Abstract
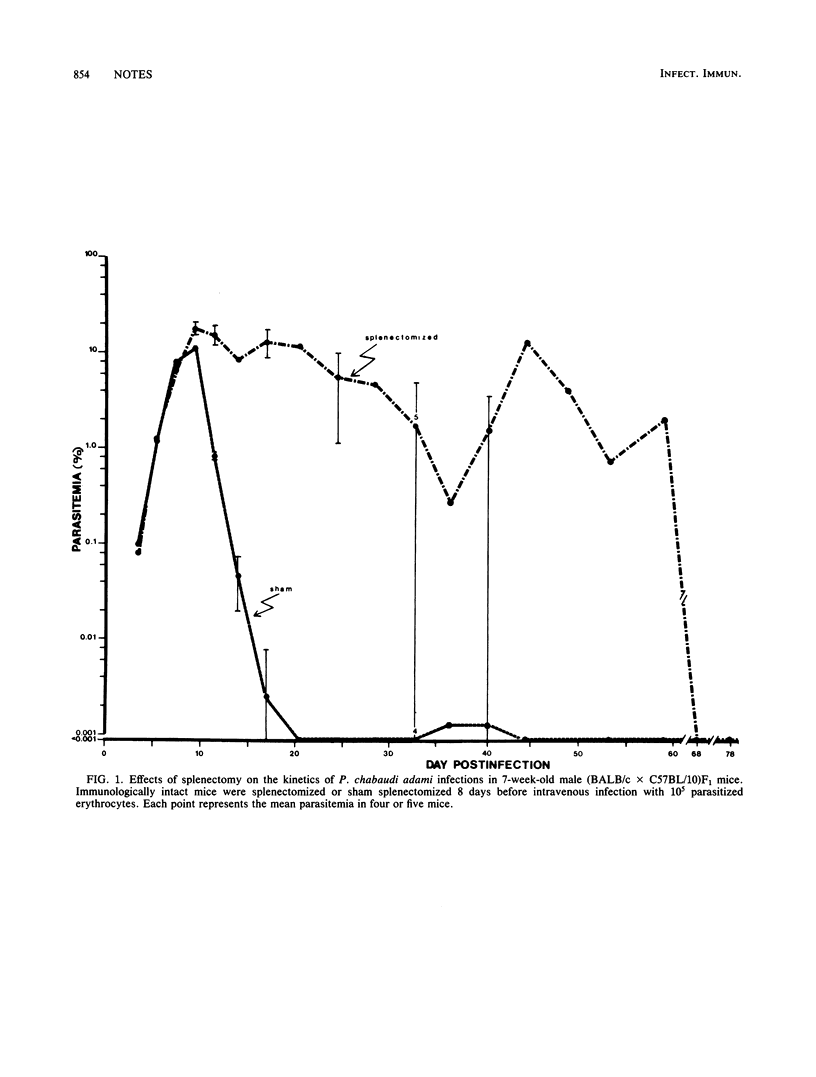
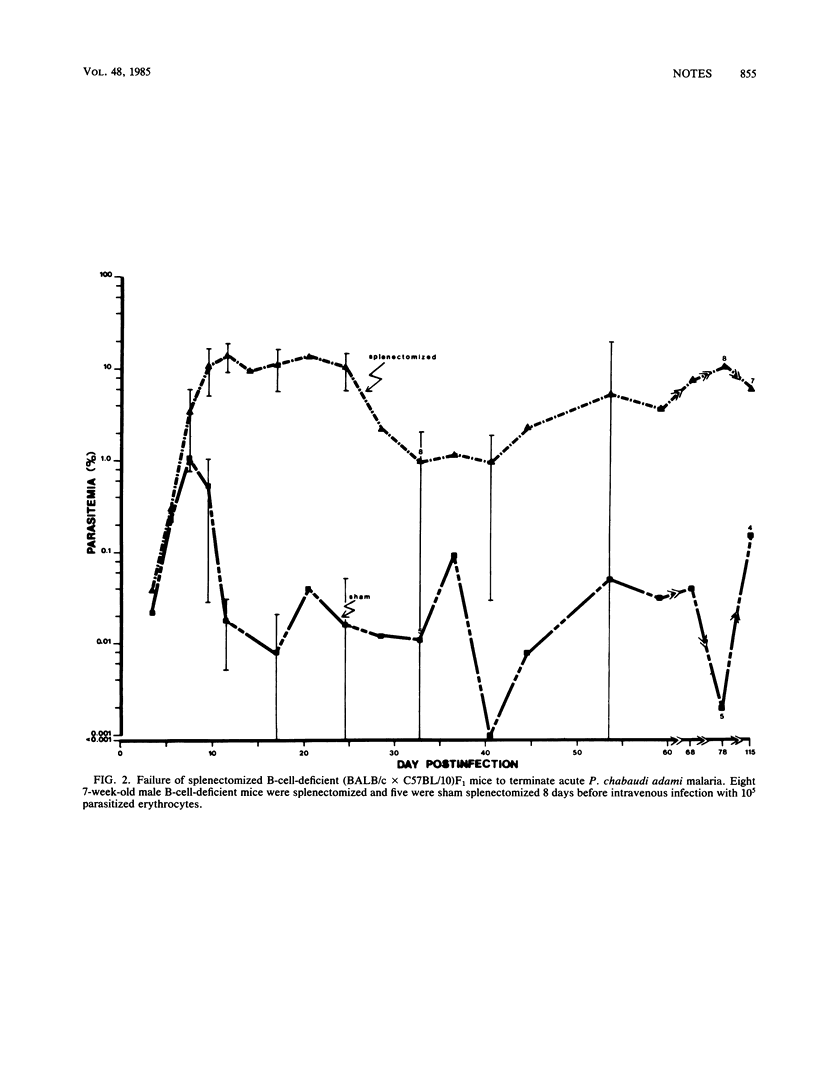
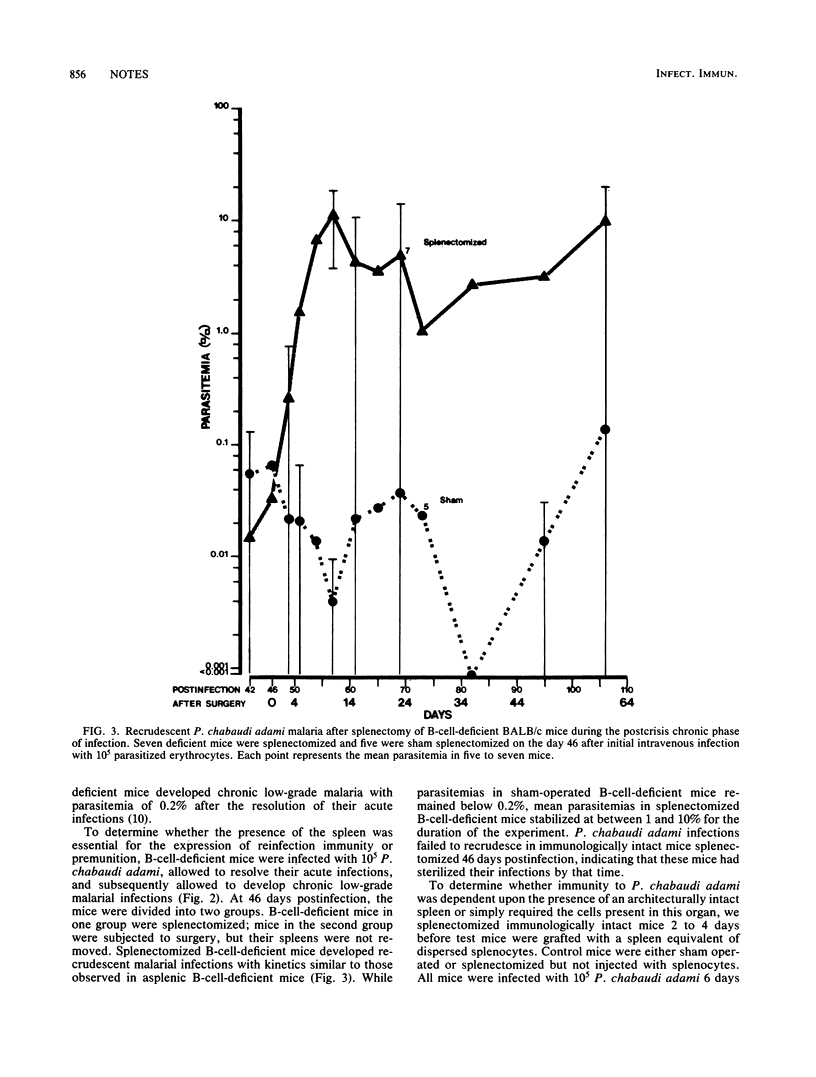
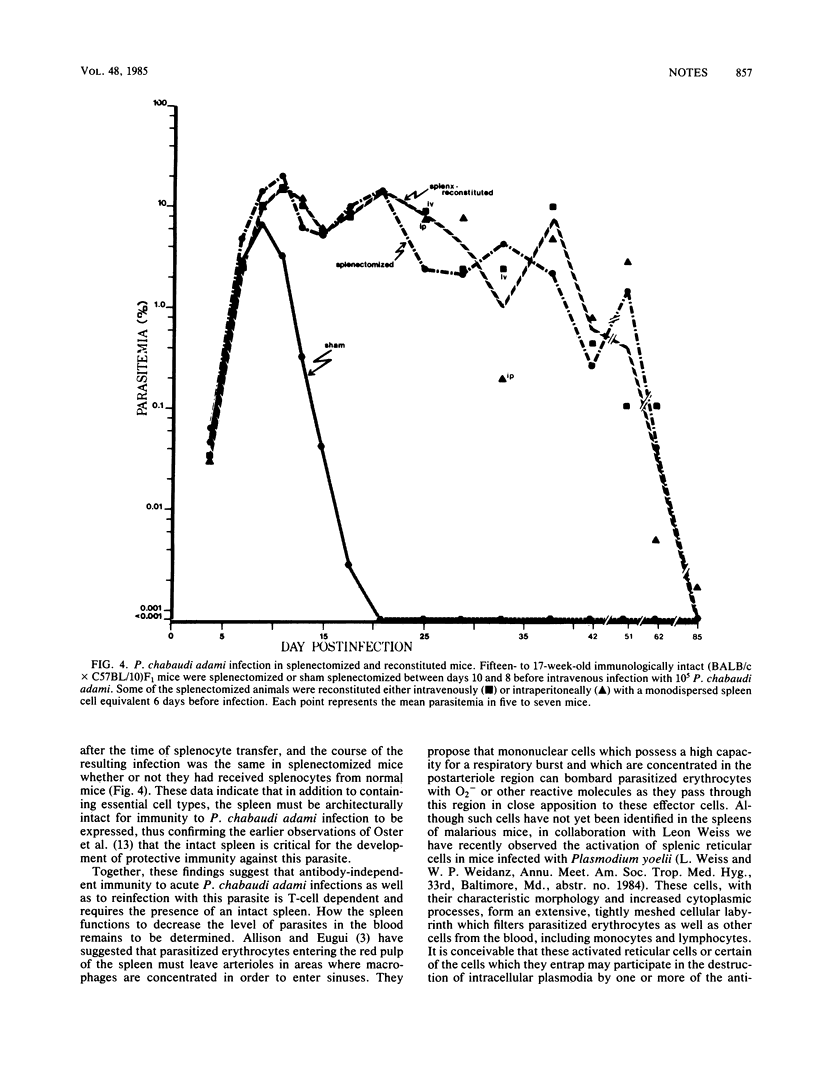
Splenectomy of B-cell-deficient mice and immunologically intact mice before infection with Plasmodium chabaudi adami led to the development of significant parasitemias which eventually resolved in the latter mice. Whereas both eusplenic B-cell-deficient mice and immunologically intact mice resolved their acute P. chabaudi adami infection, only B-cell-deficient mice subsequently developed chronic low-grade malaria. Splenectomy of B-cell-deficient mice with chronic malaria led to recrudescing infections, suggesting that the expression of antibody-independent immunity to reinfection was spleen dependent. When dispersed spleen cells were injected into splenectomized mice before challenge with P. chabaudi adami, the kinetics of the resulting infection resembled that seen in splenectomized mice which had not been grafted with normal spleen cells. This finding indicates that immunity to P. chabaudi adami requires the presence of an architecturally intact spleen.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C. Cellular immunity to malaria and babesia parasites: a personal viewpoint. Contemp Top Immunobiol. 1984;12:463–490. doi: 10.1007/978-1-4684-4571-8_12. [DOI] [PubMed] [Google Scholar]
- Allison A. C., Eugui E. M. A radical interpretation of immunity to malaria parasites. Lancet. 1982 Dec 25;2(8313):1431–1433. doi: 10.1016/s0140-6736(82)91330-7. [DOI] [PubMed] [Google Scholar]
- Allison A. C., Eugui E. M. The role of cell-mediated immune responses in resistance to malaria, with special reference to oxidant stress. Annu Rev Immunol. 1983;1:361–392. doi: 10.1146/annurev.iy.01.040183.002045. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Allison A. C., Cox F. E. Protection of mice against Babesia and Plasmodium with BCG. Nature. 1976 Jan 29;259(5541):309–311. doi: 10.1038/259309a0. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Cox F. E., Allison A. C. Protection of mice against Babesia spp. and Plasmodium spp. with killed Corynebacterium parvum. Parasitology. 1977 Feb;74(1):9–18. doi: 10.1017/s003118200004748x. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Hunt N. H. Evidence for reactive oxygen intermediates causing hemolysis and parasite death in malaria. Infect Immun. 1983 Jan;39(1):1–6. doi: 10.1128/iai.39.1.1-6.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark I. A. Protection of mice against Babesia microti with cord factor, COAM, zymosan, glucan, Salmonella and Listeria. Parasite Immunol. 1979 Autumn;1(3):179–196. doi: 10.1111/j.1365-3024.1979.tb00705.x. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Virelizier J. L., Carswell E. A., Wood P. R. Possible importance of macrophage-derived mediators in acute malaria. Infect Immun. 1981 Jun;32(3):1058–1066. doi: 10.1128/iai.32.3.1058-1066.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eling W. M. Chronic, patent Plasmodium berghei malaria in splenectomized mice. Infect Immun. 1982 Mar;35(3):880–886. doi: 10.1128/iai.35.3.880-886.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grun J. L., Weidanz W. P. Antibody-independent immunity to reinfection malaria in B-cell-deficient mice. Infect Immun. 1983 Sep;41(3):1197–1204. doi: 10.1128/iai.41.3.1197-1204.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grun J. L., Weidanz W. P. Immunity to Plasmodium chabaudi adami in the B-cell-deficient mouse. Nature. 1981 Mar 12;290(5802):143–145. doi: 10.1038/290143a0. [DOI] [PubMed] [Google Scholar]
- Jensen J. B., Boland M. T., Allan J. S., Carlin J. M., Vande Waa J. A., Divo A. A., Akood M. A. Association between human serum-induced crisis forms in cultured Plasmodium falciparum and clinical immunity to malaria in Sudan. Infect Immun. 1983 Sep;41(3):1302–1311. doi: 10.1128/iai.41.3.1302-1311.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oster C. N., Koontz L. C., Wyler D. J. Malaria in asplenic mice: effects of splenectomy, congenital asplenia, and splenic reconstitution on the course of infection. Am J Trop Med Hyg. 1980 Nov;29(6):1138–1142. doi: 10.4269/ajtmh.1980.29.1138. [DOI] [PubMed] [Google Scholar]
- Rank R. G., Weidanz W. P., Bondi A. Nonsterilizing immunity in avian malaria: an antibody-independent phenomenon. Proc Soc Exp Biol Med. 1976 Feb;151(2):257–259. doi: 10.3181/00379727-151-39186. [DOI] [PubMed] [Google Scholar]
- Roberts D. W., Weidanz W. P. T-cell immunity to malaria in the B-cell deficient mouse. Am J Trop Med Hyg. 1979 Jan;28(1):1–3. doi: 10.4269/ajtmh.1979.28.1. [DOI] [PubMed] [Google Scholar]
- Taverne J., Dockrell H. M., Playfair J. H. Endotoxin-induced serum factor kills malarial parasites in vitro. Infect Immun. 1981 Jul;33(1):83–89. doi: 10.1128/iai.33.1.83-89.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidanz W. P., Grun J. L. Antibody-independent mechanisms in the development of acquired immunity to malaria. Adv Exp Med Biol. 1983;162:409–423. doi: 10.1007/978-1-4684-4481-0_37. [DOI] [PubMed] [Google Scholar]


