Abstract
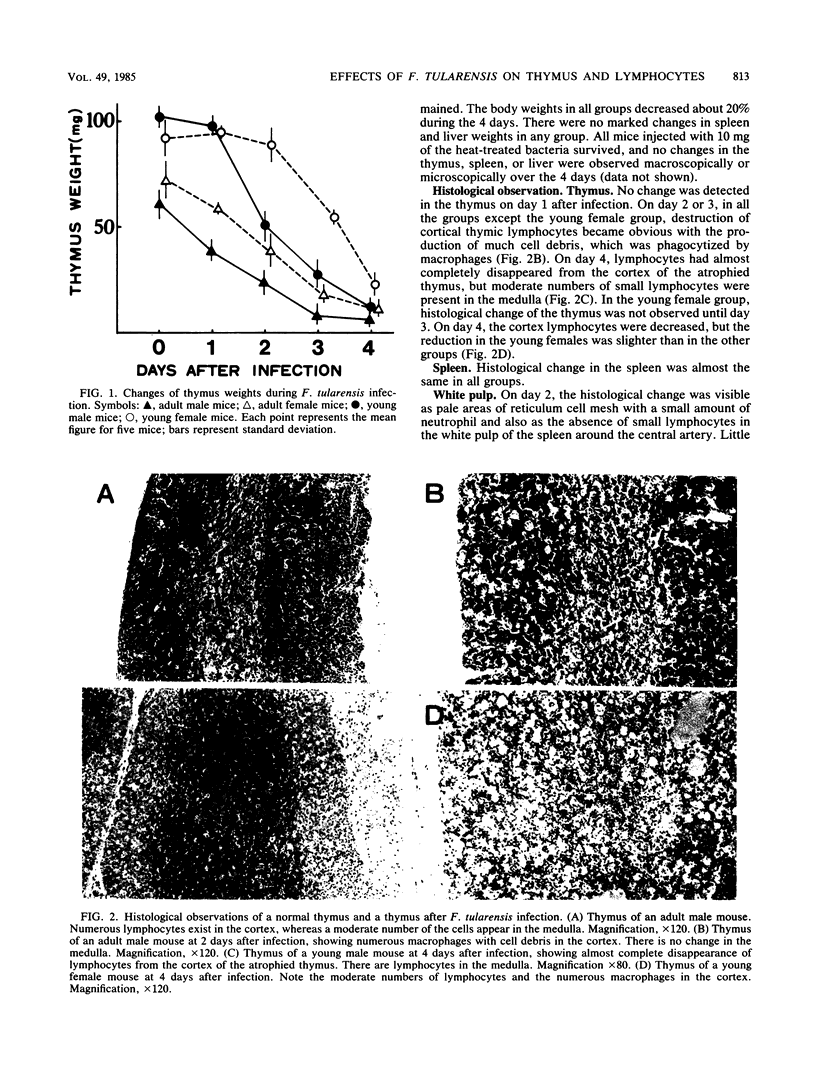
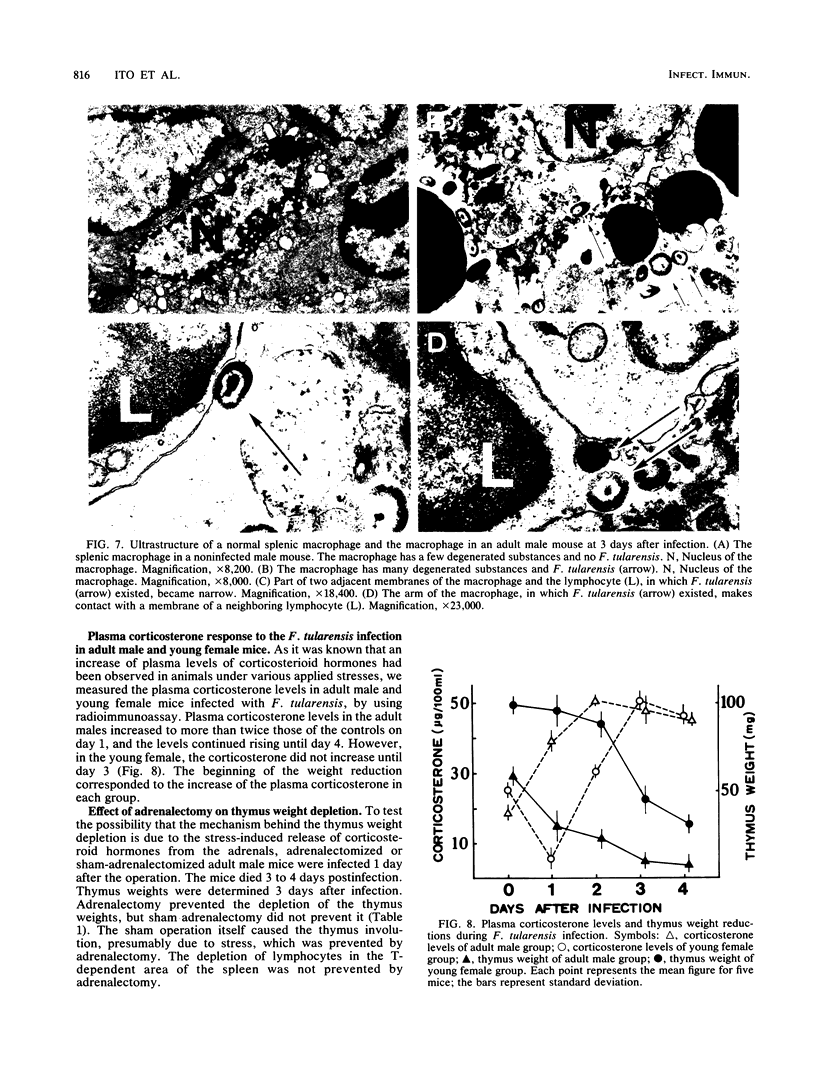
When BALB/c mice (young and adult animals of both sexes) were infected intraperitoneally with 10(3) viable cells of Francisella tularensis (10(2) 50% lethal dose), all mice in these groups died on day 4. Reductions in thymus weights and in numbers of thymic cortex lymphocytes were observed in all the groups, but the decline was not so severe in the young females. Increases of plasma corticosterone in the adult males began 1 day after infection, but in the young females, the levels did not increase until day 3, the same days on which the respective thymus weights began to decline. Depletion of the thymus weights in the infected mice was prevented by adrenalectomy. The lymphocytes of the thymus (T)-dependent areas in peripheral lymphoid tissues in all groups were destroyed. By using an electron microscope, we found a large quantity of F. tularensis within the macrophages in the T-dependent areas but not in the thymus. The destruction of lymphocytes in the T-dependent areas was not prevented by adrenalectomy. Therefore, it was concluded that the weight reduction of the thymus is due to the stress of the F. tularensis infection. However, we think other mechanisms are responsible for the depression of lymphocytes in the T-dependent areas of peripheral lymphoid tissues.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali-Khan Z. Cellular changes in the lymphoreticular tissues of C57L/J mice infected with Echinococcus multilocularis cysts. Immunology. 1978 May;34(5):831–839. [PMC free article] [PubMed] [Google Scholar]
- Bolton P. M., Kirov S. M., Donald K. J. The effects of major and minor trauma on lymphocyte kinetics in mice. Aust J Exp Biol Med Sci. 1979 Oct;57(5):479–492. doi: 10.1038/icb.1979.49. [DOI] [PubMed] [Google Scholar]
- Durkin H. G., Thorbecke G. J. Preferential destruction of germinal centers by prednisolone and x-irradiation. Lab Invest. 1972 Jan;26(1):53–62. [PubMed] [Google Scholar]
- Gross H. A., Ruder H. J., Brown K. S., Lipsett M. B. A radioimmunoassay for plasma corticosterone. Steroids. 1972 Dec;20(6):681–695. doi: 10.1016/0039-128x(72)90051-7. [DOI] [PubMed] [Google Scholar]
- HUZIWARA T., WATANABE Y., HYODO S., KONNO K. [In vitro phagocytosis of Pasteurella tularensis by guinea pig macrophages. Variations in phagocytic responses of macrophages dependent upon the nature of the irritants used]. C R Seances Soc Biol Fil. 1962;156:191–195. [PubMed] [Google Scholar]
- Hanaoka M., Suzuki S., Hotchin J. Thymus-dependent lymphocytes: destruction by lymphocytic choriomeningitis virus. Science. 1969 Mar 14;163(3872):1216–1219. doi: 10.1126/science.163.3872.1216. [DOI] [PubMed] [Google Scholar]
- Lundin P. M., Schelin U. The effect of steroids on the histology and ultrastructure of lymphoid tissue. 3. Thymus in prolonged steroid induced involution. Pathol Eur. 1969;4(1):58–68. [PubMed] [Google Scholar]
- MCELREE H., DOWNS C. M. The phagocytosis of Pasteurella tularensis by rat mononuclear cells as influenced by normal serums and various irritants. J Infect Dis. 1961 Jul-Aug;109:98–106. doi: 10.1093/infdis/109.1.98. [DOI] [PubMed] [Google Scholar]
- Mandel T. E., Cheers C. Resistance and susceptibility of mice to bacterial infection: histopathology of listeriosis in resistant and susceptible strains. Infect Immun. 1980 Dec;30(3):851–861. doi: 10.1128/iai.30.3.851-861.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OHARA S., SATO M., SATO T. On pig-liver medium and a modified medium for cultivation of Bacterium tularense. Tohoku J Exp Med. 1953;58(2):185–190. doi: 10.1620/tjem.58.185. [DOI] [PubMed] [Google Scholar]
- Parrott D. M., Ferguson A. Selective migration of lymphocytes within the mouse small intestine. Immunology. 1974 Mar;26(3):571–588. [PMC free article] [PubMed] [Google Scholar]
- Parrott D. V., De Sousa M. A., East J. Thymus-dependent areas in the lymphoid organs of neonatally thymectomized mice. J Exp Med. 1966 Jan 1;123(1):191–204. doi: 10.1084/jem.123.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptak W., Gaugas J. M., Rees R. J., Allison A. C. Immune responses in mice with murine leprosy. Clin Exp Immunol. 1970 Jan;6(1):117–124. [PMC free article] [PubMed] [Google Scholar]
- Public Health Weekly Reports for JUNE 22, 1923. Public Health Rep. 1923 Jun 22;38(25):1391–1447. [PMC free article] [PubMed] [Google Scholar]
- RINGERTZ N., FAGRAEUS A., BERGLUND K. On the action of cortisone on the thymus and lymph nodes in mice. Acta Pathol Microbiol Scand Suppl. 1952 Jun;93:44–51. [PubMed] [Google Scholar]
- Veress B., Omer A., Satir A. A., El Hassan A. M. Morphology of the spleen and lymph nodes in fatal visceral leishmaniasis. Immunology. 1977 Nov;33(5):605–610. [PMC free article] [PubMed] [Google Scholar]
- WEAVER J. A. Changes induced in the thymus and lymph nodes of the rat by the administration of cortisone and sex hormones and by other procedures. J Pathol Bacteriol. 1955 Jan-Apr;69(1-2):133–139. doi: 10.1002/path.1700690119. [DOI] [PubMed] [Google Scholar]








