Abstract
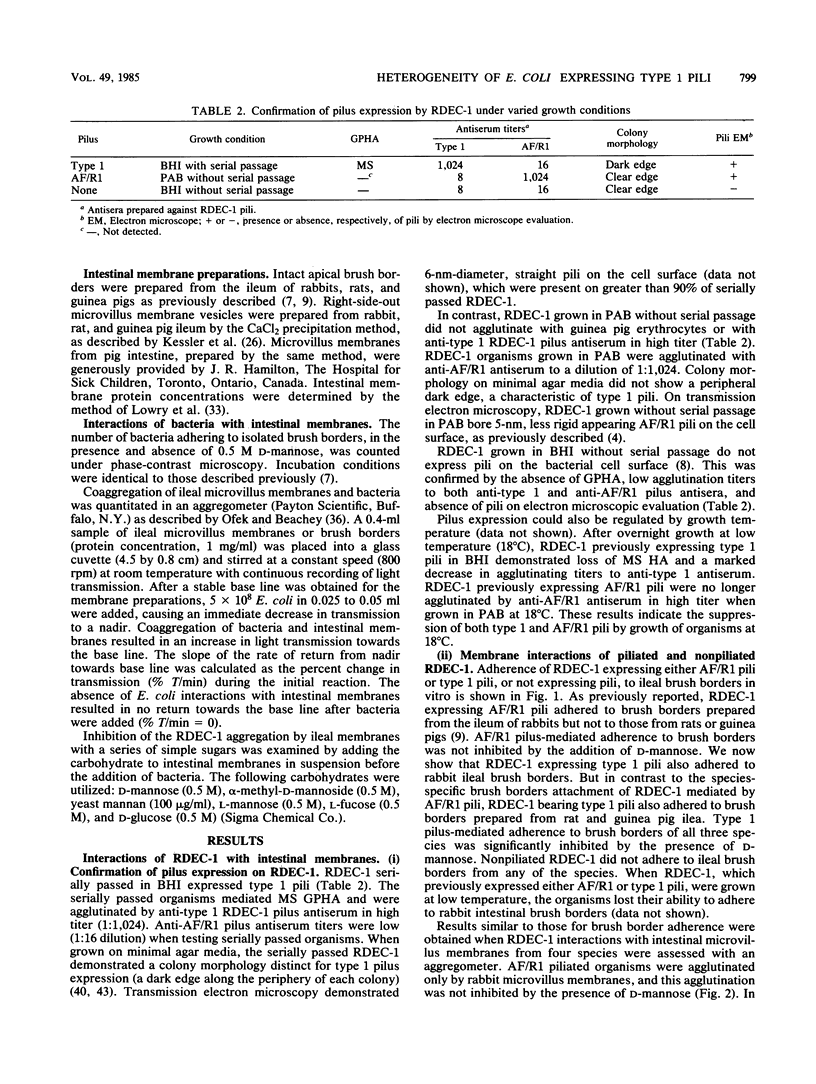
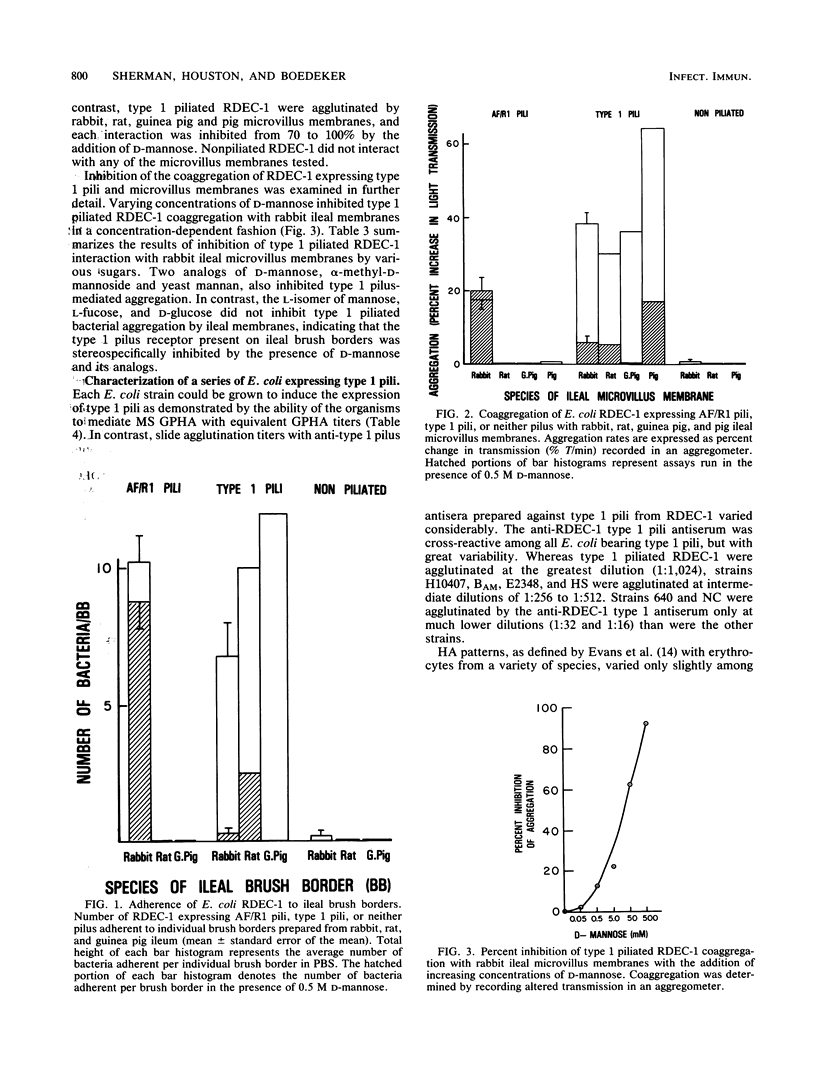
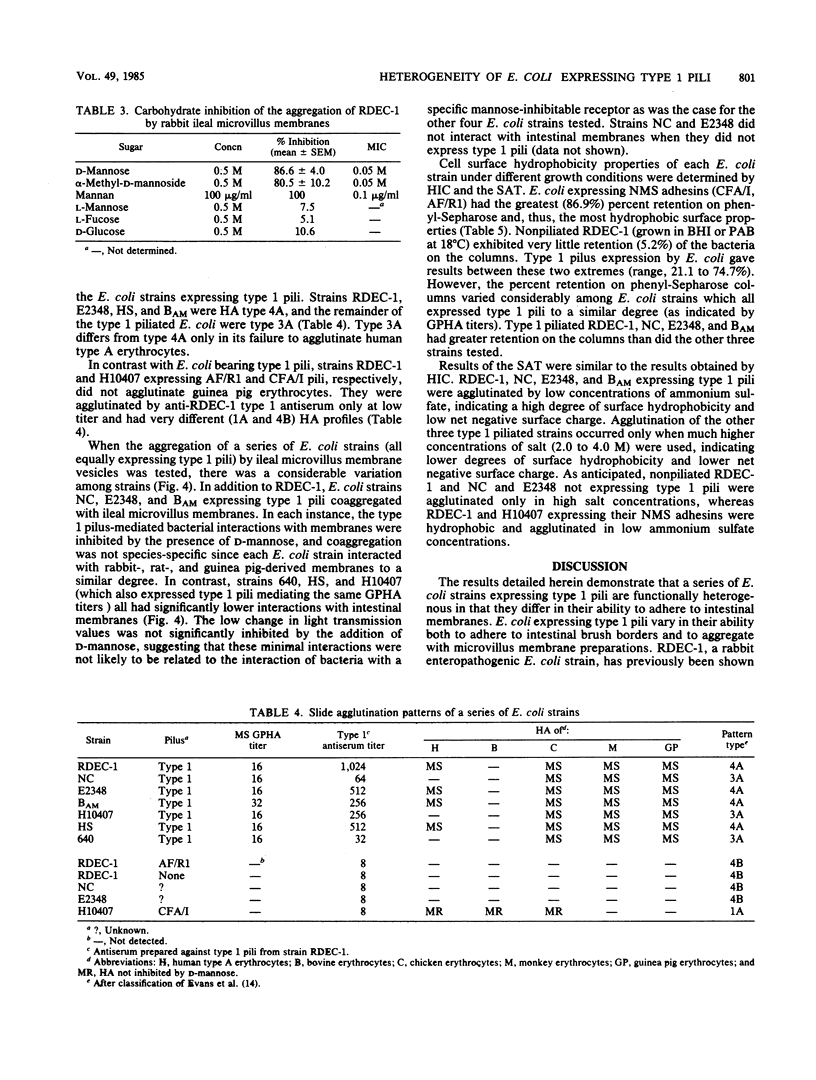
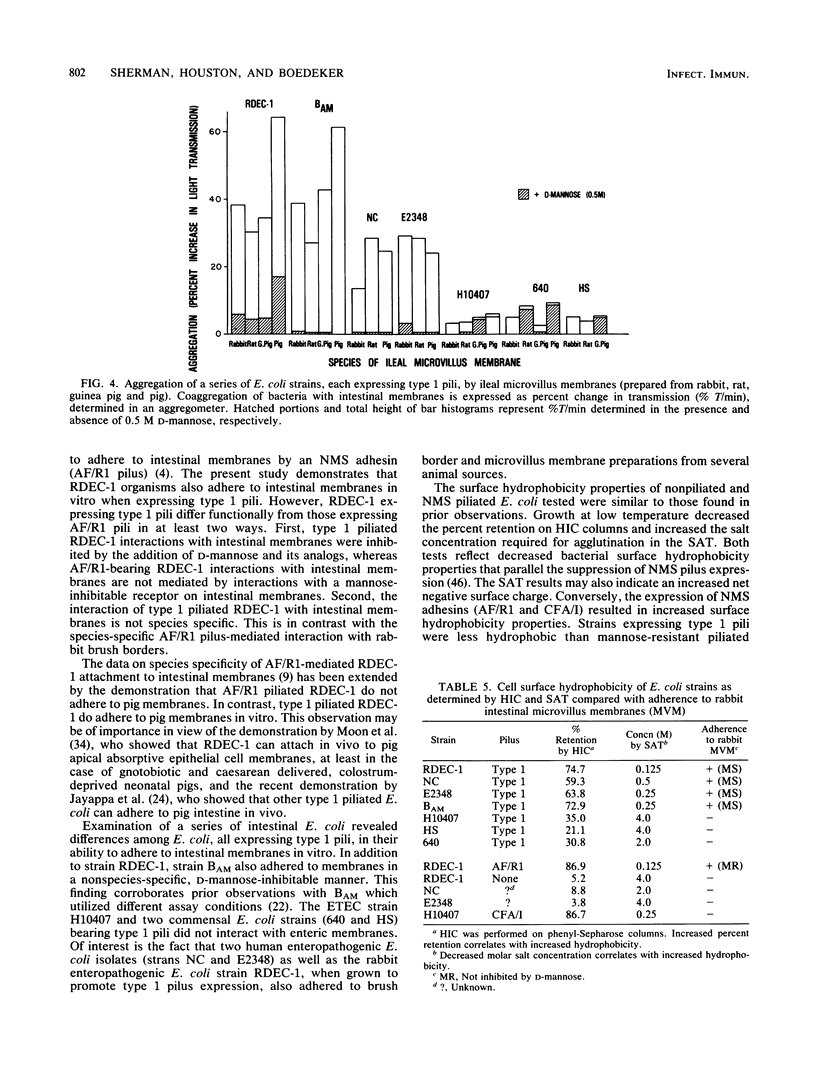
Although the role of host-specific, nonmannose-sensitive pilus adhesins in the intestinal adherence of pathogenic Escherichia coli is well established, a similar role for mannose-sensitive type 1 or common pili is less clear, since these structures can be expressed by most E. coli, even nonpathogens. We first examined whether type 1 pili, expressed by the rabbit-effacing, adherent, enteropathogenic E. coli strain RDEC-1, mediated interactions with intestinal membranes of several species and compared these interactions with those mediated by the nonmannose-sensitive adhesin of RDEC-1. We next grew a series of E. coli intestinal strains in static broth to promote type 1 pilus expression and determined whether E. coli expressing type 1 pili differed in their affinity for intestinal membranes (as measured by phase-contrast microscopy and aggregometry), hydrophobic surface properties, net negative surface charge (as measured by hydrophobic interaction chromatography and salt aggregation), and hemagglutination patterns. In contrast to the species-specific attachment to rabbit brush borders of RDEC-1 expressing its nonmannose-sensitive adhesin, type 1 pili on RDEC-1 mediated mannose-sensitive attachment to intestinal membranes of all four species tested. Expression of type 1 pili on other E. coli strains resulted in varying degrees of nonspecies-specific, mannose-sensitive attachment to intestinal membranes. This attachment correlated with increasing surface hydrophobicity rather than with hemagglutination patterns. These results indicate that various E. coli strains expressing type 1 pili are functionally heterogeneous and suggest that some type 1 pili might contribute to in vivo enteroadherence.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. J., Whitehead J. S., Kim Y. S. Interaction of Escherichia coli K88 antigen with porcine intestinal brush border membranes. Infect Immun. 1980 Sep;29(3):897–901. doi: 10.1128/iai.29.3.897-901.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad-Masalmeh M., Moon H. W., Runnels P. L., Schneider R. A. Pilus production, hemagglutination, and adhesion by porcine strains of enterotoxigenic Escherichia coli lacking K88, K99, and 987P antigens. Infect Immun. 1982 Jan;35(1):305–313. doi: 10.1128/iai.35.1.305-313.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldini M. M., Kaper J. B., Levine M. M., Candy D. C., Moon H. W. Plasmid-mediated adhesion in enteropathogenic Escherichia coli. J Pediatr Gastroenterol Nutr. 1983;2(3):534–538. doi: 10.1097/00005176-198302030-00023. [DOI] [PubMed] [Google Scholar]
- Berendson R., Cheney C. P., Schad P. A., Boedeker E. C. Species-specific binding of purified pili (AF/R1) from the Escherichia coli RDEC-1 to rabbit intestinal mucosa. Gastroenterology. 1983 Oct;85(4):837–845. [PubMed] [Google Scholar]
- Cheney C. P., Boedeker E. C. Adherence of an enterotoxigenic Escherichia coli strain, serotype O78:H11, to purified human intestinal brush borders. Infect Immun. 1983 Mar;39(3):1280–1284. doi: 10.1128/iai.39.3.1280-1284.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney C. P., Boedeker E. C., Formal S. B. Quantitation of the adherence of an enteropathogenic Escherichia coli to isolated rabbit intestinal brush borders. Infect Immun. 1979 Nov;26(2):736–743. doi: 10.1128/iai.26.2.736-743.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney C. P., Formal S. B., Schad P. A., Boedeker E. C. Genetic transfer of a mucosal adherence factor (R1) from an enteropathogenic Escherichia coli strain into a Shigella flexneri strain and the phenotypic suppression of this adherence factor. J Infect Dis. 1983 Apr;147(4):711–723. doi: 10.1093/infdis/147.4.711. [DOI] [PubMed] [Google Scholar]
- Cheney C. P., Schad P. A., Formal S. B., Boedeker E. C. Species specificity of in vitro Escherichia coli adherence to host intestinal cell membranes and its correlation with in vivo colonization and infectivity. Infect Immun. 1980 Jun;28(3):1019–1027. doi: 10.1128/iai.28.3.1019-1027.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneke C. F., Thorne G. M., Gorbach S. L. Attachment pili from enterotoxigenic Escherichia coli pathogenic for humans. Infect Immun. 1979 Oct;26(1):362–368. doi: 10.1128/iai.26.1.362-368.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr, Clegg S., Pauley J. A. Purification and characterization of the CFA/I antigen of enterotoxigenic Escherichia coli. Infect Immun. 1979 Aug;25(2):738–748. doi: 10.1128/iai.25.2.738-748.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr New surface-associated heat-labile colonization factor antigen (CFA/II) produced by enterotoxigenic Escherichia coli of serogroups O6 and O8. Infect Immun. 1978 Aug;21(2):638–647. doi: 10.1128/iai.21.2.638-647.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. J., Jr, Evans D. G., DuPont H. L. Hemagglutination patterns of enterotoxigenic and enteropathogenic Escherichia coli determined with human, bovine, chicken, and guinea pig erythrocytes in the presence and absence of mannose. Infect Immun. 1979 Feb;23(2):336–346. doi: 10.1128/iai.23.2.336-346.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. J., Jr, Evans D. G., Young L. S., Pitt J. Hemagglutination typing of Escherichia coli: definition of seven hemagglutination types. J Clin Microbiol. 1980 Aug;12(2):235–242. doi: 10.1128/jcm.12.2.235-242.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firon N., Ofek I., Sharon N. Carbohydrate-binding sites of the mannose-specific fimbrial lectins of enterobacteria. Infect Immun. 1984 Mar;43(3):1088–1090. doi: 10.1128/iai.43.3.1088-1090.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman L. R., Cantey J. R. Peyer's patch lymphoid follicle epithelial adherence of a rabbit enteropathogenic Escherichia coli (strain RDEC-1). Role of plasmid-mediated pili in initial adherence. J Clin Invest. 1984 Jul;74(1):90–95. doi: 10.1172/JCI111423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson R. E., Fusco P. C., Brinton C. C., Moon H. W. In vitro adhesion of Escherichia coli to porcine small intestinal epithelial cells: pili as adhesive factors. Infect Immun. 1978 Aug;21(2):392–397. doi: 10.1128/iai.21.2.392-397.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson R. E. K99 surface antigen of Escherichia coli: purification and partial characterization. Infect Immun. 1977 Jan;15(1):272–279. doi: 10.1128/iai.15.1.272-279.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jann K., Schmidt G., Blumenstock E., Vosbeck K. Escherichia coli adhesion to Saccharomyces cerevisiae and mammalian cells: role of piliation and surface hydrophobicity. Infect Immun. 1981 May;32(2):484–489. doi: 10.1128/iai.32.2.484-489.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayappa H. G., Goodnow R. A., Geary S. J. Role of Escherichia coli type 1 pilus in colonization of porcine ileum and its protective nature as a vaccine antigen in controlling colibacillosis. Infect Immun. 1985 May;48(2):350–354. doi: 10.1128/iai.48.2.350-354.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Rutter J. M. Role of the K88 antigen in the pathogenesis of neonatal diarrhea caused by Escherichia coli in piglets. Infect Immun. 1972 Dec;6(6):918–927. doi: 10.1128/iai.6.6.918-927.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler M., Acuto O., Storelli C., Murer H., Müller M., Semenza G. A modified procedure for the rapid preparation of efficiently transporting vesicles from small intestinal brush border membranes. Their use in investigating some properties of D-glucose and choline transport systems. Biochim Biophys Acta. 1978 Jan 4;506(1):136–154. doi: 10.1016/0005-2736(78)90440-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levine M. M., Black R. E., Brinton C. C., Jr, Clements M. L., Fusco P., Hughes T. P., O'Donnell S., Robins-Browne R., Wood S., Young C. R. Reactogenicity, immunogenicity and efficacy studies of Escherichia coli type 1 somatic pili parenteral vaccine in man. Scand J Infect Dis Suppl. 1982;33:83–95. [PubMed] [Google Scholar]
- Levine M. M., Kaper J. B., Black R. E., Clements M. L. New knowledge on pathogenesis of bacterial enteric infections as applied to vaccine development. Microbiol Rev. 1983 Dec;47(4):510–550. doi: 10.1128/mr.47.4.510-550.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M., Ristaino P., Marley G., Smyth C., Knutton S., Boedeker E., Black R., Young C., Clements M. L., Cheney C. Coli surface antigens 1 and 3 of colonization factor antigen II-positive enterotoxigenic Escherichia coli: morphology, purification, and immune responses in humans. Infect Immun. 1984 May;44(2):409–420. doi: 10.1128/iai.44.2.409-420.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M., Ristaino P., Sack R. B., Kaper J. B., Orskov F., Orskov I. Colonization factor antigens I and II and type 1 somatic pili in enterotoxigenic Escherichia coli: relation to enterotoxin type. Infect Immun. 1983 Feb;39(2):889–897. doi: 10.1128/iai.39.2.889-897.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl M., Faris A., Wadström T., Hjertén S. A new test based on 'salting out' to measure relative surface hydrophobicity of bacterial cells. Biochim Biophys Acta. 1981 Nov 5;677(3-4):471–476. doi: 10.1016/0304-4165(81)90261-0. [DOI] [PubMed] [Google Scholar]
- Moon H. W., Whipp S. C., Argenzio R. A., Levine M. M., Giannella R. A. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect Immun. 1983 Sep;41(3):1340–1351. doi: 10.1128/iai.41.3.1340-1351.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J. A., Thorns C., Scott A. C., Sojka W. J., Wells G. A. Adhesion in vitro and in vivo associated with an adhesive antigen (F41) produced by a K99 mutant of the reference strain Escherichia coli B41. Infect Immun. 1982 Jun;36(3):1146–1153. doi: 10.1128/iai.36.3.1146-1153.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hanley P. D., Cantey J. R. Surface structures of Escherichia coli that produce diarrhea by a variety of enteropathic mechanisms. Infect Immun. 1978 Sep;21(3):874–878. doi: 10.1128/iai.21.3.874-878.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H. Bacterial adherence. Adv Intern Med. 1980;25:503–532. [PubMed] [Google Scholar]
- Ofek I., Beachey E. H. Mannose binding and epithelial cell adherence of Escherichia coli. Infect Immun. 1978 Oct;22(1):247–254. doi: 10.1128/iai.22.1.247-254.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Goldhar J., Eshdat Y., Sharon N. The importance of mannose specific adhesins (lectins) in infections caused by Escherichia coli. Scand J Infect Dis Suppl. 1982;33:61–67. [PubMed] [Google Scholar]
- Ofek I., Mirelman D., Sharon N. Adherence of Escherichia coli to human mucosal cells mediated by mannose receptors. Nature. 1977 Feb 17;265(5595):623–625. doi: 10.1038/265623a0. [DOI] [PubMed] [Google Scholar]
- Old D. C., Duguid J. P. Selective outgrowth of fimbriate bacteria in static liquid medium. J Bacteriol. 1970 Aug;103(2):447–456. doi: 10.1128/jb.103.2.447-456.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salit I. E., Gotschlich E. C. Type I Escherichia coli pili: characterization of binding to monkey kidney cells. J Exp Med. 1977 Nov 1;146(5):1182–1194. doi: 10.1084/jem.146.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaney L. M., Liu Y. P., To C. M., To C. C., Ippen-Ihler K., Brinton C. C., Jr Isolation and characterization of Escherichia coli phase variants and mutants deficient in type 1 pilus production. J Bacteriol. 1977 Apr;130(1):495–505. doi: 10.1128/jb.130.1.495-505.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To S. C., Moon H. W., Runnels P. L. Type 1 pili (F1) of porcine enterotoxigenic Escherichia coli: vaccine trial and tests for production in the small intestine during disease. Infect Immun. 1984 Jan;43(1):1–5. doi: 10.1128/iai.43.1.1-5.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulshen M. H., Rollo J. L. Pathogenesis of escherichia coli gastroenteritis in man--another mechanism. N Engl J Med. 1980 Jan 10;302(2):99–101. doi: 10.1056/NEJM198001103020207. [DOI] [PubMed] [Google Scholar]
- Wadström T., Faris A., Freer J., Habte D., Hallberg D., Ljungh A. Hydrophobic surface properties of enterotoxigenic E. coli (ETEC) with different colonization factors (CFA/i, CFA/ii, K88 and K99) and attachment to intestinal epithelial cells. Scand J Infect Dis Suppl. 1980;Suppl 24:148–153. [PubMed] [Google Scholar]


