Abstract
Objective
The aim of this study was to determine if significant differences in plaque composition exist between the popliteal and tibial vessels in patients with severe peripheral arterial disease (PAD).
Methods
Forty-four patients with PAD required either above knee (n=38), below knee (n=5) or through knee (n=1) amputation for pedal sepsis/gangrene. The fifty-one vessels (anterior tibial, n=9; posterior tibial, n=10; peroneal, n=3; popliteal, n=29) were obtained, and underwent intravascular ultrasound (IVUS) evaluation ex vivo within 24 hours of amputation. Sequential IVUS data were obtained at known intervals throughout the vessel length, and then analyzed with radiofrequency techniques for quantification of plaque composition, plaque volume, and total vessel volume. Plaque composition was categorized as fibrous, fibro-fatty, necrotic core, and dense calcium. Clinical data was obtained via review of electronic records at the time of amputation. Two-sided t-tests were performed to compare components within each plaque. Results are expressed as mean percentage±SEM.
Results
Tibial vessels had more dense calcium within these plaques than popliteal arteries (33.8±5.6% v. 10.6±1.9%, P<0.001).
Consequently, distal vessels had less fibro-fatty and fibrous plaque than popliteal arteries (7.7±1.4% v. 13.1±1.2%, P<0.005; 42.4±4.7% v. 61.4±2.2%, P<0.001), respectively. Necrotic core plaque composition was found to be similar when comparing tibial verses popliteal arteries (16.1% vs. 14.9%, p = NS). Clinical factors including diabetes, hyperlipidemia and chronic renal insufficiency were not associated with plaque composition differences using a univariate analysis.
Conclusions
As we progress distally in the arterial tree of patients with PAD, calcium plaque content increases with decreasing burden of fibro-fatty plaque. Clinical and demographic factors with the exception of smoking were not found to be associated with atherosclerotic plaque composition.
Introduction
Vascular calcification is known to exist in various arterial beds throughout the circulatory system. Its presence is often associated with atherosclerosis, a chronic inflammatory disease, with its extent associated with overall burden of atherosclerosis1. Clinical factors such as diabetes are known to influence calcification2 which is a known marker of future cardiovascular events3.
Although calcification and atherosclerosis are widely known to coexist in various vessels, differences in plaque composition within the arterial tree are not fully understood. In their practice, peripheral vascular clinicians have hypothesized that there may be notable differences between arteries located above and below the knee. A recent published work4 by our group led to this hypothesis based on a number of histology slides were evaluated from both popliteal and tibial arteries.
Surface ultrasound, magnetic resonance imaging (MRI) and computed tomography (CT) poorly resolve the components and structure of the vascular wall, at least in their present state of evolution. Intravascular ultrasound (IVUS) can provide important structural information about the arterial wall. In the past several years, advances that include three-dimensional imaging reconstruction and analysis methods have made significant progress toward routine quantitative IVUS analysis5. IVUS can potentially provide atherosclerotic plaque composition information that is well correlated with histological data6, 7.
Methods
From 2004–2007 124 patients underwent a mid-calf level or higher below-knee or above-knee amputation for ongoing critical ischemia, overwhelming tissue loss, ascending gangrene or pedal sepsis precluding limb salvage. During this time frame, over 800 patients per year underwent lower extremity revascularization. Patients undergoing amputation represented approximately 4% of the overall patient population with severe PAD.
Available arterial tissue was evaluated for extent of PAD and prior surgical intervention. All patients in this study suffered from chronic severe PAD. Severely stenosed or occluded segments not permitting passage of an IVUS catheter (n=41) and those with evident surgical interventions (n=5) were excluded from analysis. 33 patients were excluded during post IVUS imaging analysis due to large vessel diameters placing a portion of the vessel outside the IVUS field of view, short available vessel segments, and/or data acquisition issues. This yielded specimens from 45 patients with 5 below-knee, 1 through-knee, and 39 above-knee amputations
At the time of amputation, an electronic review of available medical records was conducted to obtain clinical data including age, gender, diabetes, smoking, and renal function.
Human Arterial Analyses
Arteries from lower extremity amputations were harvested as part of an NIH sponsored study entitled “Clinical implications of peripheral plaque morphology” (R01 HL075721). These studies were approved by the Institutional Review Board.
Following amputation, arteries were harvested within a 24-hour period. Patent anterior tibial, posterior tibial and peroneal arteries were harvested from below-knee amputations. Additionally, patent popliteal arteries were harvested from above-knee amputations. Severely stenosed, occluded or previously intervened arterial segments were excluded from analysis.
Arteries were then placed in a computer controlled pressure perfusion system similar to a previously used described system for coronary vessels6. This system simulates in vivo conditions by perfusing vessels with PBS solution at 100mm HG and heated to 37C. Patent side branches were ligated to maintain pressure. A 6F sheath provided access to vessels for endovascular devices during experimentation.
Data Acquisition
All data was acquired using Volcano Corporation’s (Volcano Corp. Rancho Cordova, California) clinical In-Vision Gold IVUS console, 3.5F Eagle Eye phased array IVUS catheter and Trak Back II motorized pullback device. During IVUS imaging, the IVUS catheter was advanced to the most distal accessible segment of the vessel. Sequential IVUS data was then collected (one frame/second) during a motorized pullback at a rate of 0.5mm/s.
Raw sequential RF IVUS data was saved from the IVUS console on DVD’s and transferred to a PC workstation for later analysis. Grayscale images were also acquired and later assisted in the vessel contour definition process.
Data Analysis
Grayscale images were reconstructed from raw IVUS RF data on a Pentium IV PC using IVUS Lab software8. Contours defining the internal elastic lamina (IEL) and external elastic lamina (EEL) of the vessel were manually identified for each grayscale cross sectional image. Atherosclerotic plaque composition was then computed using Volcano’s latest VH-IVUS™ atherosclerotic tissue characterization algorithm, available as commercial software package labeled PCVH, yielding a colored tissue map and quantitative geometric and plaque composition information for each corresponding grayscale image. Atherosclerotic plaques were characterized as one of four types by the software: Fibrous, Fibro-Fatty, Necrotic Core, or Dense Calcium.
The output from the VH IVUS™ software yielded cross sectional area (CSA) information expressed in mm2 for each plaque throughout the length of the pullback. Plaque CSA data was provided not only for individual cross sections as well as the mean CSA over the length of the pullback. Volumes expressed in mm3 were derived using CSA data and known distances between cross sections based on the motorized pullback speed. Additionally, grayscale IVUS data including vessel, plaque plus media, and lumen CSA were provided in analysis results. Plaque burden was calculated as the plaque plus media CSA divided by vessel CSA.
An average rate of change in each of the four plaque type CSA over the length of the vessel was calculated by finding the differences between each adjacent cross section and then dividing by the distance between cross sections. The differences were then averaged and then expressed as mm2/cm.
Statistical analysis
Due to size and geometry variations between vessels, a direct comparison using geometric dimensions was not possible. This issue was solved by taking the total plaque volume and calculating the average percent composition of fibrous, fibro-fatty, necrotic core, and dense calcium over the entire vessel length. In addition, comparisons for age, diabetic status, smoking cessation, chronic renal failure, and hyperlipidemia were carried out comparing only popliteals with popliteals and tibials with tibials to remove vessel type as a potential variable.
The distribution of the demographics and clinical factors for the cohort was calculated using two sided t-tests to measure the association between demographic and clinical factors with plaque composition and atheroma burden. Results are shown as the Mean ± Standard Deviation. P values <0.05 were considered significant.
Results
An electronic review of available medical records for the 45 amputees found the mean age at amputation was 67.0 ± 13.5 years with 29 (64.4%) males and 16 (35.6%) females. The ethnicity of the group consisted of 20(44.4%) African-Americans and 25 (55.6%) Caucasians. 27 (60.0%) patients were diagnosed with non-insulin dependent diabetes mellitus (NIDDM) and 18 (40.0%) suffered from chronic renal insufficiency defined as a serum Cr > 1.5 mg/dL. Smoking documentation showed 13 (28.9%) individuals presently smoke, 12 (26.7%) quit, 14 (31.1%) never smoked, and 6 (13.3%) had no available data.
Popliteal arteries were found to have an increased mean plaque burden (Table 1) of 63.28±9.1% compared to tibial arteries at 52.6±8.0% (P<0.001). Clinical and demographic factors, with the exception of chronic renal failure, did not show any association with plaque burden although smoking, NIDDM and those aged greater than 60 years tended to have an increased plaque burden. Patients with chronic renal failure had a higher plaque burden in the tibial arteries (57.7 ± 5.1% v. 48.7 ± 8.8%, P= 0.02) with a similar trend in popliteal arteries although not significant (65.2±8.3% v. 62.3±9.5%, P=N.S.)
Table 1.
Comparison of plaque burden and composition between popliteal and tibial arteries. (n=45)
| Tibials | Popliteals | P-Value | |
|---|---|---|---|
| Plaque Burden | 52.6 ± 7.9 | 63.3 ± 9.1 | P<0.001 |
| Plaque Composition | |||
| Fibrous | 46.3 ± 19.7% | 59.3 ± 12.0%, | P=0.002 |
| Fibro-Fatty | 7.9 ± 6.6% | 13.3 ± 6.4% | P=0.002 |
| Necrotic Core | 18.7 ± 10.1 % | 15.6 ± 7.5% | P=N.S. |
| Dense Calcium | 27.2 ± 23.3% | 11.8 ± 10.1% | P<0.001 |
Atherosclerotic plaque composition within the tibial (posterior, anterior, and peroneal) arteries contained higher percentages of dense calcium than popliteal arteries. Dense calcium accounted for 27.2 ± 23.3% of plaque in the tibial arteries compared to only 11.8 ± 10.1% for popliteal arteries (P <0.001). In addition, tibial artery plaque was found to contain smaller percentages of fibrous (46.25 ± 19.7% v. 59.3 ± 12.0%, P=0.002) and fibro-fatty (7.85 ± 6.6% v. 13.3 ± 6.4%, P=0.002) plaque than popliteal arteries. No significance was noted for the percentage of necrotic core plaque between vessels (18.7±10.1 v. 15.6±7.5, P=N.S) (Table 1). 10 of the 45 patients had IVUS data collected for both a popliteal and a tibial artery allowing for direct comparison of arteries within each patient. This yielded similar results to the entire group. (Table 1a)
Table 1a.
Direct comparison of plaque burden and composition for patients with IVUS data collected on both a popliteal and a tibial artery. (n=10)
| Tibials | Popliteal | P-Value | |
|---|---|---|---|
| Plaque Burden | 52.1 ± 5.2 | 60.0 ± 7.2 | P=0.012 |
| Plaque Composition | |||
| Fibrous | 45.7 ± 19.7% | 60.0 ± 12.4%, | P=0.003 |
| Fibro-Fatty | 6.6 ± 5.3% | 12.2 ± 5.2% | P=0.037 |
| Necrotic Core | 21.9 ± 13.0% | 16.6 ± 8.9% | P=0.212 |
| Dense Calcium | 25.8±23.9% | 11.2±8.7% | P=0.022 |
Smoking was the only variable from the collected clinical and demographic factors that was associated with atherosclerotic plaque composition differences. (Table 2, Table 2a and Table 2b) Popliteal arteries in smokers had smaller percentages of fibro-fatty (6.9±3.9% v. 18.5±13.8%, P=0.019) and necrotic core plaques (11.4±5.5 v. 18.2±8.6, P=0.048). A higher composition was noted for fibro-fatty plaque in both popliteal (15.8±5.5% v. 9.0±6.0%, P=0.014) and tibial arteries (13.1±8.4% v. 5.3±4.9%, P=0.044).
Table 2.
Relation between clinical factors and plaque burden for popliteal and tibial arteries. Plaque burden is represented by Mean % ± Standard Deviation.
| Clinical Factor | Vessel | Present | Absent | P-Value |
|---|---|---|---|---|
| NIDDM | Popliteal | 65.3 ± 6.9 | 61.4 ± 10.8 | 0.192 |
| Tibials | 54.4 ± 7.3 | 47.1 ± 10.5 | 0.106 | |
| CRF | Popliteal | 65.2 ± 8.3 | 62.3 ± 9.5 | 0.344 |
| Tibials | 57.7 ± 5.1 | 48.7 ± 8.8 | 0.020 | |
| SMOKING | Popliteal | 65.6 ± 10.4 | 59.3 ± 8.1 | 0.137 |
| Tibials | 49.4 ± 11.6 | 52.2 ± 6.8 | 0.578 | |
| AGE>60 | Popliteal | 64.3 ± 8.7 | 61.9 ± 11.1 | 0.511 |
| Tibials | 51.4 ± 6.9 | 56.5 ± 10.0 | 0.176 | |
| Hyperlipidemia | Popliteal | 62.1 ± 7.8 | 64.9 ± 10.2 | 0.351 |
| Tibials | 51.9 ± 9.1 | 53.6 ± 6.5 | 0.610 | |
Table 2a.
Clinical factors: Associations with fibrous and fibro-fatty plaque compositions.
| Fibrous | Fibro Fatty | ||||||
|---|---|---|---|---|---|---|---|
| Clinical Factor | Present | Absent | P-Value | Present | Absent | P-Value | |
| NIDDM | Popliteal | 60.4±12.2 | 58.4±11.7 | 0.61 | 13.4±5.7 | 13.3±7.2 | 0.978 |
| Tibials | 43.1±4.8 | 54.0±2.0 | 0.321 | 6.1±1.6 | 12.6±1.2 | 0.067 | |
| CRF | Popliteal | 55.1±12.3 | 62.3±10.9 | 0.067 | 14.0±6.8 | 13.0±6.2 | 0.659 |
| Tibials | 42.6±22.2 | 48.3±20.1 | 0.567 | 5.2±3.6 | 9.7±8.1 | 0.165 | |
| SMOKING | Popliteal | 65.8±4.8 | 54.4±16.8 | 0.052 | 15.8±5.5 | 9.0±6.0 | 0.014 |
| Tibials | 55.2±11.1 | 35.2±20.1 | 0.036 | 13.1±8.4 | 5.3±4.9 | 0.044 | |
| AGE>60 | Popliteal | 61.6±9.9 | 57.9±11.4 | 0.346 | 13.6±6.4 | 13.96.2 | 0.898 |
| Tibials | 47.5±20.2 | 42.6±20.3 | 0.612 | 7.9±6.1 | 7.6±8.5 | 0.932 | |
| Hyperlipidemia | Popliteal | 59.6±12.2 | 59.7±12.0 | 0.97 | 13.3±5.6 | 13.5±7.2 | 0.95 |
| Tibials | 45.1±17.1 | 47.6±23.7 | 0.76 | 8.4±6.6 | 7.2±6.9 | 0.666 | |
Table 2b.
Clinical factors: Associations with dense calcium and necrotic core plaque compositions.
| Dense Calcium | Necrotic Core | ||||||
|---|---|---|---|---|---|---|---|
| Clinical Factor | Vessel | Present | Absent | P-Value | Present | Absent | P-Value |
| Popliteal | 11.5±6.3 | 11.9±10.0 | 0.905 | 14.7±6.3 | 16.4±8.7 | 0.5 | |
| NIDDM | Tibials | 29.8±19.5 | 21.6±23.8 | 0.54 | 21.0±4.0 | 11.8±11.0 | 0.106 |
| Popliteal | 14.2±11.7 | 10.1±8.5 | 0.223 | 16.8±6.7 | 14.6±7.9 | 0.389 | |
| CRF | Tibials | 33.8±24.9 | 23.2±25.1 | 0.376 | 18.4±5.8 | 18.8±13.7 | 0.94 |
| Popliteal | 6.9±3.9 | 18.5±13.8 | 0.019 | 11.4±5.5 | 18.2±8.6 | 0.048 | |
| SMOKING | Tibials | 14.8±11.0 | 38.0±27.2 | 0.055 | 16.9±9.1 | 21.5±14.8 | 0.488 |
| Popliteal | 10.0±7.2 | 12.3±10.9 | 0.467 | 14.8±7.8 | 15.9±5.9 | 0.698 | |
| AGE>60 | Tibials | 26.3±24.0 | 30.0±23.1 | 0.743 | 18.3±10.7 | 19.8±9.0 | 0.767 |
| Popliteal | 11.9±10.7 | 11.3±9.8 | 0.847 | 15.2±5.7 | 15.6±8.9 | 0.884 | |
| Hyperlipidemia | Tibials | 24.9±22.2 | 30.0±25.3 | 0.603 | 21.7±11.8 | 15.2±6.6 | 0.12 |
Mean rates of change for each plaque progressing distally were calculated. (Table 3) Dense calcium was found to increase on average at rate of 0.02 mm2/cm in popliteals and 0.13mm2/cm in tibials as we progress distally. Fibrous, fibro-Fatty, and necrotic core plaques were found to decrease in the popliteal artery at −0.43 mm2/cm, −0.05 mm2/cm, and −0.01 mm2/cm respectively. Fibrous and necrotic core plaques were found to increase at 0.04 mm2/cm and 0.14 mm2/cm in tibials while fibro-fatty plaque decreased at −0.17 mm2/cm. Differences in the mean rate of change for each plaque were present although no significance was noted between vessel types.
Table 3.
Rate of increase of plaque type progressing distally (mm2/cm).
| Dense Calcium | Fibrous | Fibro-Fatty | Necrotic Core | |
|---|---|---|---|---|
| Popliteal | 0.02 | −0.43 | −0.05 | −0.01 |
| Tibial | 0.13 | 0.04 | −0.17 | 0.14 |
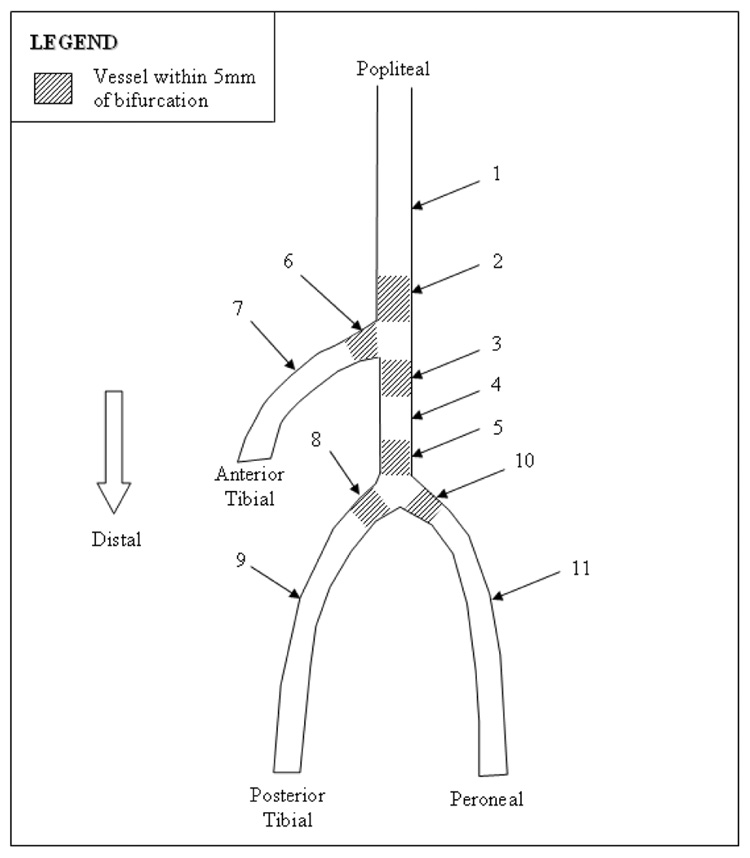
Plaque composition was also broken down to evaluate plaques within 5mm (proximally and distally) of bifurcations. (Figure 1 and Table 4). The distal popliteal showed a reduced plaque burden compared to the mid popliteal (56.2±9.9% v. 64.0±9.6%). Plaque burden was found to increase in the proximal trunk and mid-trunk to 58.1±8.6%, 59.9±9.0% respectively.
Figure 1.
Plaque composition by location.
Table 4.
Plaque burden and composition by arterial location.
| Vessel Region | Artery Region | n | Plaque Burden | Fibrous | Fibro-Fatty | Dense Calcium | Necrotic Core |
|---|---|---|---|---|---|---|---|
| 1 | Mid Popliteal | 29 | 64.0±9.6 | 60.6±11.0 | 12.8±6.6 | 12.7±10.6 | 16.6±8.0 |
| 2 | Distal Popliteal | 6 | 56.2±9.9 | 56.5±5.7 | 9.8±5.7 | 10.9±4.5 | 22.7±7.6 |
| 3 | Proximal Trunk | 5 | 58.1±8.6 | 60.0±12.6 | 14.4±6.4 | 10.2±10.3 | 15.4±9.3 |
| 4 | Mid Trunk | 3 | 59.9±9.0 | 54.6±18.5 | 11.6±2.6 | 9.3±0.3 | 15.4±4.7 |
| 5 | Distal Trunk | 4 | 51.9±4.3 | 57.9±14.2 | 10.7±6.6 | 14.5±12.6 | 16.9±5.2 |
| 6 | Proximal Anterior Tibial | N/A | N/A | N/A | N/A | N/A | N/A |
| 7 | Mid Anterior Tibial | 5 | 57.0±5.5 | 39.8±25.3 | 3.7±3.3 | 38.7±28.6 | 19.7±3.1 |
| 8 | Proximal Posterior Tibial | 2 | 49.7±0.4 | 62.8±4.8 | 11.8±8.3 | 12.0±11.9 | 13.4±1.2 |
| 9 | Mid Posterior Tibial | 2 | 49.8±4.8 | 67.3±4.1 | 7.8±6.7 | 13.4±7.0 | 11.5±3.8 |
| 10 | Proximal Peroneal | N/A | N/A | N/A | N/A | N/A | N/A |
| 11 | Mid Peroneal | 2 | 50.1±11.5 | 29.4±25.7 | 3.0±3.4 | 42.7±45.4 | 18.9±7.8 |
Discussion
Quantitative grayscale IVUS data is limited to geometric measurements that include diameters and areas that define the vessel. These measurements are based on user identified vessel contours that may be automatically detected or manually defined by an experienced user.
Grayscale IVUS cannot provide quantitative information on histologic tissue composition9. Those well versed with grayscale IVUS interpretation may make general qualitative assessments of the vessel atherosclerotic tissue components that include soft plaques typically include diffuse lipid infiltration, fibrous plaques, and calcified plaques based on tissue echogenicity. Difficulty arises due to an overlap of echogenicity between tissue types leading to potential misclassifications. Thrombus further complicates interpretation since it provides a similar grayscale representation to atherosclerotic plaques10 and its interpretation should only be considered presumptive.
Grayscale IVUS images are comprised of only a fraction of available raw IVUS data. Raw image data undergoes amplification, filtering, time gain compensation and an array of other processes to display what in reality is only the envelope of the original time-domain signal. These image processing steps are performed to yield an optimal grayscale image.
VH-IVUS™ tissue data derived from raw IVUS RF data provides additional data over conventional grayscale IVUS. VH-IVUS™ begins with raw IVUS backscattered data from tissue located within predefined IEL and EEL contours. An autoregressive method extracts frequency spectral information. Predefined spectral parameters are then assessed using an algorithm to define tissue as one of four atherosclerotic plaque types: Fibrous, Fibro-Fatty, Necrotic Core, or Dense Calcium.
Although the tissue characterization algorithm was originally developed for the coronary arteries, the CAPITAL study11 showed strong correlation between VH IVUS™ and histopathology results for carotid arteries. In addition, our group is in the process of developing a peripheral version of the tissue characterization with current preliminary unpublished accuracy results similar to that published by Nair et. al6. The published accuracies for carotid arteries combined with our peripheral results provide sufficient confidence to currently use the coronary developed algorithm in the peripherals until a peripheral version is formally available.
As we had hypothesized, atherosclerotic plaques found in the tibial arteries contain higher percentages of dense calcium than the popliteal artery. The abrupt calcification beginning just distal to the popliteal artery was felt during experimentation, but not quantitatively known until the results of the VH IVUS™ were complete. In addition, the dense calcium cross sectional area also increased progressing distally for both tibial and popliteals. With the significant difference in dense calcium, it was logical to expect other plaque types to vary.
Many studies have used electron beam computed tomography to investigate calcification and associations with demographic and clinical factors. Chronic kidney disease (CKD) has been shown to influence cardiovascular calcification7, 12–14 with severity of the disease linked to the prevalence and intensity of calcification. Although the effects were primarily studied in the coronary arteries, the pathobiology factors may be systemic. Fuchs2 et al. reported that spotty and linear calcifications are more prone in lower extremity arteries of diabetic patients. Increased age, elevated LDL, and smoking have also been shown to increase calcification15.
The lack of association between demographics and clinical factors observed in our study was unexpected. The severity of the vascular disease leading to amputation was suspected to outweigh other factors in determining the atherosclerotic plaque characteristics. Calcification amongst smokers in our study was actually lower than in non-smokers which is opposite of that reported by other studies15. Fuchs2 et al. reported that spotty and linear calcifications are more prone in lower extremity arteries of diabetic patients and while not significant; our data did tend to show higher calcification levels in the tibial arteries of diabetics.
Coronary research provides much of the knowledge we currently have on atherosclerosis and its known mechanisms due to minimal amounts of data available directly from the periphery. Coronary knowledge has been extrapolated and applied to the periphery to aid in our understanding. However, the relationship between coronary and peripheral atherosclerosis is not fully understood with some evidence suggesting possible differences in disease progression16, 17. Our data may suggest that based on sharp variations between popliteal and tibial vessels, that differences between these vessels and other arterial beds throughout the body are likely. This information may be critical in developing artery specific endovascular technologies including angioplasty balloons, specific stent design, atherectomy devices, etc. Our goal is to have a validated in vivo IVUS tool to aid the clinician in the treatment of PAD.
Our study has limitations. Given the limited sample size, we may have Type II errors. Additional samples will aid in confirming the observed correlations. Even though a majority of amputations were done for pedal sepsis, proximal arterial specimens presumably represent a more virulent process than other patients with PAD.
An additional limitation is present with the selection of vessels due to the severity of PAD. IVUS catheter access could not be obtained in many of the extreme examples of PAD to obtain data so these specimens were excluded from all analysis. This may potentially alter our findings. While an atherectomy may be possible to obtain catheter access, it is unknown what effects this may have on plaque characterization. Nair6 et al. only used vessels with no prior interventions in their research on plaque characterization.
Thus, in vivo plaque characterization during angiography is warranted and presently ongoing to confirm our results in patients with PAD requiring endovascular intervention. Further research investigating the atherosclerotic mechanisms in lower extremity atherosclerosis would be valuable to aid in explaining the differences observed in our research with respect to previously published coronary data.
Conclusion
As we progress distally in the arterial tree of patients with PAD, calcium plaque content increases with decreasing burden of fibro-fatty plaque. Clinical and demographic factors with the exception of smoking and chronic renal failure were not found to be associated with atherosclerotic plaque composition or plaque burden.
Acknowledgments
Funded by the NIH/NHLBI, R01 HL075721
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the Peripheral Vascular Surgery Society 18th Annual Winter Meeting, Snowmass, CO, February 1–3, 2008
References
- 1.Rumberger JA, Simons DB, Fitzpatrick LA, et al. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area: a histopathologic correlative study. Circulation. 1995;92:2157–2162. doi: 10.1161/01.cir.92.8.2157. [DOI] [PubMed] [Google Scholar]
- 2.Fuchs U, Caffier P, Schulz HG, et al. Arterial calcification in diabetics. Virchows Arch A Pathol Anat Histopathol. 1985;407:431–439. doi: 10.1007/BF00709989. [DOI] [PubMed] [Google Scholar]
- 3.Lehto S, Niskanen L, Suhonen M, et al. Medial artery calcification. A neglected harbinger of cardiovascular complications in non-insulin-dependent diabetes mellitus. Arterioscler Thromb Vasc Biol. 1996;16:978–983. doi: 10.1161/01.atv.16.8.978. [DOI] [PubMed] [Google Scholar]
- 4.Kashyap VS, Pavkov ML, Bishop PD, et al. Angiography underestimates peripheral atherosclerosis: lumenography revisited. J Endovasc. Ther. 2008 Feb;15(1):117–125. doi: 10.1583/07-2249R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klingensmith J, Schoenhagen P, Tajaddini A, et al. Automated three-dimensional assessment of coronary artery anatomy with intravascular ultrasound scanning. Am Heart J. 2003;145:795–805. doi: 10.1016/S0002-8703(03)00089-9. [DOI] [PubMed] [Google Scholar]
- 6.Nair A, Kuban BD, Tuzcu EM, et al. Coronary plaque classification with intravascular ultrasound radiofrequency data analysis. Circulation. 2002 Oct 22;106(17):2200–2206. doi: 10.1161/01.cir.0000035654.18341.5e. [DOI] [PubMed] [Google Scholar]
- 7.Nasu K, Tsuchikane E, Katoh O, et al. Accuracy of in vivo coronary plaque morphology assessment. A validation study of in vivo virtual histology compared with in vitro histopathology. J Amer Coll Cardiol. 2006;47:2405–2412. doi: 10.1016/j.jacc.2006.02.044. [DOI] [PubMed] [Google Scholar]
- 8.Klingensmith J, Vince D, Kuban B, et al. Assessment of coronary compensatory enlargement by three-dimensional intravascular ultrasound. Int. J Card Imaging. 2000;16:87–98. doi: 10.1023/a:1006333619358. [DOI] [PubMed] [Google Scholar]
- 9.Mintz GS, Nissen SE, Anderson WD, et al. A report of the American College of Cardiology Task Force on Clinical Expert Consensus. American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies (IVUS) [DOI] [PubMed] [Google Scholar]
- 10.Di Mario C, Go¨rge G, Peters R, et al. Clinical application and image interpretation in intracoronary ultrasound. Study Group on Intracoronary Imaging of the Working Group of Coronary Circulation and of the Subgroup on Intravascular Ultrasound of the Working Group of Echocardiography of the European Society of Cardiology. Eur Heart J. 1998;19:207–229. doi: 10.1053/euhj.1996.0433. [PMID: 9519314] [DOI] [PubMed] [Google Scholar]
- 11.Diethrich EB, Pauliina Margolis M, Reid DB, et al. Virtual Histology Intravascular Ultrasound Assessment of Carotid Artery Disease: The Carotid Artery Plaque Virtual Histology Evaluation (CAPITAL) Study. J Endovasc Ther. 2007 Oct;14(5):676–86. doi: 10.1177/152660280701400512. [DOI] [PubMed] [Google Scholar]
- 12.Goodman WG, Goldin J, Kuizon BD, et al. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med. 2000;342:1478–1483. doi: 10.1056/NEJM200005183422003. [DOI] [PubMed] [Google Scholar]
- 13.Ketteler M, Schlieper G, Floege J. Calcification and cardiovascular health: new insights into an old phenomenon. Hypertension. 2006;47:1027–1034. doi: 10.1161/01.HYP.0000219635.51844.da. [DOI] [PubMed] [Google Scholar]
- 14.Johnson RC, Leopold JA, Loscalzo J. Vascular calcification: pathobiological mechanisms and clinical implications. Circ Res. 2006;99:1044–1059. doi: 10.1161/01.RES.0000249379.55535.21. [DOI] [PubMed] [Google Scholar]
- 15.Qunibi WY. Reducing the burden of cardiovascular calcification in patients with chronic kidney disease. J Am Soc Nephrol. 2005;16 suppl 2:S95–S102. doi: 10.1681/ASN.2005060666. [DOI] [PubMed] [Google Scholar]
- 16.Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113(11):e463–e654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 17.Norgren L, Hiatt WR, Dormandy JA, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) J Vasc Surg. 2007;45 Suppl S:S5–67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]