
ROCK activity can be regulated by several distinct mechanisms (Table 1). The kinase activity is increased after Rho binding [54, 73, 82]. The interaction between RBD and active GTP-bound form of Rho disrupts the interaction between the catalytic domain and the inhibitory carboxyl-terminal region of ROCK. The Rho/ROCK pathway is activated by numerous extracellular stimuli. The consequence of Rho-dependent ROCK activation is highly cell type-dependent, ranging from a change in contractility, cell permeability, migration, proliferation to apoptosis.
Table 1.
Known mechanisms of ROCK activation and inactivation
| Regulators | Site of interaction on ROCK | Effects | Ref |
|---|---|---|---|
| Rho | interacts with RBD, ROCK1 and ROCK2 | activation | [54,73,82] |
| AA | interacts with the PH domain, ROCK2 | activation | [29,35] |
| SPC | interacts with the PH domain, ROCK2 | activation | [126] |
| PI3,4,5P3, PI4,5P2 |
interact with the PH domain, ROCK2 only | activation | [151] |
| Gem, Rad | interact with the coiled-coil domain, ROCK1 and ROCK2 |
inactivation | [145] |
| Caspase 3 | cleaves at DETD1113, ROCK1 only | activation | [16,119] |
| RhoE | interacts between amino acids 1-420, ROCK1 only | inactivation | [101,112] |
| STK15 | phosphorylates at serine residues between amino acids 726 -1,034, ROCK1 |
inactivation | [25] |
| Granzyme B | cleaves at IGLD1131, ROCK2 only | activation | [118] |
| Caspase 2 | cleavage site is not known, ROCK2 only | activation | [117] |
| Raf-1 | site of interaction is not known, ROCK2 only | inactivation | [107] |
| p21Cip1/WAF1 | site of interaction is not known, ROCK2 | inactivation | [81] |
Lipid mediators such as AA [29,35] and SPC [126] can interact with the ROCK carboxyl-terminal inhibitory domain and stimulate ROCK activity independently of Rho. At the cellular level, the activation of AA or SPC/ROCK pathway was mainly observed in smooth muscle cells to increase calcium sensitivity [92, 126]. In addition, inositol phospholipids such as PI3,4,5P3 and PI4,5P2 can interact with the PH domain of ROCK2, but not ROCK1, suggesting that ROCK2 may be regulated by PI-3 kinase activity [151]. Dimerization and/or transphosphorylation may also play a role in regulating ROCK activity by regulating its affinity for ATP [24].
ROCK can be activated constitutively by proteolytic cleavage of the inhibitory carboxyl-terminal domain. ROCK1 is cleaved by caspase 3 at the cleavage site DETD1113 during apoptosis [16, 119]. This consensus sequence for caspase 3 cleavage is conserved in human, rat and mouse, but is not present in ROCK2. Caspase 3 is believed to be responsible for activating ROCK1 in apoptotic cells, as evidenced by the absence of ROCK1 cleavage in caspase 3-deficient MCF-7 breast carcinoma cells and the presence of ROCK1 cleavage after restoring procaspase 3 expression [119]. In addition, ROCK1 cleavage by caspase 3 can be inhibited by caspase inhibitors in a variety of apoptotic cells [16, 23, 70, 86, 104, 119, 127, 139, 140, 155]. However, caspase 3-independent cleavage of ROCK1 was observed in extracellular ATP-induced and P2X7 ATP receptor-mediated apoptosis [91] and in cancer cells subjected to combined BGC9331 (a thymidylate synthase inhibitor) and SN-38 (a topoisomerase I inhibitor) treatment [17]. The nature of the proteases involved and the cleavage site for caspase 3-independent cleavage of ROCK1 remain to be identified [17, 91]. On the other hand, during cytotoxic lymphocyte granule-induced cell death, human ROCK2 can be cleaved by the proapoptotic protease granzyme B at IGLD1131 site, and this site is not present in ROCK1 [118]. Human ROCK2, but not ROCK1, can also be activated by caspase 2-dependent cleavage in endothelial cells in response to thrombin, but the cleavage site remains to be identified [117].
Besides these positive regulators, negative control of ROCK activity has also been described (Table 1). The small GTP-binding protein RhoE interacts with the N-terminal region of ROCK1 (amino acids 1-420) and prevents Rho binding to RBD [101, 112]. Other negative regulators have been found to bind to and inhibit ROCK activity, such as the small GTP-binding proteins, Gem and Rad [145], Raf-1 [107], p21(Cip1/WAF1) [81] and Aurora-A/serine/threonine kinase 15 (STK15) [25]. However, their mechanisms of action are not defined.
As discussed below, the caspase 3/ROCK pathway is believed to play a major role in regulating membrane blebbing [16, 119], a characteristic feature of apoptotic cells, in a variety of cell types, independent of apoptotic stimuli. An apoptotic role for other ROCK activation pathway such as Rho/ROCK [59, 89], granzyme B/ROCK2 [118], caspase 2/ROCK2 [117] may be highly cell type-dependent and/or apoptotic stimulus-dependent. Importantly, ROCK1 and ROCK2 can be distinctly activated or inhibited by a number of positive or negative regulators, and in turn can have distinct cellular or physiological functions.
ROCK1- and ROCK2-knockout mice
Recently ROCK1-knockout (ROCK1-/-) and ROCK2-knockout (ROCK2-/-) mice have been generated [115, 122, 135, 153] and their phenotypes are different. ROCK1-/- mice under C57BL/6N genetic background exhibit eyelid open at birth (EOB) and omphalocele phenotype due to disorganization of actin filament in the epithelial cells of the eyelid and of the umbilical ring [122]. ROCK2-/- mice in a mixed genetic background between 129/SvJ and C57BL/6N are embryonic lethal because of placental dysfunction, and have intrauterine growth retardation caused by thrombus formation in the labyrinth layer of the placenta, indicating that there is no compensation for the loss of ROCK2 by ROCK1 [135]. Interestingly, ROCK2-/- mice under C57BL/6N genetic background exhibit not only the placental phenotype but also EOB and omphalocele phenotype [136], indicating that genetic background affects the EOB and omphalocele phenotype in ROCK2-/- mice. In addition the shared EOB and omphalocele phenotype in ROCK1-/- and ROCK2-/- mice under C57BL/6N genetic background indicates that they act together to regulate the assembly of actin bundles essential for closure of eyelid and ventricular body wall in mouse embryos.
The genetic background may also affect the EOB and omphalocele phenotype in ROCK1-/- mice, as this phenotype was not observed in ROCK1-/- mice under the mixed C57BL/6N-129/SvJ or FVB background [153], although the differences in targeting vector may also contribute to the different phenotype of ROCK1-/- mice generated by different laboratories [115, 122, 153]. In addition, the ROCK1-/- mice under FVB background have no detected anatomical abnormalities from embryonic day 9.5 to adulthood, although they are underrepresented, possibly due to lethality at very early developmental stages [153]. Further studies with ROCK1 deletion in various strain backgrounds and at early developmental stages (prior to E9.5) should provide additional information on the developmental role of ROCK1. A common characteristic of ROCK1-/- and ROCK2-/- mice is that after surviving their intrauterine and perinatal problems these mice develop normally and are apparently healthy and fertile [115, 122, 135, 153]. In addition there is no compensatory up-regulation of the ROCK1 expression in ROCK2-/- mice and vice versa.
Systemic and conditional ROCK1- and ROCK2-knockout mice offer a unique opportunity to analyze in vivo physiological and pathological functions of ROCK1 and ROCK2 as ROCK inhibitors do not distinguish these two isoforms. A recent study has reported that ROCK1+/- mice exhibit cardiac hypertrophy but decreased cardiac fibrosis in response to angiotensin II infusion [115]. We have observed that ROCK1-/- mice exhibit cardiac hypertrophy but reduced cardiac fibrosis in response to pressure overload [153]. These observations suggest an important role for ROCK1 in the development of cardiac fibrosis, but not hypertrophy. The molecular and cellular mechanisms underlying the cardiac fibrotic role of ROCK1 remain to be defined. On the other hand, renal fibrosis induced by unilateral ureteral obstruction is not reduced by ROCK1 deletion suggesting that the presence of ROCK2 may compensate for the loss of ROCK1 in this renal fibrosis model [34]. Interestingly, in vivo study with ROCK1-/- mice also revealed an important role for ROCK1 in cardiomyocyte apoptosis [13], which is a critical contributing factor of heart failure progression [31]. Deletion of ROCK1 indeed prevents or delays the development of dilated cardiomyopathy in several pathological models (Shi et al., unpublished observations). These studies strongly suggest that ROCK1 and ROCK2 have some non-overlapping in vivo functions.
The role of ROCK in regulating morphological apoptotic events
ROCK is recognized as a major regulator of the morphological events that occur during the execution phase of apoptosis, including cell contraction, dynamic membrane blebbing, nuclear disintegration and fragmentation of apoptotic cells into apoptotic bodies (Fig. 3).
Membrane blebbing
The execution phase of apoptosis is characterized by a number of morphological changes, which consist of three stages: release, membrane blebbing, and condensation. During the release stage, a dying cell loses contact with the extracellular matrix and surrounding cells, and begins to round up. This retraction stage is followed by plasma membrane blebbing. After a period of membrane blebbing which is normally transient, cells move into the condensation stage; where cells either condense into a single apoptotic body or fragment into multiple apoptotic bodies. Plasma membrane blebbing is regulated by MLC phosphorylation and actomyosin contraction [88]. A role for caspase 3-mediated cleavage and activation of ROCK1 in the regulation of membrane blebbing of apoptotic cells was first demonstrated in anti-Fas antibody-treated Jurkat cells and TNFα-treated NIH3T3 [16, 119]. This caspase 3-mediated ROCK1 activation is both necessary and sufficient for the formation of membrane blebs through increasing MLC phosphorylation and actomyosin contraction [16, 119]. The importance of this pathway in bleb formation was then confirmed by other studies in a variety of cell types, independent of apoptotic stimuli [23, 70, 86, 104, 127, 155]. In addition, ROCK1-mediated blebbing facilitates cellular fragmentation and/or apoptotic cell clearance as discussed below.
Even though caspase 3-mediated ROCK1 activation appears to be a major pathway inducing membrane blebbing in apoptotic cells, membrane blebbing could occur via Rho-dependent and caspase-independent activation of ROCK in some apoptotic conditions [59, 89]. Both ROCK1 and ROCK2 could be involved in this process as both can be activated by Rho to regulate MLC phosphorylation and actomyosin contraction. In agreement with this, over-expression of wild-type ROCK2 or a constitutively active mutant of ROCK2 is able to induce membrane blebbing through activation of actin–myosin contractility [130]. Finally, ROCK2, but not ROCK1, can be activated by granzyme B-mediated cleavage (at Asp 1131) in a context of cytotoxic lymphocyte granule-induced apoptosis. This cleaved (activated) ROCK2 is able to induce membrane blebbing independent of caspase 3 activation [118].
Fragmentation of apoptotic cells
During the execution phase of apoptosis, a cell packages itself into apoptotic bodies therefore its contents are not released. The resulting apoptotic bodies are removed from the surrounding tissue through phagocytosis [71]. Apoptotic cells undergo a number of changes to prepare for phagocytosis. These include nuclear fragmentation, relocation of fragmented DNA into membrane blebs, shrinkage and/or fragmentation into apoptotic bodies and they express phagocytic markers on their surface. ROCK activation and subsequent MLC phosphorylation are required for apoptotic nuclear disintegration [18] as well as relocation of fragmented DNA into blebs and apoptotic bodies [16] in NIH3T3 cells. ROCK activity and actomyosin contraction also contribute to the formation of endoplasmic reticulum and chromatin-containing blebs in the late apoptotic cells which are likely the progenitors of apoptotic bodies [70]. Moreover, inhibition of ROCK with Y27632 prevents fragmentation of apoptotic cells and blocks Golgi fragmentation in apoptotic COS-7 and PC12 cells respectively, and these ROCK-dependent execution events require actomyosin contraction[102,103]. Interestingly, ROCK controls surface expression phagocytic markers independently of actomyosin contraction [102]. Importantly, cells dying in the presence of Y-27632 are less efficiently phagocytized than those dying without the inhibitor, this suggests that ROCK plays an important role in the preparation of dead cells for phagocytosis [102]. Additionally, siRNA knockdown of ROCK1 but not ROCK2 inhibits fragmentation of dying cells, it is consistent with ROCK1, but not ROCK2, being cleaved and activated by caspases [103]. However, Jurkat cells dying in the presence of Y27632 are phagocytosed by macrophages as efficiently as those dying without the inhibitor [127], suggesting that importance of ROCK in the preparation of dead cells for phagocytosis may depend on cell type and/or apoptotic stimulus.
Phagocytosis of apoptotic cells
Deletion of apoptotic cells from tissues involves their phagocytosis by macrophages, dendritic cells, and tissue cells. The rapid and efficient phagocytosis of apoptotic cells plays a critical role in preventing secondary necrosis, inflammation as well as in tissue remodeling and regulating immune responses. Phagocytic clearance of apoptotic cells consists of four distinct steps: accumulation of phagocytes at the site where apoptotic cells are located in response to specific attraction signals released by cells undergoing apoptosis; recognition by phagocytes through a number of bridge molecules and receptors; engulfment of apoptotic cells; and degradation of engulfed cells within phagocytes. Recent studies have shown that Rho and ROCK are involved in the clearance of apoptotic cells through the regulation of actin cytoskeleton [26, 138].
A decrease in Rho activity was observed during engulfment of apoptotic cells [138]. In addition, Rho seems to negatively affect basal phagocytosis, such that inhibition of Rho-mediated signaling enhances the uptake of apoptotic cells. ROCK seems to be primarily responsible for this inhibitory effect on engulfment [138]. The negative effect of Rho/ROCK during engulfment of apoptotic cells differs from other types of phagocytosis mediated via the complement receptor and Fc receptor, respectively. Rho and ROCK have been shown to be critical for complement receptor-mediated phagocytosis, and but not for Fc receptor-mediated phagocytosis [12, 100]. Recently, ROCk2 activity, but not ROCK1 activity, has been shown to facilitate phagocytic uptake of fibronectin-coated beads [151].
During the degradation of apoptotic cells within macrophages or fibroblasts, the Rho/ROCK/ERM signaling pathway contributes to the rapid maturation of phagosomes, as blockage of Rho/ROCK/ERM signaling delays maturation rates of phagosomes containing apoptotic cells [26]. The maturation of phagosomes containing cells taken up through Fc receptors is slower and is independent of Rho and ROCK.
The role of ROCK in mediating apoptotic signals
Although a role for ROCK in regulating morphological apoptotic events during the execution phase of apoptosis is well recognized, the importance of ROCK in regulating apoptotic caspase cascades is highly cell type-dependent and/or apoptotic stimulus-dependent. Under some conditions, ROCK activation or inhibition is not important for mediating apoptotic signals. For example, inhibition of ROCK does not affect caspase 3 activation and progression of apoptosis in anti-Fas antibody-treated Jurkat cells and TNFα-treated NIH3T3 [16, 119]. Inhibition of ROCK disrupts actin stress fibers but does not induce apoptosis in 3T3 cells [90]. Expression of the cleaved (activated) ROCK1 is sufficient to induce membrane blebbing, but does not result in chromatine condensation in NIH 3T3 cells [16, 18] and Jurkat cells [119]. ROCK activation is required for membrane blebbing, but not for other apoptotic events such as the loss of mitochondrial respiration and cytochrome c release induced by eosinophil peroxidase in lung epithelial cells [86]. However, there is increasing recognition that the actin cytoskeleton rearrangement induced by ROCK activation may be involved not only in end-stage execution of apoptosis, but in the intracellular signaling involved in the initiating stages, as well (Fig. 3). ROCK-mediated apoptotic signaling may involve cell death domain receptor-dependent extrinsic pathway (e.g. regulation of assembly of death receptor complex) and/or mitochondrial-dependent intrinsic pathway (e.g. regulation of expression of apoptotic or anti-apoptotic molecules).
In vitro evidence
Several studies have shown that Rho/ROCK activation is required for endothelial cell death caused by cytokines or drugs, which induce MLC phosphorylation and actin cytoskeletal alterations. ROCK inhibition by Y27632 or Rho inhibition by the overexpression of dominant negative Rho significantly attenuates TNFα-induced apoptosis and caspase 8 activity through reduction of MLC phosphorylation and actomyosin contraction, which may be critical for the assembly of the TNFα death receptor complex in bovine pulmonary artery endothelial cells[105,106]. Rho and ROCK activations by combretastatin A-4-phosphate, a tumor vascular-targeting agent responsible for microtubule depolymerization, lead to increased MLC phosphorylation and actomyosin contractility, which then induce membrane blebbing and loss of cell adherence in human umbilical vein endothelial cells [59]. Consistent with this observation, up-regulation of ROCK activity via inhibition of ERK-MAPK signaling during tumor vascularization promotes endothelial cell retraction and death [85].
Several studies also point to a pro-apoptotic role of ROCK in other cell types. ROCK-mediated MLC phosphorylation acts as an upstream event required for membrane contraction and the subsequent caspase 8, 10 and 3 activation during phorbol-12-myristate-13-acetate (PMA) stimulation in erythroblastic TF-1 cells [67]. In addition to MLC phosphorylation, ROCK activation mediates other molecular events in PMA-treated apoptotic cells such as prevention of nuclear distribution of phospho-ERK, inhibition of induction of the cell cycle inhibitor p21(Cip1/Waf1) [68], and cytosolic translocation of heterogeneous nuclear ribonucleoprotein C1 and C2, which are two nuclear restricted pre-mRNA binding proteins [72]. In primary thymocytes, apoptosis induced by activation of the receptor for thromboxane A(2), which is coupled to Gα(12/13) subunits, is inhibited by deletion of Lsc RhoGEF (an activator of GTPases of the Rho family), associated with inactivation of Rho/ROCK and increased Akt phosphorylation [47]. Ethanol is an important teratogen that induces marked central nervous system dysfunctions and ethanol-induced ROCK activation in astrocytes promotes MLC phosphorylation and actomyosin contraction thereby leading to apoptosis induced by loss of anchorage [89].
As we already know, the majority of the studies have been performed with ROCK inhibitors or dominant negative mutants of ROCK, which inhibit both ROCK1 and ROCK2. However, ROCK1 and ROCK2 may not share identical apoptotic functions. For example, ROCK2 activation has been found to promote apoptosis through increasing ezrin phosphorylation, leading to Fas clustering and membrane expression in Raf-1 deficient embryonic fibroblasts [107]. Reduced ROCK2 expression by siRNA reduced Fas-induced apoptosis of Raf-1 deficient embryonic fibroblasts [107]. On the other hand, ROCK1 physically associates with STK15, which is implicated in the regulation of the centrosome replication cycle. Suppression of ROCK1 expression by siRNA bypasses G(2)/M cell cycle arrest after STK15 depletion and alleviates apoptosis in HeLa cells [25].
ROCK1 has been demonstrated to be a key mediator which links pro-apoptotic stimuli to apoptosis in cultured neonatal cardiomyocytes [13]. Multiple pro-apoptotic stimuli result in the caspase 3-dependent proteolytic cleavage and concomitant activation of ROCK1 in cardiomyocytes. Additionally, reduction of ROCK1 activity via siRNA treatment blocks ceramide-induced caspase 3 activation and apoptosis. Moreover, expression of an activated ROCK1 mutant is sufficient to induce caspase 3 activation in cultured neonatal rat cardiomyocytes suggesting a positive feed-forward regulatory loop. The mechanism underlying the apoptotic role of ROCK1 in amplification of caspase 3 remains to be defined, and may be mediated by PTEN activation. In contrast, a previous study has shown that treatment of cultured rat neonatal cardiomyocytes with Y27632 promoted cardiomyocyte apoptosis [98], and inhibition of both ROCK1 and ROCK2 by this chemical inhibitor may contribute to the different observations compared with our study.
In vivo evidence
In a variety of animal disease models, inhibition of ROCK produces protective effects including reduced apoptosis which is accompanied in most cases by reduced inflammatory responses. Inhibition of ROCK significantly reduces medial smooth muscle cell apoptosis, inflammatory cell infiltration and cytokine production, and attenuates abdominal aortic aneurysm formation induced by angiotensin II [144]. ROCK mediates production of reactive oxygen species and inflammatory cytokines which are substantially involved in the pathogenesis of hepatocellular necrosis and apoptosis induced by cold ischemia-reperfusion injury after liver transplantation in rats [125]. ROCK mediates infiltration of leukocytes as well as production of TNFα and CXC chemokines and hepatocellular apoptosis in endotoxemic liver injury (challenged with lipopolysaccharide and D-galactosamine) [134]. The inhibition of ROCK by Y27632 during acute ischemia/reperfusion injury significantly reduces cardiomyocyte apoptosis, prevents down-regulation of Bcl-2 protein, and attenuates inflammatory responses [8]. ROCK inhibition by fasudil reduces endothelin-1-induced myocardial injury, such as multifocal myocardial necrosis with infiltration of neutrophils and macrophages in the rabbit [149]. The underlying mechanisms for the apoptotic role of ROCK in these studies are not defined, and effects of ROCK inhibitors in other cellular events such as inflammatory cell infiltration and cytokine production may be related to the anti-apoptotic effect of ROCK inhibitors. Consistent with the studies mentioned above, ROCK is involved in the regulation of inflammatory responses, such as production of inflammatory cytokines, inflammatory cell infiltration and vehicle secretions, in a number of experimental models [48, 50, 117, 133], and these inflammatory responses may subsequently promote apoptosis. Finally, ROCK also regulates oxidative stress [49, 125, 150], which in turn can induce apoptosis.
The role of ROCK in mediating cell survival signals
There is increasing evidence that ROCK-mediated focal adhesion and integrin activation are involved in cell survival [32, 45, 142]. Perturbations of the actin cytoskeleton integrity via ROCK inhibition can initiate events that commit a cell to apoptosis.
In vitro evidence
ROCK-mediated survival effect has been documented in epithelial, cancer and endothelial cells as well as in other cell types. In airway epithelial cells, inhibition with either Y-27632 or HA1077 induces membrane ruffling, loss of actin stress fibers, and apoptosis that can be blocked by inhibiting caspase function or by inhibiting protein synthesis [90]. Rho/ROCK activation also plays a pro-survival role during oxidative stress (H2O2)-induced intestinal epithelial cell injury via activation of PKCδ and PKD[129]. In anaplastic thyroid cancer cells, inhibition of Rho by lovastatin or inhibition of ROCK by Y27632 induces cytochrome c release, activation of caspases 3 and 9, and apoptosis which required de novo protein synthesis [154]. Inhibition of Rho or ROCK results in activation of caspases 3, 8 and 9, and glioma cell apoptosis [110]. Treatment of human umbilical vein endothelial cells with ROCK inhibitors Y-27632 and HA-1077 causes dose-dependent cell death, which is dependent on de novo protein synthesis and is associated with an increase in p53 protein level [76].
ROCK may play a pro-survival role in hepatic stellate cells (HSC) which play a central role in the development of hepatic fibrosis. Inhibition of ROCK by Y27632 causes apoptosis with increased DNA fragmentation, condensation of nuclear chromatin and caspase 3 activity in rat cultured HSCs, possibly through decreasing cell spreading and attachment [53]. However, another study using ROCK inhibitor fasudil has shown that inhibition of ROCK does not induce apoptosis of rat cultured HSCs, but inhibits cell spreading, suppresses collagen production and enhances collagenase activity [38].
ROCK has been shown to regulate cell survival of the central nervous system. Activation of G-protein-coupled neurotransmitter receptors including cholinergic muscarinic receptors can provide neuroprotection from a wide variety of potentially lethal toxic insults. In human neuroblastoma SH-SY5Y cells, ROCK activation by muscarinic receptor stimulation is critical for the protective effect of muscarinic receptor activation against H2O2 (oxidative stress)- and camptothecin (DNA damage)-induced apoptosis [20].
In vivo evidence
A pro-survival role of ROCK in vascular smooth muscle cells was first observed in neointima formation after balloon injury in the rat carotid artery [120]. Y27632 treatment significantly increases the neointimal TUNEL-positive smooth muscle cells at 7 and 14 day after vascular injury [120], and this increased apoptosis is associated with significantly up-regulation of the proapoptotic Bax, suggesting that ROCK inhibition induces neointimal smooth muscle cell apoptosis through Bax up-regulation [121]. Consistent with this finding, the inhibition of ROCK with fasudil enhances smooth muscle cell apoptosis in the neointima after stent implantation in the porcine coronary artery, and this increased apoptosis is associated with down-regulation of the anti-apoptotic protein Bcl-2 [84]. Moreover, inhibition of ROCK with fasudil significantly enhances smooth muscle cell apoptosis in autologous vein grafts in rabbits[40] and in pulmonary hypertension in rats [1], further supporting a pro-survival role of ROCK in smooth muscle cells. Increased smooth muscle cell apoptosis along with inhibition of smooth muscle cell proliferation, inflammatory cell infiltration and/or extracellular matrix protein production contribute to the inhibition of neointima formation via ROCK inhibition [40, 84, 120]. Therefore, these studies suggest that ROCK plays a pro-survival role in neointimal smooth muscle cells. The underlying mechanisms are not defined, and effects of ROCK inhibitors in other cellular events such as smooth cell proliferation, inflammatory cell infiltration and cytokine production may be related to their effect on smooth muscle cell apoptosis.
ROCK also plays an essential role in the survival of the spinal motor neurons during mammalian embryogenesis as conditional expression of a dominant-negative form of ROCK (inhibits both ROCK1 and ROCK2) in spinal motor neurons in transgenic mice increases apoptosis [64]. Moreover, Rho/ROCK pathway is involved negatively in the regulation of glioma cell death pathway as inhibition of Rho or ROCK induces apoptosis of C6 glioma cell tumor cells and results in a significantly smaller tumor mass in vivo [110].
Conclusions and future directions
ROCK plays an important role in the regulation of morphological changes of apoptotic cells by regulating the cytoskeleton and actomyosin contractility. ROCK, either as a proapoptotic or anti-apoptotic regulator, has also been reported in a variety of cell types. However, how the basic components of the apoptotic machinery are regulated by ROCK is not completely understood in many instances, and is likely different depending on the cell type and the apoptotic stimulus. Clearly, more studies are needed in order to understand the molecular and cellular mechanisms through which ROCK regulates cell death and survival as well as in association with its roles in physiology and patho-physiology.
ROCK1 and ROCK2 not only function in a redundant manner, each is able to compensate for the loss of the other in some systems, but also may have their own distinct roles in some tissues during embryonic development (e.g. placenta) or under certain patho-physiological conditions in adulthood (e.g. cardiac fibrosis, cardiomyocyte apoptosis). There are a number of factors that could contribute to their distinct functions including the differences in their tissue distribution, subcellular localization, activation by upstream signals (e.g. caspases) and interaction with downstream molecules. The difficulty in studying specific cellular function of the two ROCK isoforms is due to the high degree of their sequence homology in the kinase domain and the lack of isoform specific inhibitors. Genetic targeting approaches using systemic and/or conditional ROCK1 and ROCK2 knockout mice coupled with molecular analysis using RNA interference and gene transfer strategies will become a focus of future efforts to understand distinct cellular, physiological and patho-physiological functions of the two ROCK isoforms. A greater understanding of physiological and patho-physiological roles of each ROCK isoform is much desired for better evaluating beneficial and side effects of ROCK inhibitors in animal and clinical studies.
Acknowledgements
This work was supported by National Institutes of Health grant HL-72897 and by the Riley Children’s Foundation and the Lilly Endowment. We are grateful to Dr. Michael Rubart and Lelia J. Summers for comments on the manuscript.
References
- 1.Abe K, Shimokawa H, Morikawa K, Uwatoku T, Oi K, Matsumoto Y, Hattori T, Nakashima Y, Kaibuchi K, Sueishi K, Takeshit A. Long-term treatment with a Rho-kinase inhibitor improves monocrotaline-induced fatal pulmonary hypertension in rats. Circ Res. 2004;94:385–393. doi: 10.1161/01.RES.0000111804.34509.94. [DOI] [PubMed] [Google Scholar]
- 2.Amano M, Chihara K, Kimura K, Fukata Y, Nakamura N, Matsuura Y, Kaibuchi K. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science. 1997;275:1308–1311. doi: 10.1126/science.275.5304.1308. [DOI] [PubMed] [Google Scholar]
- 3.Amano M, Chihara K, Nakamura N, Kaneko T, Matsuura Y, Kaibuchi K. The COOH terminus of Rho-kinase negatively regulates rho-kinase activity. J Biol Chem. 1999;274:32418–32424. doi: 10.1074/jbc.274.45.32418. [DOI] [PubMed] [Google Scholar]
- 4.Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J Biol Chem. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- 5.Asano T, Ikegaki I, Satoh S, Suzuki Y, Shibuya M, Takayasu M, Hidaka H. Mechanism of action of a novel antivasospasm drug, HA1077. J Pharmacol Exp Ther. 1987;241:1033–1040. [PubMed] [Google Scholar]
- 6.Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 7.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 8.Bao W, Hu E, Tao L, Boyce R, Mirabile R, Thudium DT, Ma XL, Willette RN, Yue TL. Inhibition of Rho-kinase protects the heart against ischemia/reperfusion injury. Cardiovasc Res. 2004;61:548–558. doi: 10.1016/j.cardiores.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Begum N, Sandu OA, Ito M, Lohmann SM, Smolenski A. Active Rho kinase (ROK-alpha ) associates with insulin receptor substrate-1 and inhibits insulin signaling in vascular smooth muscle cells. J Biol Chem. 2002;277:6214–6222. doi: 10.1074/jbc.M110508200. [DOI] [PubMed] [Google Scholar]
- 10.Breitenlechner C, Gassel M, Hidaka H, Kinzel V, Huber R, Engh RA, Bossemeyer D. Protein kinase A in complex with Rho-kinase inhibitors Y-27632, Fasudil, and H-1152P: structural basis of selectivity. Structure (Camb) 2003;11:1595–1607. doi: 10.1016/j.str.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Brown JH, Del Re DP, Sussman MA. The Rac and Rho hall of fame: a decade of hypertrophic signaling hits. Circ Res. 2006;98:730–742. doi: 10.1161/01.RES.0000216039.75913.9e. [DOI] [PubMed] [Google Scholar]
- 12.Caron E, Hall A. Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science. 1998;282:1717–1721. doi: 10.1126/science.282.5394.1717. [DOI] [PubMed] [Google Scholar]
- 13.Chang J, Xie M, Shah VR, Schneider MD, Entman ML, Wei L, Schwartz RJ. Activation of Rho-associated coiled-coil protein kinase 1 (ROCK-1) by caspase-3 cleavage plays an essential role in cardiac myocyte apoptosis. Proc Natl Acad Sci USA. 2006;103:14495–14500. doi: 10.1073/pnas.0601911103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen XQ, Tan I, Ng CH, Hall C, Lim L, Leung T. Characterization of RhoA-binding kinase ROKalpha implication of the pleckstrin homology domain in ROKalpha function using region-specific antibodies. J Biol Chem. 2002;277:12680–12688. doi: 10.1074/jbc.M109839200. [DOI] [PubMed] [Google Scholar]
- 15.Chevrier V, Piel M, Collomb N, Saoudi Y, Frank R, Paintrand M, Narumiya S, Bornens M, Job D. The Rho-associated protein kinase p160ROCK is required for centrosome positioning. J Cell Biol. 2002;157:807–817. doi: 10.1083/jcb.200203034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coleman ML, Sahai EA, Yeo M, Bosch M, Dewar A, Olson MF. Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat Cell Biol. 2001;3:339–345. doi: 10.1038/35070009. [DOI] [PubMed] [Google Scholar]
- 17.Coudray AM, Louvet C, Kornprobst M, Raymond E, Andre T, Tournigand C, Faivre S, De Gramont A, Larsen AK, Gespach C. Increased anticancer activity of the thymidylate synthase inhibitor BGC9331 combined with the topoisomerase I inhibitor SN-38 in human colorectal and breast cancer cells: induction of apoptosis and ROCK cleavage through caspase-3-dependent and -independent mechanisms. Int J Oncol. 2005;27:553–561. [PubMed] [Google Scholar]
- 18.Croft DR, Coleman ML, Li S, Robertson D, Sullivan T, Stewart CL, Olson MF. Actin-myosin-based contraction is responsible for apoptotic nuclear disintegration. J Cell Biol. 2005;168:245–255. doi: 10.1083/jcb.200409049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 20.De Sarno P, Shestopal SA, Zmijewska AA, Jope RS. Anti-apoptotic effects of muscarinic receptor activation are mediated by Rho kinase. Brain Res. 2005;1041:112–115. doi: 10.1016/j.brainres.2005.01.081. [DOI] [PubMed] [Google Scholar]
- 21.Dempsey PW, Doyle SE, He JQ, Cheng G. The signaling adaptors and pathways activated by TNF superfamily. Cytokine Growth Factor Rev. 2003;14:193–209. doi: 10.1016/s1359-6101(03)00021-2. [DOI] [PubMed] [Google Scholar]
- 22.Di Cristofano A, Pandolfi PP. The multiple roles of PTEN in tumor suppression. Cell. 2000;100:387–390. doi: 10.1016/s0092-8674(00)80674-1. [DOI] [PubMed] [Google Scholar]
- 23.Domnina LV, Ivanova OY, Pletjushkina OY, Fetisova EK, Chernyak BV, Skulachev VP, Vasiliev JM. Marginal blebbing during the early stages of TNF-induced apoptosis indicates alteration in actomyosin contractility. Cell Biol Int. 2004;28:471–475. doi: 10.1016/j.cellbi.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 24.Doran JD, Liu X, Taslimi P, Saadat A, Fox T. New insights into the structure-function relationships of Rho-associated kinase: a thermodynamic and hydrodynamic study of the dimer-to-monomer transition and its kinetic implications. Biochem J. 2004;384:255–262. doi: 10.1042/BJ20040344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Du J, Hannon GJ. Suppression of p160ROCK bypasses cell cycle arrest after Aurora-A/STK15 depletion. Proc Natl Acad Sci USA. 2004;101:8975–8980. doi: 10.1073/pnas.0308484101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Erwig LP, McPhilips KA, Wynes MW, Ivetic A, Ridley AJ, Henson PM. Differential regulation of phagosome maturation in macrophages and dendritic cells mediated by Rho GTPases and ezrin-radixin-moesin (ERM) proteins. Proc Natl Acad Sci USA. 2006;103:12825–12830. doi: 10.1073/pnas.0605331103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 28.Feng J, Ito M, Ichikawa K, Isaka N, Nishikawa M, Hartshorne DJ, Nakano T. Inhibitory phosphorylation site for Rho-associated kinase on smooth muscle myosin phosphatase. J Biol Chem. 1999;274:37385–37390. doi: 10.1074/jbc.274.52.37385. [DOI] [PubMed] [Google Scholar]
- 29.Feng J, Ito M, Kureishi Y, Ichikawa K, Amano M, Isaka N, Okawa K, Iwamatsu A, Kaibuchi K, Hartshorne DJ, Nakano T. Rho-associated kinase of chicken gizzard smooth muscle. J Biol Chem. 1999;274:3744–3752. doi: 10.1074/jbc.274.6.3744. [DOI] [PubMed] [Google Scholar]
- 30.Flink IL, Oana S, Maitra N, Bahl JJ, Morkin E. Changes in E2F complexes containing retinoblastoma protein family members and increased cyclin-dependent kinase inhibitor activities during terminal differentiation of cardiomyocytes. J Mol Cell Cardiol. 1998;30:563–578. doi: 10.1006/jmcc.1997.0620. [DOI] [PubMed] [Google Scholar]
- 31.Foo RS, Mani K, Kitsis RN. Death begets failure in the heart. J Clin Invest. 2005;115:565–571. doi: 10.1172/JCI24569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frisch SM, Screaton RA. Anoikis mechanisms. Curr Opin Cell Biol. 2001;13:555–562. doi: 10.1016/s0955-0674(00)00251-9. [DOI] [PubMed] [Google Scholar]
- 33.Fritz G, Kaina B. Rho GTPases: promising cellular targets for novel anticancer drugs. Curr Cancer Drug Targets. 2006;6:1–14. [PubMed] [Google Scholar]
- 34.Fu P, Liu F, Su S, Wang W, Huang XR, Entman ML, Schwartz RJ, Wei L, Lan HY. Signaling Mechanism of Renal Fibrosis in Unilateral Ureteral Obstructive Kidney Disease in ROCK1 Knockout Mice. J Am Soc Nephrol. 2006;17:3105–3114. doi: 10.1681/ASN.2005121366. [DOI] [PubMed] [Google Scholar]
- 35.Fu X, Gong MC, Jia T, Somlyo AV, Somlyo AP. The effects of the Rho-kinase inhibitor Y-27632 on arachidonic acid-, GTPgammaS-, and phorbol ester-induced Ca2+-sensitization of smooth muscle. FEBS Lett. 1998;440:183–187. doi: 10.1016/s0014-5793(98)01455-0. [DOI] [PubMed] [Google Scholar]
- 36.Fujisawa K, Fujita A, Ishizaki T, Saito Y, Narumiya S. Identification of the Rho-binding domain of p160ROCK, a Rho-associated coiled-coil containing protein kinase. J Biol Chem. 1996;271:23022–23028. doi: 10.1074/jbc.271.38.23022. [DOI] [PubMed] [Google Scholar]
- 37.Fukata Y, Oshiro N, Kinoshita N, Kawano Y, Matsuoka Y, Bennett V, Matsuura Y, Kaibuchi K. Phosphorylation of adducin by Rho-kinase plays a crucial role in cell motility. J Cell Biol. 1999;145:347–361. doi: 10.1083/jcb.145.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fukushima M, Nakamuta M, Kohjima M, Kotoh K, Enjoji M, Kobayashi N, Nawata H. Fasudil hydrochloride hydrate, a Rho-kinase (ROCK) inhibitor, suppresses collagen production and enhances collagenase activity in hepatic stellate cells. Liver Int. 2005;25:829–838. doi: 10.1111/j.1478-3231.2005.01142.x. [DOI] [PubMed] [Google Scholar]
- 39.Furukawa N, Ongusaha P, Jahng WJ, Araki K, Choi CS, Kim HJ, Lee YH, Kaibuchi K, Kahn BB, Masuzaki H, Kim JK, Lee SW, Kim YB. Role of Rho-kinase in regulation of insulin action and glucose homeostasis. Cell Metab. 2005;2:119–129. doi: 10.1016/j.cmet.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 40.Furuyama T, Komori K, Shimokawa H, Matsumoto Y, Uwatoku T, Hirano K, Maehara Y. Long-term inhibition of Rho kinase suppresses intimal thickening in autologous vein grafts in rabbits. J Vasc Surg. 2006;43:1249–1256. doi: 10.1016/j.jvs.2006.02.035. [DOI] [PubMed] [Google Scholar]
- 41.Goto H, Kosako H, Tanabe K, Yanagida M, Sakurai M, Amano M, Kaibuchi K, Inagaki M. Phosphorylation of vimentin by Rho-associated kinase at a unique amino-terminal site that is specifically phosphorylated during cytokinesis. J Biol Chem. 1998;273:11728–11736. doi: 10.1074/jbc.273.19.11728. [DOI] [PubMed] [Google Scholar]
- 42.Gross A, Yin XM, Wang K, Wei MC, Jockel J, Milliman C, Erdjument-Bromage H, Tempst P, Korsmeyer SJ. Caspase cleaved BID targets mitochondria and is required for cytochrome c release, while BCL-XL prevents this release but not tumor necrosis factor-R1/Fas death. J Biol Chem. 1999;274:1156–1163. doi: 10.1074/jbc.274.2.1156. [DOI] [PubMed] [Google Scholar]
- 43.Hagerty L, Weitzel DH, Chambers J, Fortner CN, Brush MH, Loiselle D, Hosoya H, Haystead TA. ROCK1 phosphorylates and activates ZIP kinase. J Biol Chem. 2006;282:4884–4893. doi: 10.1074/jbc.M609990200. [DOI] [PubMed] [Google Scholar]
- 44.Hall A. Small GTP-binding proteins and the regulation of the actin cytoskeleton. Annu Rev Cell Biol. 1994;10:31–54. doi: 10.1146/annurev.cb.10.110194.000335. [DOI] [PubMed] [Google Scholar]
- 45.Han S, Sidell N, Roman J. Fibronectin stimulates human lung carcinoma cell proliferation by suppressing p21 gene expression via signals involving Erk and Rho kinase. Cancer Lett. 2005;219:71–81. doi: 10.1016/j.canlet.2004.07.040. [DOI] [PubMed] [Google Scholar]
- 46.Hanks SK, Quinn AM, Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988;241:42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- 47.Harenberg A, Girkontaite I, Giehl K, Fischer KD. The Lsc RhoGEF mediates signaling from thromboxane A2 to actin polymerization and apoptosis in thymocytes. Eur J Immunol. 2005;35:1977–1986. doi: 10.1002/eji.200425769. [DOI] [PubMed] [Google Scholar]
- 48.Hattori T, Shimokawa H, Higashi M, Hiroki J, Mukai Y, Tsutsui H, Kaibuchi K, Takeshita A. Long-term inhibition of Rho-kinase suppresses left ventricular remodeling after myocardial infarction in mice. Circulation. 2004;109:2234–2239. doi: 10.1161/01.CIR.0000127939.16111.58. [DOI] [PubMed] [Google Scholar]
- 49.Higashi M, Shimokawa H, Hattori T, Hiroki J, Mukai Y, Morikawa K, Ichiki T, Takahashi S, Takeshita A. Long-term inhibition of Rho-kinase suppresses angiotensin II-induced cardiovascular hypertrophy in rats in vivo: effect on endothelial NAD(P)H oxidase system. Circ Res. 2003;93:767–775. doi: 10.1161/01.RES.0000096650.91688.28. [DOI] [PubMed] [Google Scholar]
- 50.Horton JW, Maass DL, Ballard-Croft C. Rho-associated kinase modulates myocardial inflammatory cytokine responses. Shock. 2005;24:53–58. doi: 10.1097/01.shk.0000167109.96000.7f. [DOI] [PubMed] [Google Scholar]
- 51.Hu E, Lee D. Rho kinase as potential therapeutic target for cardiovascular diseases: opportunities and challenges. Expert Opin Ther Targets. 2005;9:715–736. doi: 10.1517/14728222.9.4.715. [DOI] [PubMed] [Google Scholar]
- 52.Huser M, Luckett J, Chiloeches A, Mercer K, Iwobi M, Giblett S, Sun XM, Brown J, Marais R, Pritchard C. MEK kinase activity is not necessary for Raf-1 function. Embo J. 2001;20:1940–1951. doi: 10.1093/emboj/20.8.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ikeda H, Nagashima K, Yanase M, Tomiya T, Arai M, Inoue Y, Tejima K, Nishikawa T, Omata M, Kimura S, Fujiwara K. Involvement of Rho/Rho kinase pathway in regulation of apoptosis in rat hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2003;285:G880–886. doi: 10.1152/ajpgi.00039.2003. [DOI] [PubMed] [Google Scholar]
- 54.Ishizaki T, Maekawa M, Fujisawa K, Okawa K, Iwamatsu A, Fujita A, Watanabe N, Saito Y, Kakizuka A, Morii N, Narumiya S. The small GTP-binding protein Rho binds to and activates a 160 kDa Ser/Thr protein kinase homologous to myotonic dystrophy kinase. Embo J. 1996;15:1885–1893. [PMC free article] [PubMed] [Google Scholar]
- 55.Ishizaki T, Naito M, Fujisawa K, Maekawa M, Watanabe N, Saito Y, Narumiya S. p160ROCK, a Rho-associated coiled-coil forming protein kinase, works downstream of Rho and induces focal adhesions. FEBS Lett. 1997;404:118–124. doi: 10.1016/s0014-5793(97)00107-5. [DOI] [PubMed] [Google Scholar]
- 56.Ishizaki T, Uehata M, Tamechika I, Keel J, Nonomura K, Maekawa M, Narumiya S. Pharmacological properties of Y-27632, a specific inhibitor of rho-associated kinases. Mol Pharmacol. 2000;57:976–983. [PubMed] [Google Scholar]
- 57.Jugdutt BI, Idikio HA. Apoptosis and oncosis in acute coronary syndromes: assessment and implications. Mol Cell Biochem. 2005;270:177–200. doi: 10.1007/s11010-005-4507-9. [DOI] [PubMed] [Google Scholar]
- 58.Kaneko T, Amano M, Maeda A, Goto H, Takahashi K, Ito M, Kaibuchi K. Identification of calponin as a novel substrate of Rho-kinase. Biochem Biophys Res Commun. 2000;273:110–116. doi: 10.1006/bbrc.2000.2901. [DOI] [PubMed] [Google Scholar]
- 59.Kanthou C, Tozer GM. The tumor vascular targeting agent combretastatin A-4-phosphate induces reorganization of the actin cytoskeleton and early membrane blebbing in human endothelial cells. Blood. 2002;99:2060–2069. doi: 10.1182/blood.v99.6.2060. [DOI] [PubMed] [Google Scholar]
- 60.Kawabata S, Usukura J, Morone N, Ito M, Iwamatsu A, Kaibuchi K, Amano M. Interaction of Rho-kinase with myosin II at stress fibres. Genes Cells. 2004;9:653–660. doi: 10.1111/j.1356-9597.2004.00749.x. [DOI] [PubMed] [Google Scholar]
- 61.Kawano Y, Fukata Y, Oshiro N, Amano M, Nakamura T, Ito M, Matsumura F, Inagaki M, Kaibuchi K. Phosphorylation of myosin-binding subunit (MBS) of myosin phosphatase by Rho-kinase in vivo. J Cell Biol. 1999;147:1023–1038. doi: 10.1083/jcb.147.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- 63.Kitazawa T, Eto M, Woodsome TP, Brautigan DL. Agonists trigger G protein-mediated activation of the CPI-17 inhibitor phosphoprotein of myosin light chain phosphatase to enhance vascular smooth muscle contractility. J Biol Chem. 2000;275:9897–9900. doi: 10.1074/jbc.275.14.9897. [DOI] [PubMed] [Google Scholar]
- 64.Kobayashi K, Takahashi M, Matsushita N, Miyazaki J, Koike M, Yaginuma H, Osumi N, Kaibuchi K, Kobayashi K. Survival of developing motor neurons mediated by Rho GTPase signaling pathway through Rho-kinase. J Neurosci. 2004;24:3480–3488. doi: 10.1523/JNEUROSCI.0295-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kosako H, Goto H, Yanagida M, Matsuzawa K, Fujita M, Tomono Y, Okigaki T, Odai H, Kaibuchi K, Inagaki M. Specific accumulation of Rho-associated kinase at the cleavage furrow during cytokinesis: cleavage furrow-specific phosphorylation of intermediate filaments. Oncogene. 1999;18:2783–2788. doi: 10.1038/sj.onc.1202633. [DOI] [PubMed] [Google Scholar]
- 66.Kureishi Y, Kobayashi S, Amano M, Kimura K, Kanaide H, Nakano T, Kaibuchi K, Ito M. Rho-associated kinase directly induces smooth muscle contraction through myosin light chain phosphorylation. J Biol Chem. 1997;272:12257–12260. doi: 10.1074/jbc.272.19.12257. [DOI] [PubMed] [Google Scholar]
- 67.Lai JM, Hsieh CL, Chang ZF. Caspase activation during phorbol ester-induced apoptosis requires ROCK-dependent myosin-mediated contraction. J Cell Sci. 2003;116:3491–3501. doi: 10.1242/jcs.00660. [DOI] [PubMed] [Google Scholar]
- 68.Lai JM, Wu S, Huang DY, Chang ZF. Cytosolic retention of phosphorylated extracellular signal-regulated kinase and a Rho-associated kinase-mediated signal impair expression of p21(Cip1/Waf1) in phorbol 12-myristate-13-acetate-induced apoptotic cells. Mol Cell Biol. 2002;22:7581–7592. doi: 10.1128/MCB.22.21.7581-7592.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lakhani SA, Masud A, Kuida K, Porter GA, Jr., Booth CJ, Mehal WZ, Inayat I, Flavell RA. Caspases 3 and 7: key mediators of mitochondrial events of apoptosis. Science. 2006;311:847–851. doi: 10.1126/science.1115035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lane JD, Allan VJ, Woodman PG. Active relocation of chromatin and endoplasmic reticulum into blebs in late apoptotic cells. J Cell Sci. 2005;118:4059–4071. doi: 10.1242/jcs.02529. [DOI] [PubMed] [Google Scholar]
- 71.Lauber K, Blumenthal SG, Waibel M, Wesselborg S. Clearance of apoptotic cells: getting rid of the corpses. Mol Cell. 2004;14:277–287. doi: 10.1016/s1097-2765(04)00237-0. [DOI] [PubMed] [Google Scholar]
- 72.Lee HH, Chien CL, Liao HK, Chen YJ, Chang ZF. Nuclear efflux of heterogeneous nuclear ribonucleoprotein C1/C2 in apoptotic cells: a novel nuclear export dependent on Rho-associated kinase activation. J Cell Sci. 2004;117:5579–5589. doi: 10.1242/jcs.01482. [DOI] [PubMed] [Google Scholar]
- 73.Leung T, Chen XQ, Manser E, Lim L. The p160 RhoA-binding kinase ROK alpha is a member of a kinase family and is involved in the reorganization of the cytoskeleton. Mol Cell Biol. 1996;16:5313–5327. doi: 10.1128/mcb.16.10.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Leung T, Manser E, Tan L, Lim L. A novel serine/threonine kinase binding the Ras-related RhoA GTPase which translocates the kinase to peripheral membranes. J Biol Chem. 1995;270:29051–29054. doi: 10.1074/jbc.270.49.29051. [DOI] [PubMed] [Google Scholar]
- 75.Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 76.Li X, Liu L, Tupper JC, Bannerman DD, Winn RK, Sebti SM, Hamilton AD, Harlan JM. Inhibition of protein geranylgeranylation and RhoA/RhoA kinase pathway induces apoptosis in human endothelial cells. J Biol Chem. 2002;277:15309–15316. doi: 10.1074/jbc.M201253200. [DOI] [PubMed] [Google Scholar]
- 77.Li Z, Dong X, Wang Z, Liu W, Deng N, Ding Y, Tang L, Hla T, Zeng R, Li L, Wu D. Regulation of PTEN by Rho small GTPases. Nat Cell Biol. 2005;7:399–404. doi: 10.1038/ncb1236. [DOI] [PubMed] [Google Scholar]
- 78.Loirand G, Guerin P, Pacaud P. Rho kinases in cardiovascular physiology and pathophysiology. Circ Res. 2006;98:322–334. doi: 10.1161/01.RES.0000201960.04223.3c. [DOI] [PubMed] [Google Scholar]
- 79.Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- 80.Maekawa M, Ishizaki T, Boku S, Watanabe N, Fujita A, Iwamatsu A, Obinata T, Ohashi K, Mizuno K, Narumiya S. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science. 1999;285:895–898. doi: 10.1126/science.285.5429.895. [DOI] [PubMed] [Google Scholar]
- 81.Manapov F, Muller P, Rychly J. Translocation of p21(Cip1/WAF1) from the nucleus to the cytoplasm correlates with pancreatic myofibroblast to fibroblast cell conversion. Gut. 2005;54:814–822. doi: 10.1136/gut.2003.036491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Matsui T, Amano M, Yamamoto T, Chihara K, Nakafuku M, Ito M, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Rho-associated kinase, a novel serine/threonine kinase, as a putative target for small GTP binding protein Rho. Embo J. 1996;15:2208–2216. [PMC free article] [PubMed] [Google Scholar]
- 83.Matsui T, Maeda M, Doi Y, Yonemura S, Amano M, Kaibuchi K, Tsukita S, Tsukita S. Rho-kinase phosphorylates COOH-terminal threonines of ezrin/radixin/moesin (ERM) proteins and regulates their head-to-tail association. J Cell Biol. 1998;140:647–657. doi: 10.1083/jcb.140.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Matsumoto Y, Uwatoku T, Oi K, Abe K, Hattori T, Morishige K, Eto Y, Fukumoto Y, Nakamura K, Shibata Y, Matsuda T, Takeshita A, Shimokawa H. Long-term inhibition of Rho-kinase suppresses neointimal formation after stent implantation in porcine coronary arteries: involvement of multiple mechanisms. Arterioscler Thromb Vasc Biol. 2004;24:181–186. doi: 10.1161/01.ATV.0000105053.46994.5B. [DOI] [PubMed] [Google Scholar]
- 85.Mavria G, Vercoulen Y, Yeo M, Paterson H, Karasarides M, Marais R, Bird D, Marshall CJ. ERK-MAPK signaling opposes Rho-kinase to promote endothelial cell survival and sprouting during angiogenesis. Cancer Cell. 2006;9:33–44. doi: 10.1016/j.ccr.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 86.McElhinney B, Poynter ME, Shrivastava P, Hazen SL, Janssen-Heininger YM. Eosinophil peroxidase catalyzes JNK-mediated membrane blebbing in a Rho kinase-dependent manner. J Leukoc Biol. 2003;74:897–907. doi: 10.1189/jlb.0103028. [DOI] [PubMed] [Google Scholar]
- 87.Mikula M, Schreiber M, Husak Z, Kucerova L, Ruth J, Wieser R, Zatloukal K, Beug H, Wagner EF, Baccarini M. Embryonic lethality and fetal liver apoptosis in mice lacking the c-raf-1 gene. Embo J. 2001;20:1952–1962. doi: 10.1093/emboj/20.8.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mills JC, Stone NL, Erhardt J, Pittman RN. Apoptotic membrane blebbing is regulated by myosin light chain phosphorylation. J Cell Biol. 1998;140:627–636. doi: 10.1083/jcb.140.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Minambres R, Guasch RM, Perez-Arago A, Guerri C. The RhoA/ROCK-I/MLC pathway is involved in the ethanol-induced apoptosis by anoikis in astrocytes. J Cell Sci. 2006;119:271–282. doi: 10.1242/jcs.02723. [DOI] [PubMed] [Google Scholar]
- 90.Moore M, Marroquin BA, Gugliotta W, Tse R, White SR. Rho kinase inhibition initiates apoptosis in human airway epithelial cells. Am J Respir Cell Mol Biol. 2004;30:379–387. doi: 10.1165/rcmb.2003-0019OC. [DOI] [PubMed] [Google Scholar]
- 91.Morelli A, Chiozzi P, Chiesa A, Ferrari D, Sanz JM, Falzoni S, Pinton P, Rizzuto R, Olson MF, Di Virgilio F. Extracellular ATP causes ROCK I-dependent bleb formation in P2X7-transfected HEK293 cells. Mol Biol Cell. 2003;14:2655–2664. doi: 10.1091/mbc.02-04-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Morikage N, Kishi H, Sato M, Guo F, Shirao S, Yano T, Soma M, Hamano K, Esato K, Kobayashi S. Cholesterol primes vascular smooth muscle to induce Ca2 sensitization mediated by a sphingosylphosphorylcholine-Rho-kinase pathway: possible role for membrane raft. Circ Res. 2006;99:299–306. doi: 10.1161/01.RES.0000235877.33682.e9. [DOI] [PubMed] [Google Scholar]
- 93.Moroni MC, Hickman ES, Denchi E.Lazzerini, Caprara G, Colli E, Cecconi F, Muller H, Helin K. Apaf-1 is a transcriptional target for E2F and p53. Nat Cell Biol. 2001;3:552–558. doi: 10.1038/35078527. [DOI] [PubMed] [Google Scholar]
- 94.Mueller BK, Mack H, Teusch N. Rho kinase, a promising drug target for neurological disorders. Nat Rev Drug Discov. 2005;4:387–398. doi: 10.1038/nrd1719. [DOI] [PubMed] [Google Scholar]
- 95.Nakagawa O, Fujisawa K, Ishizaki T, Saito Y, Nakao K, Narumiya S. ROCK-I and ROCK-II, two isoforms of Rho-associated coiled-coil forming protein serine/threonine kinase in mice. FEBS Lett. 1996;392:189–193. doi: 10.1016/0014-5793(96)00811-3. [DOI] [PubMed] [Google Scholar]
- 96.Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- 97.Noma K, Oyama N, Liao JK. Physiological role of ROCKs in the cardiovascular system. Am J Physiol Cell Physiol. 2006;290:C661–668. doi: 10.1152/ajpcell.00459.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ogata Y, Takahashi M, Takeuchi K, Ueno S, Mano H, Ookawara S, Kobayashi E, Ikeda U, Shimada K. Fluvastatin induces apoptosis in rat neonatal cardiac myocytes: a possible mechanism of statin-attenuated cardiac hypertrophy. J Cardiovasc Pharmacol. 2002;40:907–915. doi: 10.1097/00005344-200212000-00012. [DOI] [PubMed] [Google Scholar]
- 99.Ohashi K, Nagata K, Maekawa M, Ishizaki T, Narumiya S, Mizuno K. Rho-associated kinase ROCK activates LIM-kinase 1 by phosphorylation at threonine 508 within the activation loop. J Biol Chem. 2000;275:3577–3582. doi: 10.1074/jbc.275.5.3577. [DOI] [PubMed] [Google Scholar]
- 100.Olazabal IM, Caron E, May RC, Schilling K, Knecht DA, Machesky LM. Rho-kinase and myosin-II control phagocytic cup formation during CR, but not FcgammaR, phagocytosis. Curr Biol. 2002;12:1413–1418. doi: 10.1016/s0960-9822(02)01069-2. [DOI] [PubMed] [Google Scholar]
- 101.Ongusaha PP, Kim HG, Boswell SA, Ridley AJ, Der CJ, Dotto GP, Kim YB, Aaronson SA, Lee SW. RhoE is a pro-survival p53 target gene that inhibits ROCK I-mediated apoptosis in response to genotoxic stress. Curr Biol. 2006;16:2466–2472. doi: 10.1016/j.cub.2006.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 102.Orlando KA, Pittman RN. Rho kinase regulates phagocytosis, surface expression of GlcNAc, and Golgi fragmentation of apoptotic PC12 cells. Exp Cell Res. 2006 doi: 10.1016/j.yexcr.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 103.Orlando KA, Stone NL, Pittman RN. Rho kinase regulates fragmentation and phagocytosis of apoptotic cells. Exp Cell Res. 2006;312:5–15. doi: 10.1016/j.yexcr.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 104.Parent N, Sane AT, Droin N, Bertrand R. Procaspase-2S inhibits procaspase-3 processing and activation, preventing ROCK-1-mediated apoptotic blebbing and body formation in human B lymphoma Namalwa cells. Apoptosis. 2005;10:313–322. doi: 10.1007/s10495-005-0805-7. [DOI] [PubMed] [Google Scholar]
- 105.Petrache I, Crow MT, Neuss M, Garcia JG. Central involvement of Rho family GTPases in TNF-alpha-mediated bovine pulmonary endothelial cell apoptosis. Biochem Biophys Res Commun. 2003;306:244–249. doi: 10.1016/s0006-291x(03)00945-8. [DOI] [PubMed] [Google Scholar]
- 106.Petrache I, Verin AD, Crow MT, Birukova A, Liu F, Garcia JG. Differential effect of MLC kinase in TNF-alpha-induced endothelial cell apoptosis and barrier dysfunction. Am J Physiol Lung Cell Mol Physiol. 2001;280:L1168–1178. doi: 10.1152/ajplung.2001.280.6.L1168. [DOI] [PubMed] [Google Scholar]
- 107.Piazzolla D, Meissl K, Kucerova L, Rubiolo C, Baccarini M. Raf-1 sets the threshold of Fas sensitivity by modulating Rok-alpha signaling. J Cell Biol. 2005;171:1013–1022. doi: 10.1083/jcb.200504137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Potts MB, Vaughn AE, McDonough H, Patterson C, Deshmukh M. Reduced Apaf-1 levels in cardiomyocytes engage strict regulation of apoptosis by endogenous XIAP. J Cell Biol. 2005;171:925–930. doi: 10.1083/jcb.200504082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Potts PR, Singh S, Knezek M, Thompson CB, Deshmukh M. Critical function of endogenous XIAP in regulating caspase activation during sympathetic neuronal apoptosis. J Cell Biol. 2003;163:789–799. doi: 10.1083/jcb.200307130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rattan R, Giri S, Singh AK, Singh I. Rho/ROCK pathway as a target of tumor therapy. J Neurosci Res. 2006;83:243–255. doi: 10.1002/jnr.20707. [DOI] [PubMed] [Google Scholar]
- 111.Ricci JE, Munoz-Pinedo C, Fitzgerald P, Bailly-Maitre B, Perkins GA, Yadava N, Scheffler IE, Ellisman MH, Green DR. Disruption of mitochondrial function during apoptosis is mediated by caspase cleavage of the p75 subunit of complex I of the electron transport chain. Cell. 2004;117:773–786. doi: 10.1016/j.cell.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 112.Riento K, Guasch RM, Garg R, Jin B, Ridley AJ. RhoE binds to ROCK I and inhibits downstream signaling. Mol Cell Biol. 2003;23:4219–4229. doi: 10.1128/MCB.23.12.4219-4229.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol. 2003;4:446–456. doi: 10.1038/nrm1128. [DOI] [PubMed] [Google Scholar]
- 114.Riento K, Totty N, Villalonga P, Garg R, Guasch R, Ridley AJ. RhoE function is regulated by ROCK I-mediated phosphorylation. Embo J. 2005;24:1170–1180. doi: 10.1038/sj.emboj.7600612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rikitake Y, Oyama N, Wang CY, Noma K, Satoh M, Kim HH, Liao JK. Decreased perivascular fibrosis but not cardiac hypertrophy in ROCK1+/-haploinsufficient mice. Circulation. 2005;112:2959–2965. doi: 10.1161/CIRCULATIONAHA.105.584623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sanchis D, Mayorga M, Ballester M, Comella JX. Lack of Apaf-1 expression confers resistance to cytochrome c-driven apoptosis in cardiomyocytes. Cell Death Differ. 2003;10:977–986. doi: 10.1038/sj.cdd.4401267. [DOI] [PubMed] [Google Scholar]
- 117.Sapet C, Simoncini S, Loriod B, Puthier D, Sampol J, Nguyen C, Dignat-George F, Anfosso F. Thrombin-induced endothelial microparticle generation: identification of a novel pathway involving ROCK-II activation by caspase-2. Blood. 2006;108:1868–1876. doi: 10.1182/blood-2006-04-014175. [DOI] [PubMed] [Google Scholar]
- 118.Sebbagh M, Hamelin J, Bertoglio J, Solary E, Breard J. Direct cleavage of ROCK II by granzyme B induces target cell membrane blebbing in a caspase-independent manner. J Exp Med. 2005;201:465–471. doi: 10.1084/jem.20031877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sebbagh M, Renvoize C, Hamelin J, Riche N, Bertoglio J, Breard J. Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing. Nat Cell Biol. 2001;3:346–352. doi: 10.1038/35070019. [DOI] [PubMed] [Google Scholar]
- 120.Shibata R, Kai H, Seki Y, Kato S, Morimatsu M, Kaibuchi K, Imaizumi T. Role of Rho-associated kinase in neointima formation after vascular injury. Circulation. 2001;103:284–289. doi: 10.1161/01.cir.103.2.284. [DOI] [PubMed] [Google Scholar]
- 121.Shibata R, Kai H, Seki Y, Kusaba K, Takemiya K, Koga M, Jalalidin A, Tokuda K, Tahara N, Niiyama H, Nagata T, Kuwahara F, Imaizumi T. Rho-kinase inhibition reduces neointima formation after vascular injury by enhancing Bax expression and apoptosis. J Cardiovasc Pharmacol. 2003;42(Suppl 1):S43–47. doi: 10.1097/00005344-200312001-00011. [DOI] [PubMed] [Google Scholar]
- 122.Shimizu Y, Thumkeo D, Keel J, Ishizaki T, Oshima H, Oshima M, Noda Y, Matsumura F, Taketo MM, Narumiya S. ROCK-I regulates closure of the eyelids and ventral body wall by inducing assembly of actomyosin bundles. J Cell Biol. 2005;168:941–953. doi: 10.1083/jcb.200411179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shimokawa H, Takeshita A. Rho-kinase is an important therapeutic target in cardiovascular medicine. Arterioscler Thromb Vasc Biol. 2005;25:1767–1775. doi: 10.1161/01.ATV.0000176193.83629.c8. [DOI] [PubMed] [Google Scholar]
- 124.Shintani T, Klionsky DJ. Autophagy in health and disease: a double-edged sword. Science. 2004;306:990–995. doi: 10.1126/science.1099993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shiotani S, Shimada M, Suehiro T, Soejima Y, Yosizumi T, Shimokawa H, Maehara Y. Involvement of Rho-kinase in cold ischemia-reperfusion injury after liver transplantation in rats. Transplantation. 2004;78:375–382. doi: 10.1097/01.tp.0000128618.41619.e7. [DOI] [PubMed] [Google Scholar]
- 126.Shirao S, Kashiwagi S, Sato M, Miwa S, Nakao F, Kurokawa T, Todoroki-Ikeda N, Mogami K, Mizukami Y, Kuriyama S, Haze K, Suzuki M, Kobayashi S. Sphingosylphosphorylcholine is a novel messenger for Rho-kinase-mediated Ca2+ sensitization in the bovine cerebral artery: unimportant role for protein kinase C. Circ Res. 2002;91:112–119. doi: 10.1161/01.res.0000026057.13161.42. [DOI] [PubMed] [Google Scholar]
- 127.Shiratsuchi A, Mori T, Nakanishi Y. Independence of plasma membrane blebbing from other biochemical and biological characteristics of apoptotic cells. J Biochem (Tokyo) 2002;132:381–386. doi: 10.1093/oxfordjournals.jbchem.a003233. [DOI] [PubMed] [Google Scholar]
- 128.Sin WC, Chen XQ, Leung T, Lim L. RhoA-binding kinase alpha translocation is facilitated by the collapse of the vimentin intermediate filament network. Mol Cell Biol. 1998;18:6325–6339. doi: 10.1128/mcb.18.11.6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Song J, Li J, Lulla A, Evers BM, Chung DH. Protein kinase D protects against oxidative stress-induced intestinal epithelial cell injury via Rho/ROK/PKC-delta pathway activation. Am J Physiol Cell Physiol. 2006;290:C1469–1476. doi: 10.1152/ajpcell.00486.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Song Y, Hoang BQ, Chang DD. ROCK-II-induced membrane blebbing and chromatin condensation require actin cytoskeleton. Exp Cell Res. 2002;278:45–52. doi: 10.1006/excr.2002.5565. [DOI] [PubMed] [Google Scholar]
- 131.Sordella R, Classon M, Hu KQ, Matheson SF, Brouns MR, Fine B, Zhang L, Takami H, Yamada Y, Settleman J. Modulation of CREB activity by the Rho GTPase regulates cell and organism size during mouse embryonic development. Dev Cell. 2002;2:553–565. doi: 10.1016/s1534-5807(02)00162-4. [DOI] [PubMed] [Google Scholar]
- 132.Sumi T, Matsumoto K, Nakamura T. Specific activation of LIM kinase 2 via phosphorylation of threonine 505 by ROCK, a Rho-dependent protein kinase. J Biol Chem. 2001;276:670–676. doi: 10.1074/jbc.M007074200. [DOI] [PubMed] [Google Scholar]
- 133.Suzuki J, Jin ZG, Meoli DF, Matoba T, Berk BC. Cyclophilin A is secreted by a vesicular pathway in vascular smooth muscle cells. Circ Res. 2006;98:811–817. doi: 10.1161/01.RES.0000216405.85080.a6. [DOI] [PubMed] [Google Scholar]
- 134.Thorlacius K, Slotta JE, Laschke MW, Wang Y, Menger MD, Jeppsson B, Thorlacius H. Protective effect of fasudil, a Rho-kinase inhibitor, on chemokine expression, leukocyte recruitment, and hepatocellular apoptosis in septic liver injury. J Leukoc Biol. 2006;79:923–931. doi: 10.1189/jlb.0705406. [DOI] [PubMed] [Google Scholar]
- 135.Thumkeo D, Keel J, Ishizaki T, Hirose M, Nonomura K, Oshima H, Oshima M, Taketo MM, Narumiya S. Targeted disruption of the mouse rho-associated kinase 2 gene results in intrauterine growth retardation and fetal death. Mol Cell Biol. 2003;23:5043–5055. doi: 10.1128/MCB.23.14.5043-5055.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Thumkeo D, Shimizu Y, Sakamoto S, Yamada S, Narumiya S. ROCK-I and ROCK-II cooperatively regulate closure of eyelid and ventral body wall in mouse embryo. Genes Cells. 2005;10:825–834. doi: 10.1111/j.1365-2443.2005.00882.x. [DOI] [PubMed] [Google Scholar]
- 137.Tominaga T, Ishizaki T, Narumiya S, Barber DL. p160ROCK mediates RhoA activation of Na-H exchange. Embo J. 1998;17:4712–4722. doi: 10.1093/emboj/17.16.4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tosello-Trampont AC, Nakada-Tsukui K, Ravichandran KS. Engulfment of apoptotic cells is negatively regulated by Rho-mediated signaling. J Biol Chem. 2003;278:49911–49919. doi: 10.1074/jbc.M306079200. [DOI] [PubMed] [Google Scholar]
- 139.Ueda H, Morishita R, Itoh H, Narumiya S, Mikoshiba K, Kato K, Asano T. Galpha11 induces caspase-mediated proteolytic activation of Rho-associated kinase, ROCK-I, in HeLa cells. J Biol Chem. 2001;276:42527–42533. doi: 10.1074/jbc.M102529200. [DOI] [PubMed] [Google Scholar]
- 140.Ueda H, Morishita R, Narumiya S, Kato K, Asano T. Galphaq/11 signaling induces apoptosis through two pathways involving reduction of Akt phosphorylation and activation of RhoA in HeLa cells. Exp Cell Res. 2004;298:207–217. doi: 10.1016/j.yexcr.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 141.Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- 142.Valenick LV, Schwarzbauer JE. Ligand density and integrin repertoire regulate cellular response to LPA. Matrix Biol. 2006;25:223–231. doi: 10.1016/j.matbio.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 143.Van Aelst L, D’Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11:2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- 144.Wang YX, Martin-McNulty B, da Cunha V, Vincelette J, Lu X, Feng Q, Halks-Miller M, Mahmoudi M, Schroeder M, Subramanyam B, Tseng JL, Deng GD, Schirm S, Johns A, Kauser K, Dole WP, Light DR. Fasudil, a Rho-kinase inhibitor, attenuates angiotensin II-induced abdominal aortic aneurysm in apolipoprotein E-deficient mice by inhibiting apoptosis and proteolysis. Circulation. 2005;111:2219–2226. doi: 10.1161/01.CIR.0000163544.17221.BE. [DOI] [PubMed] [Google Scholar]
- 145.Ward Y, Yap SF, Ravichandran V, Matsumura F, Ito M, Spinelli B, Kelly K. The GTP binding proteins Gem and Rad are negative regulators of the Rho-Rho kinase pathway. J Cell Biol. 2002;157:291–302. doi: 10.1083/jcb.200111026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wei L, Roberts W, Wang L, Yamada M, Zhang S, Zhao Z, Rivkees SA, Schwartz RJ, Imanaka-Yoshida K. Rho kinases play an obligatory role in vertebrate embryonic organogenesis. Development. 2001;128:2953–2962. doi: 10.1242/dev.128.15.2953. [DOI] [PubMed] [Google Scholar]
- 147.Wibberley A, Chen Z, Hu E, Hieble JP, Westfall TD. Expression and functional role of Rho-kinase in rat urinary bladder smooth muscle. Br J Pharmacol. 2003;138:757–766. doi: 10.1038/sj.bjp.0705109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wolfrum S, Dendorfer A, Rikitake Y, Stalker TJ, Gong Y, Scalia R, Dominiak P, Liao JK. Inhibition of Rho-kinase leads to rapid activation of phosphatidylinositol 3-kinase/protein kinase Akt and cardiovascular protection. Arterioscler Thromb Vasc Biol. 2004;24:1842–1847. doi: 10.1161/01.ATV.0000142813.33538.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yamamoto Y, Ikegaki I, Sasaki Y, Uchida T. The protein kinase inhibitor fasudil protects against ischemic myocardial injury induced by endothelin-1 in the rabbit. J Cardiovasc Pharmacol. 2000;35:203–211. doi: 10.1097/00005344-200002000-00005. [DOI] [PubMed] [Google Scholar]
- 150.Yang X, Doser TA, Fang CX, Nunn JM, Janardhanan R, Zhu M, Sreejayan N, Quinn MT, Ren J. Metallothionein prolongs survival and antagonizes senescence-associated cardiomyocyte diastolic dysfunction: role of oxidative stress. Faseb J. 2006;20:1024–1026. doi: 10.1096/fj.05-5288fje. [DOI] [PubMed] [Google Scholar]
- 151.Yoneda A, Multhaupt HA, Couchman JR. The Rho kinases I and II regulate different aspects of myosin II activity. J Cell Biol. 2005;170:443–453. doi: 10.1083/jcb.200412043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yoneda A, Ushakov D, Multhaupt HA, Couchman JR. Fibronectin Matrix Assembly Requires Distinct Contributions from Rho Kinases I and -II. Mol Biol Cell. 2007;18:66–75. doi: 10.1091/mbc.E06-08-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhang YM, Bo J, Taffet GE, Chang J, Shi J, Reddy AK, Michael LH, Schneider MD, Entman ML, Schwartz RJ, Wei L. Targeted deletion of ROCK1 protects the heart against pressure overload by inhibiting reactive fibrosis. Faseb J. 2006;20:916–925. doi: 10.1096/fj.05-5129com. [DOI] [PubMed] [Google Scholar]
- 154.Zhong WB, Wang CY, Chang TC, Lee WS. Lovastatin induces apoptosis of anaplastic thyroid cancer cells via inhibition of protein geranylgeranylation and de novo protein synthesis. Endocrinology. 2003;144:3852–3859. doi: 10.1210/en.2003-0098. [DOI] [PubMed] [Google Scholar]
- 155.Zihni C, Mitsopoulos C, Tavares IA, Ridley AJ, Morris JD. Prostate-derived sterile 20-like kinase 2 (PSK2) regulates apoptotic morphology via C-Jun N-terminal kinase and Rho kinase-1. J Biol Chem. 2006;281:7317–7323. doi: 10.1074/jbc.M513769200. [DOI] [PubMed] [Google Scholar]